Abstract
Titanium carbide ceramic particles could act as inoculants to generate fine microstructure and helps to increase the wear resistance for steel. In this article, the effect of titanium addition on the microstructure, wear and mechanical properties of Ni-Hard4 white cast iron was studied. The study was undertaken with five laboratory pieces of cast iron made with different titanium amounts. The microstructures were examined by optical microscopy, scanning electron microscopy equipped by EDS. The impact energy, hardness and wear resistance of the samples were determined. Thermodynamic calculation showed that solute titanium atoms can react with carbon and form TiC in modified samples. The results indicated that the morphology of chromium carbides can be improved by adding a suitable amount of titanium. This improvement was correlated with the emergence of TiC ceramic particles. These particles can act as the substrates for heterogeneous nucleation of primary chromium carbides, which result in significant refinement of the final average carbide diameter. In addition, the hardness and wear resistance were improved without significant variations in fracture toughness. The wear resistance was increased with titanium addition owing to change microstructure and the precipitation hardening of fine ceramic particles in the martensitic matrix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ceramic particle reinforced metal matrix composite has higher mechanical properties and wear resistance than its matrix material because the ceramic particles can strongly resist abrasive wear [1, 2]. Recently, TiC ceramic particulate locally reinforced metal matrix composites fabricated by different methods such as conventional melting and casting [3], powder metallurgy [3], reactive sintering [4] and Self-propagating high-temperature synthesis [5]. Hence, casting compared to other methods offers advantages with respect to simplicity and economy for industrial applications.
Decrease in common shutdown of apparatus for replacement of worn out components and a subsequent decrease in costs, have encouraged engineers to evaluate candidate alloys which can provide much better abrasive wear resistance along with adequate toughness [6].
Ni-Hard4 white cast iron (NHWCI) is excellent wear-resistant material and has been widely used in wear-affected equipment operated under extreme conditions, such as in mineral processing, cement, copper and iron manufacturing. NHWCI is also used to produce the liners of the AG mill in Chadormalu mining and Industrial Company in Iran, (the most dominant iron ore concentrate producer in the Middle East). Most of the tribological research on NHWCI indicates that wear resistance of NHWCI results primarily from the high volume fraction of hard chromium carbides, although the toughness of the matrix also contributes to the wear resistance [7].
Researchers have reported improvements in the wear resistance and mechanical properties of cast iron without reduction in impact toughness by adding strong carbide-forming elements, such as vanadium, tungsten, titanium and niobium [8, 9]. Zhong et al. [10] reported the improvement of hardness and wear resistance of the gray cast iron by the addition of in situ synthesized TiC particulates in the iron matrix. Chung et al. [11] reported the effects of titanium addition on microstructure and wear resistance of hypereutectic high chromium cast iron. Moreover, Wu et al. [12] reported the effects of titanium on the morphology of primary M7C3 carbides in hypereutectic high chromium white iron. Mousavi et al. [13] reported the effects of tungsten on erosion-corrosion behavior of high chromium white cast iron. Zhi et al. [14] worked on the effect of niobium on the microstructure and mechanical properties of hypereutectic high chromium cast irons.
The aim of these studies was refinement of primary chromium carbides and increasing the carbide nucleation rate to obtain dispersed fine carbides. This improved the hardness of the matrix without reduction in impact toughness and improved the wear resistant of NHWCI by means of in situ formation of the TiC ceramic particles in casting process by a liquid reaction during the solidification process. This promoted an adequate bonding between the matrix and the reinforcements. However, no systematic work has been performed to study the effects of titanium addition on structure and properties of NHWCI. In the present study, various percentages of titanium were added to NHWCI, and the effect of titanium carbide ceramic particles on the microstructure, mechanical properties and wear resistance of NHWCI were investigated.
2 Experimental
The alloys were prepared in a 400 kg capacity medium frequency induction furnace. Initial charge materials were clean steel scrap, low silicon pig iron and Ferro-alloys such as Fe-65%Cr and Fe-35%Ti which was added to a slag-free molten steel to minimize the oxidation loss and the slag formation. The melt, after removal of any dross and slag, was poured at 1480 °C into the CO2-silicate moulds. The size of the specimen was 100 mm × 100 mm × 200 mm. Alloys were heat treated in an electric furnace in an ambient atmosphere at 840 °C for 2 h, followed by air quenching to room temperature. The microstructures of the specimen were characterized by using the Olympus Optical Microscope (OM) and Scanning Electron Microscopy (SEM) in a Philips XL30 at an accelerating voltage of 20 kV equipped with an energy dispersive X-ray spectrometer (EDS). Metallographic samples (15 × 15 × 10 mm) for OM and SEM observations were etched with Nital 2 % etchant. The volume fraction of carbides and average carbide diameter (D) were measured by using Clemex Image Analyzer software from 10 OM images of each specimen (randomly selected) at magnification of 200×. The following equation was used to determine carbide diameter refined due to the effect of titanium:
where A is the area of the carbides (µm2) and N is the number of the carbides [14].
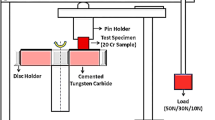
Mechanical properties were also studied according to standard ASTM: A370. Impact toughness tests were carried out on a Galdabini Charpy impact universal testing machine; all tests were performed on three samples. In addition, the samples hardness was measured on EMCO Rockwell hardness tester and 10 measurements were taken across each sample to obtain the average value of hardness. The wear resistance of samples were evaluated using a dry sand/rubber wheel abrasion wear test method in accordance with ASTM: G65. AFS 50/70 sand was used, and the sand flow rate was adjusted to 300–400 g/min. A testing load of 130 N was selected. The total wear distance and rotation speed were 4500 m and 250 rpm, respectively. The wear resistances of alloys were ranked in terms of the weight loss [11–14].
3 Results and discussion
The chemical composition of all samples which were used in the study is presented in Table 1. While similar amounts of carbon, chromium, nickel, molybdenum, manganese and silicon were obtained for the alloys; the only variable element was titanium whose amount varied from 0 to 1.05 %. Since the pouring condition and cooling rate were the same for all the alloys, variations in microstructure as well as in mechanical properties was considered to be due to the effect of different titanium carbide amounts.
3.1 Thermodynamic of formation TiC ceramic particles
The standard free energy variation for each reaction \(\left( {\varDelta G_{f}^{0} } \right)\) depends on the standard enthalpy change \(\left( {\varDelta H_{f}^{0} } \right)\) and the standard entropy change \(\left( {\varDelta S_{f}^{0} } \right)\) as follows:
It is well known that TiC is the most probable carbide of Ti (melting point 3340 K), [15–18]. Richardson [18] reported for the first time, the Gibbs energy of formation of TiC corresponding to the reaction Ti + C \(\to\) TiC to be:
\(\varDelta G_{f}^{0}\) (TiC) = −44,600 + 3.16 T Cal/mole (±3000 Cal) in the temperature range 1150–2000 K. In addition, three carbides of Cr have been reported [18]: Cr23C6 (melting point 1848 K), Cr7C3 (melting point 2038 K), and Cr3C2 (melting point 2083 K). Measured \(\varDelta G_{f}^{0}\) for the reaction\(\frac{23}{6}\) Cr + C \(\to \frac{1}{6}\) Cr23C6 is:
\(\varDelta G_{f}^{0}\) (Cr23C6) = −12,833 − 3.05T Cal/mole (±1200 Cal) for the temperature range 1150–1300 K, for the reaction Cr23C6 + C \(\to \frac{23}{7}\) Cr7C3 is:
\(\varDelta G_{f}^{0}\) (Cr7C3) = −29,985 − 7.41T Cal/mole (±400 Cal) for the temperature range 1100–1720 K and for the reaction Cr7C3 + C \(\to \frac{7}{5}\) Cr3C2 is:
\(\varDelta G_{f}^{0}\) (Cr3C2) = −9840 − 2.64T Cal/mole (±400 Cal) for the temperature range 1300–1500 K.
Moreover, the result of thermodynamic analysis indicates that the change in free energy for the formation of TiC is much more negative than other possible reactions such as
It is clear that the formation of TiC ceramic particles can occur before any phenomena during solidification. Consequently, the formation of TiC may begin at higher temperatures than the formation of chromium carbides. Therefore, it is inevitable for the high melting point particles, such as, TiC to act as heterogeneous nucleation site for chromium carbides. Uniform distribution of TiC in the matrix, ensures significant refinement and distribution of the chromium carbides [19].
3.2 Microstructure of (NHWCI)
Chromium and nickel are the main alloying elements in NHWCI; chromium increases the hardness and wear resistance and nickel improves the combination of toughness, strength and hardenability [20]. The as-cast and heat treated microstructures of the titanium free NHWCI (sample Ti0), are shown in Fig. 1. The SEM micrographs of the as-cast condition consist of austenite (γ) matrix and primary chromium carbides (Fig. 1a, b in different magnification). Tabrett et al. [21] has clearly summarized the solidification path followed by these kinds of alloys; solidification process starts with the formation of austenite dendrites which grow while the temperature decreases and the remaining liquid reaches the eutectic composition; then the eutectic reaction occurs and the coupled austenite/carbide eutectic develops. In the as-cast microstructure, austenitic matrix remains as a metastable phase at room temperature due to the high amount of alloying elements, particularly nickel and carbon that lowers the martensite start temperature (M S) to temperatures below room temperature. After heat treatment, the precipitation of the secondary chromium carbide in the austenite matrix occurs and absorbs carbon from its surroundings area. Therefore, the carbon content of matrix decreases. The depletion of carbon in the austenite matrix increases the M S temperature, which allows austenite to transform into martensite during cooling after heat treatment [22]. The microstructure of the sample Ti0 after heat treatment is shown in Fig. 1c, d. This is a typical microstructure of NHWCI, which consists of chromium carbides in the martensitic matrix and the amount of retained austenite. The figure confirms the transformation of austenite phase (γ) in the as-cast structure to martensite after the heat treatment. The typical microstructure of the matrix should consist of austenite and martensite [6]. Moreover, hardness measurements give values of 39 ± 3 HRC in the as-cast condition and 50 ± 3 HRC after heat treatment. The increase in hardness after heat treatment can be attributed to the formation of the martensitic matrix that is harder than austenite. The improvement of hardness can be correlated qualitatively to the improvement of wear resistance. It has been reported that after heat treatments, in general, higher abrasion wear resistance is achieved [19, 22].
3.3 Influence of titanium on the microstructure
The microstructural changes of the NHWCI with various amounts of titanium addition are illustrated in Fig. 2. The microstructural changes after titanium addition is a fragmentation of the primary chromium carbide network, which changes the carbides morphology and decreases the average carbide diameters due to a partial replacement of the primary chromium carbide with TiC ceramic particles. Figure 3 shows the effect of titanium concentration on the volume fraction and the average diameters of carbides. It can be seen that the chromium carbides are refined gradually with the increase of titanium concentration.
Titanium is wildly used in steel and cast iron manufacturing as an effective alloying element for partitioning the matrix as well as modifying the carbides [8, 11]. Titanium is one of the strong carbide forming elements; therefore, TiC can easily be incorporated into the melt during solidification. It may be deduced that TiC ceramic particles are formed at some temperature higher than austenite dendrite formation (during solidification) and that these small carbides are entrapped by the growing austenite dendrites. Hence TiC particles are the first to solidify (based on thermodynamic calculation results). So they play a very important role as heterogeneous nuclei of the chromium carbides [23]. In other word, the formation of the first precipitation of TiC depletes certain carbon, thus reducing the volume fraction of chromium carbides.
In Fig. 4, it has been proved based on results of SEM micrograph and EDS analysis that TiC can act as the heterogeneous nuclei of chromium carbides. EDS analysis by a fine electron beam indicates only the presence of carbon and titanium in the particle. Presence of any other element in the EDS analysis undertaken by SEM may be due to the matrix interaction with the beam. The nucleation substrate and matrix solids have to be coherent or semi coherent, during solidification. The energy on the newly formed interface must be lower than the surface energy of the same area, if the interface is formed directly during the solidification process. However, strain energy is present if there is no full compliance between the nucleation substrate and matrix solids. To reduce this strain energy, it requires maximum atom accordance on the interface. So the lattice disregistry between two phases has a great importance in determining whether the new substance can be the substrate for the heterogeneous nucleation [24, 25]. According to the disregistry theory of Turnbull and Vonnegut, some inclusions can act as the heterogeneous nucleus of the new crystalline phase. A mathematical model of the two phases’ lattice parameter misfit is [12]:
In this regard, δ is the disregistry of lattice parameter, a N and a C are low-index planes of the nucleated solid phase and the substrate, respectively. According to Bramfitt [26], during heterogeneous nucleation, the nuclei are most effective with a disregistry below 6 %, while those with δ between 6 and 12 % are medium effective and those with δ above 12 % are ineffective. TiC has a face-centered cubic (fcc) lattice like NaCl lattice parameter a = 0.46 nm. The chromium carbide (M7C3) has close-packed hexagonal structure with the lattice parameter a = 0.688 nm and c = 0.454 nm [27]. Thus with a disregistry of 1.32 %; TiC can act as an effective heterogeneous nuclei of M7C3 carbide.
3.4 Influence of titanium on the mechanical properties
The bulk hardness of the samples have been measured to evaluate the effect of TiC ceramic particles on the bulk hardness of the NHWCI. Results of the measurements are presented in Fig. 5. Figure 5 shows that the hardness increases with increasing amount of TiC ceramic particles. It appears that the dispersion strengthening of matrix strongly influences the increase in bulk hardness in spite of the finer structure resulting from titanium additions. In addition, the dispersed TiC particles, which are harder than chromium carbides, embedded in a fine eutectic microstructure, further enhances the bulk hardness. Therefore, Ti addition promotes a partial replacement of the primary chromium carbide and also a fragmentation of the carbide network in accordance with the average carbide diameter (Fig. 3). This is the most important mechanism for increasing the bulk hardness in modified alloys with titanium. Moreover, an increase in the amount of titanium dissolved in matrix, may have contributed to the matrix strengthening by solid solution; however, such a contribution is negligible compared to the dispersion strengthening. The impact energy does not show a clear trend as the amount of TiC ceramic particles increases in the NHWCI. Increase in bulk hardness can imply a decrease in fracture toughness. However an increasing hardness, seemingly accompanied by effective parameters such as particle dispersion, decrease in volume fraction of carbide, and structure refinement, can also prevent the reduction of impact toughness of NHWCI.
3.5 Influence of titanium on the wear resistance
The weight losses of the NHWCI specimens with different nominal concentrations of TiC ceramic during abrasive wear testing are measured and the results are illustrated in Fig. 6. Figure 6 reveals that the concentration of titanium dominates the wear resistance of the NHWCI. Generally, wear resistance depends on matrix microstructure, carbide types and their volume fraction, fracture toughness and the hardness of the alloys. Abrasive like SiC (2600 HV 0.05), much harder than both the matrix and carbide phases of Ni-Hard 4 specimens, is able to penetrate into the surface of the specimens and remove significant material during sliding. This result indicates that TiC ceramic particles plays an important role in improving the wear resistance of the NHWCI due to change in the microstructure and hardness. The cross-sectional microstructures of two samples (Ti 0 and Ti 100) after wear test are compared in Fig. 7. It illustrates that carbides protrude from the surface further than the martensitic matrix due to its higher hardness. These results demonstrate that carbide particles improve the degree of resistivity to wear. These figures also show the nucleation sites and propagation paths of cracks during the wear process. The microcracks are initiated at the interfaces between the martensite matrix and carbide particles. The micro cracks propagate through the carbide particles and are blocked by the matrix because carbides possess poor ductility and have a large difference in hardness from the martensite matrix. Bradley et al. [28] believed that the decrease in the fraction of the eutectic carbides, which forms a network, improved the fracture toughness by shortening the crack propagation distance. Based on these results, the wear sequence may be deduced as follows: in the first step, the martensite matrix is worned out such that, the carbide particles protrude out of the surface. Secondly, the stress concentration on the protruded carbide particles lead to the creation of cracks near or at the carbide particles, after which the carbide particles finally fall off the martensite matrix.
4 Conclusions
This paper was carried out to investigate the effect of titanium concentration on the microstructure, mechanical properties and wear resistance in Ni-Hard4 white cast iron. The following conclusions can be derived from experiments:
-
The addition of titanium causes the formation of titanium ceramic particles that promote a partial replacement of the primary chromium carbide and also a fragmentation of the primary carbide network because TiC can act as heterogeneous nucleation site for chromium carbides in the reaction, and they lead to significant refinement and distribution the chromium carbides.
-
By Ti addition, without any sharp decreases in impact toughness, hardness of sample increases due to the formation of hard TiC particle and fragmentation of chromium carbide network. It is also possible that the titanium dissolved in matrix, may have contributed to the matrix strengthening by solid solution.
-
The wear resistance of the Ni-hard cast improves with the addition of Ti for the following reasons: (a) formation of hard TiC ceramic particles (b) significant refinement of the primary chromium network.
-
The wear sequence of the Ni-hard cast iron is as follows: firstly, the martensite matrix is worned out such that chromium carbides and TiC ceramic particles protrude from the surface. Secondly, the stress concentration on the protruded carbides particles create cracks near or on them.
References
Liu J and Liu Z, Mater Lett 64 (2010) 684.
Cook B A, Peters J S, Harringa J L, and Russell A M, Wear 271 (2011) 640.
Das K, Bandyopadhyay T K, and Das S, J Mater Sci 37 (2012) 3881.
Shi L, Zhang J, Wang L, Jiang W, and Chen L, J Mater Sci Technol 27 (2011) 239.
Liang Y, Han Z, Zhang Z, Li X, and Ren L, Mater Des 40 (2012) 64.
Jinzhu L, Shizhuo L, and Yongfa M, Wear 166 (1993) 37.
Tang X H, Chung R, Li D Y, Hinckley B, and Dolman K, Wear 267 (2009) 116.
Bedolla-Jacuinde A, Correa R, Quezada J G, and Maldonado C, Mater Sci Eng A 398 (2005) 297.
F. M. M., Hem Ind 68 (2014) 413.
Zhong L, Xu Y, Hojamberdiev M, Wang J, and Wang J, Mater Des 32 (2011) 3790.
Chung R J, Tang X, Li D Y, Hinckley B, and Dolman K, Wear 267 (2009) 356.
Wu X, Xing J, Fu H, and Zhi X, Mater Sci Eng A 457 (2007) 180.
Mousavi Anijdan S H, Bahrami A, Varahram N, and Davami P, Mater Sci Eng A 454–455 (2007) 623.
Zhi X, Xing J, Fu H, and Xiao B, Mater Lett 62 (2008) 857.
Shatynski S, Oxid Met 13 (1979) 105.
Rudy E, Compendium of Phase Diagram Data. Air Force Materials Laboratory, Metals and Ceramics Division, Ohio (1967).
Hansen M, Constitution of Binary Alloys. McGraw-Hill, New York (1936).
Natesan K, and Kassner T F, Metall Trans 4 (1973) 2557.
Zhi X, Xing J, Gao Y, Fu H, Peng J, and Xiao B, Mater Sci Eng A 487 (2008) 171.
Chen X, and Li Y, Mater Sci Eng A 528 (2010) 770.
Tabrett C P, Sare I R, and Ghomashchi M R, Int Mater Rev 41 (1996) 59.
Scandian C, Boher C, de Mello J D B, and Rézaï-Aria F, Wear 267 (2009) 401.
Zhi X, Xing J, Fu H, and Gao Y, Mater Charact 59 (2008) 1221.
Mohammadnezhad M, Javaheri V, Shamanian M, Naseri M, and Bahrami M, Mater Des 49 (2013) 888.
Javaheri V, Shahri F, Mohammadnezhad M, Tamizifar M, and Naseri M, J Mater Eng Perform 23 (2014) 3558.
Bramfitt B L, Metall Trans 1 (1970) 1987.
Hetzner D W, and Van Geertruyden W, Mater Charact 59 (2008) 825.
Bradley W L, and Srinivasan M N, Int Mater Rev 35 (1990) 129.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Javaheri, V., Mohammadnezhad, M. & Bahrami, M. Microstructures, Wear Behavior and Mechanical Properties of the TiC Ceramic Particulate Locally Reinforced Ni-Hard4 White Cast Iron Matrix. Trans Indian Inst Met 69, 1571–1578 (2016). https://doi.org/10.1007/s12666-015-0731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0731-5