Abstract
The St. Mauritius mineral spring has been used since the late Bronze Age times (1410/1411 BC), as dated on the wooden headworks using radiocarbon and tree ring (dendrochronology) methods. It became eminent during the late Middle Ages epoch of curative treatments. The water is renowned for its high iron content and high amounts of dissolved and gaseous CO2. This special character of the Mauritius spring made it attractive to be analyzed by esteemed chemists over more than two centuries. By these analyses transferred in coherent units, a comparison of the chemical composition of this Mauritius mineral spring during the entire interval from 1788 to 2022 became possible. It reveals that the chemical composition of the Mauritius mineral spring water has largely remained stable and apart from some minor changes in water quality caused by alterations to the spring capture headworks since that time. These capture remediation actions were aimed to maintain the original composition of the Mauritius mineral spring water, which since its first utilization is threatened by the prevailing mixing processes with the shallow, low mineralized groundwater. The mineral water can be characterized as being an acidic calcium–sodium–magnesium–bicarbonate-type water, rich in iron, with a total mineralization of about 1.8 g/l. The spring discharge with a temperature of about 4–6 °C and contains about 2500 mg/l of free gaseous carbon dioxide. This high concentration of free outgassing of CO2 is due to the emission of this gas from a great depth from the Engadine Fault Line which crosses the spring discharge area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The St. Moritz mineral spring is situated in the Engadine Valley in southeastern Switzerland. It has an astonishingly long history of use from the Bronze Age to the present. Its famous Europe-wide reputation of curative effects made it attractive to be analyzed by esteemed chemists from the eighteenth century to the present. The available 18 analyses cover a time interval from 1788 to 2022, for a total of 234 years. The main aspects of the chemical analytical methods and their used units of length, volume, and weights, as also the literature research are described in the chapter “Materials and methods”.
In the next chapters, the following main aspects were discussed:
-
Hydrogeology and Geology
-
History of spring capture, from the first headworks in the Bronze Age until today
-
Medical/physiological properties and mythological background
-
Chemical properties of the Mauritius mineral spring water
-
Impacts of man-made activities on mineral water composition as identified by the chemical analyses
-
Mixing history: the struggle for the most “strong” mineral water
-
Conclusions: origin of Mauritius spring mineral water
-
Re-establishment of the original Mauritius mineral water spring. This chapter with a final discussion and outline of Mauritius mineral spring’s actual importance within today’s wellness trends concludes this publication.
Materials and methods
Literature research
Geology and hydrogeology
The selected literature covers the main works of current alpine geology from Kissling and Schlunegger (2018), Schmid et al. (2004) and Froitzheim and Manatschal (1996). Other sources of information are the regional geological studies by Peters (2005), and the local scale geological studies by Ohlendorf (1998). Information from the only geothermal exploration borehole (the San Gian borehole) was provided by Aemissegger (1993).
Sources of information for the historical use of the Mauritius mineral water (i.e., the tapping history)
Within this overview, the tapping/capture history of the spring site from the Bronze Age period to the present is outlined. Therein the latest archeological and hydrogeological information (within the historical time frame of continuous use since its discovery and first capture in 1411 BC by Bronze Age settlers to today) are presented. The information is based on the work of Oberhänsli (2017) as well as on the publications issued by the spa resort and by municipality and tourism organizations. These include the Overview of Forum Paracelsus, a travel guide, issued by Engadin St. Moritz Tourismus AG (2020); and the spa history by Hofmann (2014, 2017) The whole aspects of the excavation of the original wood headworks for the spring are outlined in the publication of Heierli (1907). Further historical aspects of Bronze Age culture as published by Leuzinger (2014), and unpublished documents and photographs of the municipality of St. Moritz, Department of infrastructures and, Margadant, Maier, Lütscher (1993, 2022), were also consulted.
Main medical/physiological properties
The medical and physiological aspects were based on the prominent publications of Käss (2008), named Deutsches Bäderbuch [with contributions of Michel (1997) and Hartmann (2008)], as also Litchfield (2006). Concerning the arsenic health limits, the publication by European Food Safety Authority (2014), De Boer and Hale (2000) and Apps et al. (2009) and the WHO Fact Sheet (2018) as well as direct information in the EAWAG News (2002) and two books by Hofmann (Heilbad-History (2014) and Gesundheits-Mythos St. Moritz 2017, 3rd Edition), were consulted. For the health effects of magnesium, the publication of Rosanoff (2013) was considered.
The aspects of CO2 concentration in air to human health are provided by Table 1 according to Kaiterra publications, https://learn.kaiterra.com/.
Concerning the radon concentration in water and indoor limits, the publications of Eidgenössische Kommission für Strahlenschutz (2017) were consulted. Concerning its therapeutic application for bronchial and rheumatic diseases by alternative natural medical practices, the references of Erickson (2007), Franke et al. (2007) are referenced. Concerning the radon health risks, the safety guidelines of the radiation health protection agencies were adopted. [e.g., by the German Federal Office for Radiation Protection, https://www.bfs.de/DE/themen/ion/umwelt/radon/wirkungen/heilkuren.html, 21.12.2022].
Mythological background of the prehistoric Mauritius spring site
The Mythological aspects of Mauritius mineral spring are mainly described in the study of Oberhänsli (2017) including Bronze Age votive treasures.
Another Bronze Age spring capture is described by Neyses-Eiden (2004) for the Trier Römersprudel.
Concerning the important aspect of red color deposits of iron oxide (hematite, ochre) for the Bronze Age people) reference is made to Roebroeks et al. (2012) and Wreschner et al. (1980).
The aspect of the cultic attraction of the uprising CO2-rich water springs was followed by a publication concerning antique Greek mythology (cultures of about the same time) by Verros et al. (2007), Jeffery (1976), De Boer and Hale (2000) and Stewart and Piccardi (2017).
Approach and methods of chemical analyses in the 18th century
To get a full record of the chemical parameters of all available analyses ranging from 1788 to 2022, a detailed recovery of all existing available literature containing old analyses since the eighteenth century was made as presented in: 12.1) References, Part 1: Bibliography of the analyses of the Mauritius spring water including some important notes about the methods applied in early chemical analyses.
This task included the recovery and understanding of the methods used by the analytical chemists as well as the conversion of the original published analyses expressed in local units and alchemistic terms into the chemical expressions and units used presently.
At the period of the first analysis of 1788, the methods for the investigation of natural waters had just commenced. It was also the period chemical principles and experimental approaches and laws began to evolve into modern-day chemical principles.
The different steps of analysis are mainly described within the referenced analysis reports. A quite complete bibliography of the analyses of the Mauritius spring water and of some also important notes about the methods applied in early chemical analyses is given in a separate Part 12.1 of the References. The complete story of the evolution of chemical knowledge from the early concepts of the Age of Enlightenment (late Renaissance period) to the beginning of the science of modern chemistry in the early twentieth century is described and discussed in detail by Pattison Muir (1907), re-edited in 1976.
As the methods of chemical analyses in the eighteenth century differ in concepts and available techniques from today’s analytical detection capabilities, they are here just described roughly. In general, the following main principles were applied:
-
The main instrumental physical methods consisted in balancing, and measuring weight, volumes, barometric pressure, and temperature.
-
The applied units depend on the period of execution of the analyses.
-
The chemical principles were applied according to the known properties of the reactions of the inorganic compounds. They included:
-
Precipitation,
-
Bringing in the solution of a substance to analyze under controlled conditions, concerning temperature, acidity, and concentration,
-
The controlled addition of specific acids, or specific bases (e.g., soda ash, sodium hydroxide),
-
The addition of a surplus of a reactant to force its precipitation, or in reverse sometimes adding a reactant, e.g., an acid to provoke dissolution,
-
Bringing solutions to the boiling point, also stepwise fractionation by distillation,
-
Filtration of the product of provoked crystallization to remove it before the next steps of separation were executed,
-
Provoke gaseous emanations from the water sample under investigation, e.g., by heating, but also by the addition of reagents such as sulfuric acid, and hydrogen chloride,
-
Adding dissolved substances to the water to force the precipitation of some compounds.
-
These different steps of analysis are mainly described within the referenced analysis reports. They had to be executed in successive order, with the final target that only one substance/product remains at the end, e.g., the most soluble, the least soluble, the densest, or heaviest.
An example of this quite difficult task of analyzing natural mineral water during the eighteenth century is provided in the oldest chemical analysis report dating from 1752 for the mineral water of Fachingen (Burggravens, Jo, Le Cerf, C., Senkenberg, J., C., cert. 1747, 1752) which has a quite similar composition to the St. Mauritius mineral spring water.
An example of the described analytical process is shown in the original published manuscript entitled: «Chemische Untersuchung einiger der bekanntern und besuchtern Gesundbrunnen und Bäder der Schweiz, insbesonders des Cantons Bern», by Morell, Carl Friedrich, Bern, 1788, (Zweyter Abschnitt, page 356–362). This analysis was also reported in Wettstein (1824), translated into Italian. A specific description and calculations according to today’s concepts are presented in a separate Appendix 1, which is not part of this publication.
Overview of ancient used weights and volumes
To compare this analysis of Morell 1788 with the analyses of the later centuries, the original units were converted into metrical units using the following factors: (Daniel-stieger.ch/masseinheiten.htm).
1 Unzen (1 troy ounce) = 31.1034768 g,
1 Civilpfund = 16 Unzen = = 497.6556288 g,
1 Apotheker Pfund = 12 Unzen = 373.2417216 g = 373.24 g,
1 Schoppen = 0.5 l \(\approx\) 500 g,
1 Gran (Grain*) equals: 64.799 mg,
1 Fuss (foot) = 1' = 12 Zoll (inch) = 0.31385 m,
1 Paris Zoll (inch) equals exactly 27.07 mm.
1 Paris cubic Zoll (cubic inch) equals 1728 Paris cubic lines = 19.835 cm3
According to the dimension and weight ordonnance of 16.5.1816, the grain is defined as:
1 preussisches Gran = 60.9 mg, and 16.42 preussische Gran = 1 g,
16 preuss. Unzen = 7680 Gran = 32 Loth = 1 Prussian (Civil Pound) = 467,711 g,
1 Loth = 14.616 g, therefore, the Prussian (Civil Pound) = 467,711 g,
1 Prussian Cubikzoll = 17,891 cm3.
(Geoscience Reference In-Depth Information: http://what-when-how.com/Tutorial/topic-65u94bp/Hydrogeologie-165.html (20.12.2022) and Aldefeld, C.K., 1835(Die älteren und neuen Maasse und Gewichte der Königlich Preussischen Rheinprovinz, Achen und Leipzig, 1835, by Jacob Anton Mayer).
Within the English/American books of standards, different definitions of the above mass units are stated:
“A grain is a unit of measurement of mass, and in the troy weight, avoirdupois, and Apothecaries' system, equal to exactly 64.79891 mg” (CRC, Handbook of Chemistry and Physics, 60th Edition, 1979–1980, Weast, R.C., Astle, M.J.: CONVERSION FACTORS).
According to NIST Handbook 44 2012 (the “grain” is same in avoirdupois, troy, and apothecaries’ units of mass), with the following conversions:
16 ounces = 1 pound (lb) = 256 drams = 7000 grains = 453.59237 g; therefore, 1/7000 pound equals a troy grain or 64.79891 mg.
12 oz troy = 1 pound troy (lb t) = 5760 grains = 373.24172 g; therefore, 1/5760 pound (lb t) equals also a troy grain or 64.79891 mg \(\to\) these units are equal to the 1 Apotheker Pfund = 12 Unzen = 373.2417216 g = 373.24 g, as used by Morell (1788).
Since the implementation of the international yard and pound agreement of 1 July 1959, the grain or troy grain (symbol: gr) measure has been defined in terms of units of mass in the International System of Units as precisely 64.79891 mg. One gram is thus approximately equivalent to 15.43236 grains.
National Institute of Standards and Technology (October 2011). Butcher, Tina; Cook, Steve; Crown, Linda, et al. eds. “Appendix C – General Tables of Units of Measurement” (PDF). Specifications, Tolerances, and Other Technical Requirements for Weighing and Measuring Devices. NIST Handbook. 44 (2012 ed.). Washington, D.C.: U.S. Department of Commerce, Technology Administration, National Institute of Standards and Technology. ISSN 0271-4027. OCLC OCLC 58927093.

Comment: These definitions in Furrer A., (1889) deviate slightly from the older units as stated in the Prussian Dimension and weight ordonnance of (16.5.1816) and as also provided by the definitions of the National Institute of Standards and Technology (October 2011). These deviations result from the change by merging the old units with the metrical dimension of:
1 Pfund equal to 16 Ounces equal to 500 g, and
1 Apothecaries pound equal to 12 Ounces to 375 g.
Finally, the conversion factors as presented in Morell (1788) of the Apothecaries system with
1 Apothecaries pound = 12 Ounces = 373.2417216 g = 373.24 g were used to convert the analyzed compounds original units in Gran (grain) per 12 Unzen (ounces) into metrical units as presented in a separate Appendix 1, which is not part of this publication.
Estimation of the accuracy of chemical analyses during the period of 1788 to 2022
The 18 available analyses date from 1788 to 2022.
In the first analysis of Morell (1788), the smallest indicated unit is 1/4 of grain. That would mean that if 1/1 Gran (Grain) equals: 64.79891 mg, an amount of 16,19973 mg. It is assumed that a skilled scientist could measure to about a differencing weight of 10 mg (about 1/8 grain).
From the nineteenth century to the beginning of the twentieth century, the precision of weighting was increased to about 1 mg. This precision was in later times further increased using electronic devices.
With the available analytical techniques developed towards the end of the 20th and up to the twenty-first century for chemical analyses, increased precision using new methods and new techniques such as Atomic Adsorption Spectroscopy (AAS), Ion-Chromatography (IC), Inductively Coupled Plasma, with Optical Emission Spectroscopy (ICP-OES), or Inductively Coupled Plasma Mass Spectrometry (ICP-MS) very much lowered the detection levels.
To compare all 18 analyses for the period 1788 to 2022 in a fair way, only an interpretation based on graphical representations is made, based on a relative qualification of the analysis results as presented in Table 2.
In addition, in Fig. 19, the concentrations are represented with error bars according to an estimated relative error of 10%, Figs. 21, 23, and 24 with estimated relative errors of 5%.
In considering the difficult processes of sampling free CO2 gas and of the Iron analysis, the analyzed concentrations are represented with error bars according to an estimated relative error of 10% in Figs. 20 and 22.
Geological and hydrogeological context of the origin of the Mauritius mineral water spring
Geology and tectonics of the Engadine area
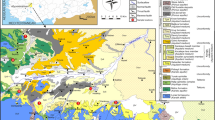
The Engadine valley is situated in the Eastern part of the Swiss Alps (Fig. 1). The mountainous complexes of this area were formed by the process of the Tertiary alpine orogeny in Europe, that have resulted from the collision of the Eurasian with the Adriatic (African) Plates, Kissling and Schlunegger (2018). Through these processes, sedimentary and crystalline rock units of the Paleozoic to Cretaceous age were shifted along major thrust lines above other units, leading to today’s structure of stapled and folded, so-called over-thrusted tectonic units or napes, Okaya et al. (1996) and Schmid et al. (2004). This shape of mountains is characteristic of the areas around the crystalline massifs in the center of the alps, such as the Aar Massif and the Mont Blanc Massif with the Helvetikum and Prealpes Napes in the North and the Penninic and Austroalpine napes units in the South (Fig. 2).

© Swisstopo2008).
 Position of St. Moritz,
Position of St. Moritz,
 trace line of the cross-section in Fig. 2
trace line of the cross-section in Fig. 2
Geological and Tectonic Map of Switzerland with main tectonic units, the Aar- and Mont Blanc massifs (crystalline core area), sedimentary filling of molasse basin, sedimentary over-thrusted napes complexes of Helvetikum, Prealpes in the North, the Penninic and Austroalpine crystalline and metamorphic sedimentary nape units in the South of the alpine Massifs. (According to Swiss Federal Topographical Survey,

© swisstopo 2008). Also shown is the position of the Engadine Fault Line/EFL (scale: approximate horizontal extent 300 km, vertical 40 km)
Large-scale geological-tectonic N–S cross-section of Switzerland (trace line in Fig. 1) illustrating the situation of tectonic units and shape of the alps in the central to the eastern part of Switzerland, according to the Swiss Geological Survey and the Seismological Agency of Switzerland (
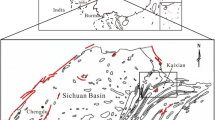
The situation of tectonic units and the shape of the alps in the central to the eastern part of Switzerland is illustrated by the large-scale geological-tectonic cross-section in Fig. 2 along the trace line in Fig. 1. It reveals the internal structure of the Alpine areas as deduced from surface geological mapping and deep seismological profiles (Naturforschende Gesellschaft in Zürich (1988) and Okayaet al. (1996). The Engadine Valley with the Engadine Fault Line (EFL) is situated on this cross-section as the valley South of the mountain range of Piz Platta and North of the Bergamo intrusion into the Penninic napes (Bergamo granite/granodiorite of Tertiary age, Valtellina), and the Austroalpine nape complex Liniger et al. (1996) and Peters (2005).
Legend of presented tectonic units:
-
Molasse Basin: mainly tertiary sediments,
-
Helvetikum: displaced cretaceous sediments
-
Penninikum: Napes mainly of metamorphic, Mesozoic sediments, including Bündner schists (metamorphic shales)
-
Valtellina: Bergamo intrusive granitic rocks
-
Austroalpine Units: mainly sediments
-
Alpi meridionali: Southern tectonic sedimentary units
-
External Massifs: units of central Alpine Aar- and Gotthard crystalline Massifs
-
Lower basement units are named according to the current seismo-tectonic interpretation of alpine orogenetic structure:
-
European Upper Crust, European Lower Crust, and Lithospheric Mantle in the Northern part and upper and lower Adriatic crust in the Southern part
Hydrogeology of the Upper Engadine Area
On the detailed geological map (Aemissegger 1993) in Fig. 3, the area of the Upper Engadine Valley (including the villages of Silvaplana in the west, St. Moritz, and Celerina in the east) is represented. This sketch map includes the trace of the Engadine Fault line and the main eastern (Austro)-Alpine tectonic units. On this map, the sites of the Mauritius mineral spring and of the deep borehole drilled for geothermal exploration are also indicated.
Detailed map of the investigated area of St. Moritz with tectonic and geological/petrographic units (Legend below, Modified after Aemissegger (1993), by permission of R. Heinz, Ed. Swiss Bulletin für Angewandte Geologie)
The Mauritius spring outflow place and the exploration borehole site are situated south of St. Moritz Lake (San Murezzan). They are situated at the edge of the alluvial plain, just at the contact with the outcropping crystalline rocks of the Corvatsch–Err Crystalline unit (Fig. 3).
Both sites are located on the local branch of the Engadine Fault Line (EFL) which is also the main conduit for uprising CO2 that causes the observed enriched CO2 gas contents in the Mauritius mineral water (Fanger 2013), as referenced in Oberhänsli, p. 30 (2017), and Querfurth (1977), Fricke (1977) in Aemissegger (1993).
The local groundwater is contained within unconsolidated fluvioglacial Quaternary sediments. These were to a large part deposited by the events of the last glaciation. During that period, the Inn Glacier flowed downwards the Inn-valley. Through this process, the bedrock of the valley bottom was eroded by the effects of the advancing glacier to a depth of about 50 m below the valley surface. During the glacier growth, a bottom layer consisting of hard clay to loamy sediments with blocks of eroded material from the bedrock itself was deposited immediately above the bedrock. These deposits (considered to be glacial till or ground moraine) consist of clay and blocks of dolomite and gypsum sedimentary and granitic and syenite crystalline rocks (belonging to the Corvatsch tectonic nape unit). During the later retreat of the glacier, fluvioglacial unconsolidated sediments such as gravel and sand were deposited above this layer within the eroded valley bottom. At the top of this sedimentary filling after the retreat of the glacier, the lakes (Lei) of Silvaplana and St. Moritz (Lei da San Murezzan) were formed.
The hydrogeological situation of the St. Moritz Mauritius mineral spring site itself is presented in the cross-section in Fig. 4 (Oberhänsli 2017), which reveals the following situation:
Hydrogeological schematic cross-section of the St. Moritz area with first prehistoric capture 1412/1411 BC (tree trunk tube Nr. 3) of St. Mauritius mineral spring (Oberhänsli 2017, Abb. 137, copyright, and permission: Archäologischer Dienst Graubünden)
Above the inclined granitic bedrock (belonging to Corvatsch–Err Crystalline unit), the confining loamy, clay ground (basal) moraine layer continues upwards to reach the nearby valley flank but pinches out at about 2 m below the surface level of the valley (Fanger 2013; Oberhänsli 2017). It is assumed that the mineral water ascends below this boundary layer of the hard-compressed loamy ground moraine (basal till layer). It originates by upward migration of the below-seated zone of altered, brittle granitic rock. In this zone, the alteration of the crystalline rock has created some additional preferential pathways and increased porosity. Within this uppermost part of this underlying (fractured) bedrock, the mineral water circulates and uprises admixed with CO2 gas, which originates from the Engadine Fault Line (Wyss et al. 2002). At the spring outflow site, the confining moraine clay layer pinches out below the ground surface. This is now the place where the mineral spring naturally discharges in a geyser-like feature and was selected by the Bronze Age settlers as the place for their first capture.
However, this mineral spring outflow place is situated also within the sedimentary fill of the Inn Valley (Fig. 4). This resulting fill material consists of porous, unconsolidated fluvioglacial sediments (containing calcareous and crystalline components) that were deposited by the retreating Inn glacier (Ohlendorf 1998). Groundwater in these sediments is fed by infiltrating water from precipitation as well as surface water from lakes and local creeks.
Overview of capture history from the first spring headworks in the Bronze Age until today
Description and historical importance of the first Bronze Age headworks
During the Bronze Age, the elevation of the land surface in the St. Moritz valley was several meters lower than today (Oberhänsli 2017). Therefore, the mineral water (of the later named St. Mauritius spring) discharged naturally at the soil surface and emerged from a small pool. This naturally emerging bubbling spring (perhaps even forming a small geyser fountain), with sparkling ferruginous water admixed with high amounts of gaseous CO2 at the soil surface, must have attracted the dwellers at the Bronze Age period. This aspect, as well as the presence of red iron oxide deposits, might have been the reason, that the dwellers living and exploring this area of the Upper Engadine have built a first spring capture.
To construct the first headworks, these dwellers used a large hollow tree trunk (Fig. 4, Tube Nr. 3 in Fig. 5, respectively) to capture the spring flow. This tree trunk had an outside diameter of 0.81 m with a total of 135 tree rings, and a total height of 1.624 m. It was hollowed out up to a remaining outside wall of 2–4 cm and was emplaced in an excavated vertical trench with the function of a standpipe precisely placed on the outflow position of the mineral spring water (Fig. 4). According to the dating based on tree ring record analysis, calibrated with radiocarbon (14C) measurements, this first wooden spring capture headworks was emplaced in 1412/1411 BC (Oberhänsli 2017). But only about 1 year later (1411/1410 BC), a new more sophisticated spring capture headwork was constructed.

(Adapted from Oberhänsli 2017, Abb. 139, Copyright, and permission: Archäologischer Dienst Graubünden)
Hydrogeological cross-section showing the reconstructed Bronze Age spring site with emplacement of the first tree trunk capture of 1412/1411 BC (tube Nr. 3) and the second capture work (tube Nr. 1 and Nr. 2) emplaced by the first capture improvements in 1411/1410 BC.
This second Bronze Age capture headworks (hydrogeological cross-section in Fig. 5) consisted of a nearly rectangular blockhouse construction made of horizontally laid round tree stems splined at the four edges (Fig. 6). It was placed in an excavated pit directly penetrating the layer of the loamy ground moraine in the spring discharge zone (hydrogeological cross-section in Fig. 5). This construction was enforced on its inner side by a wooden plank/ board functioning like a caisson (Figs. 6 and 7). Inside of this wooden construction, two huge larch tree trunks (tubes) were emplaced. Tree trunk tube Nr. 2 functioned as the main water collecting inflow standpipe for the mineral spring water. It had, according to a reconstruction of the capture remains excavated in 1907 (Figs. 6 and 7), an outside diameter of 1.148 m at the bottom end and 1.116 m at the top end. The trunk had an inside hollowed core, with an outside remaining ring wall being between 2 and 4 cm thick, and having a total height of originally 2.55 m. According to the hydrogeological cross-section (Fig. 5), another tree trunk tube (Nr. 1) was emplaced further downwards and did not penetrate through the confining clay layer, and was not functioning at any time. In Fig. 7, the functional reconstruction of the original capture work is presented with the external rectangular blockhouse construction. The inclined-laid tree stem with pinched-out steps is thought to have functioned as a ladder for cleaning the spring discharge area.
Recovered original Prehistoric capture of 1411/1410 BC by the excavation in 1907 (out of Gesundheits—Mythos St. Moritz (Hofmann 2017, 3rd Edition)
Elements of construction of the prehistoric capture of and its maintenance. According to Oberhänsli 2017 Archäologischer Dienst Graubünden, Copyright and permission by M. Stünzi Visualisierungen GmbH, Zürich
Capture remediation actions and activities before and within the period covered by chemical analyses of 1788 to 2022
Since its initial construction in 1411/1410 BC up until 1907, the Bronze Age headworks underwent several minor documented modifications but remained principally unchanged. In particular, the external appearance of the spring headworks was changed during this period.
One of the first descriptions of the headworks at the spring site was provided in 1703 by Joh. Jacob Scheuchzer (1672–1733). He described: “a Sour (water) fountain” with “strong emanating gas bubbles appearing from the soil at the hillside, in the corner of a wet, swampy meadow, about one-quarter of a mile distant to the St. Moritz village. The fountain is contained in a rectangular square shaft by four granite plaques of 1½ inch (about 27.5 cm) in width. This fountain place itself is covered by a small house like a chapel and so protected from outside influences such as rain and wind.” (Oberhänsli 2017, p. 35, free translation). This aspect of a small pond with bubbling CO2, emanating from the spring basin or small pool may have had a similar shape to that represented in Fig. 8).
Within this period, in the year 1740, the first historically documented spring site modification took place, unfortunately, just before the first analysis was made by Morell in 1788. Therefore, no influences on changes in Mauritius spring water composition are known.
The next further maintenance action took place in the year 1853, already after the first analyses of Morell (1788), Capeller (1822), Balard (1824) and Planta and Kekule (1853).
At this time, the Mauritius mineral spring site was well known as shown by the painting of Hans Conrad Escher von der Lindt made on 27. August 1819 (Fig. 9). It shows the modest Mauritius spring building that was constructed in about 1770. Another contemporary painting dated to 1820 (Fig. 10) shows the social life at that time at the Mauritius spring site inside the building. It presents a man distributing the water from the open spring basin with a bailer into a cup to the well-dressed cure guests.
Contemporary watercolor Painting by Hans Conrad Escher von der Lindt made on 27. August 1819, showing the Mauritius spring building constructed about 1770. In the background, the village of St. Moritz (today St. Moritz Dorf) is represented. (Out of Gesundheits—Mythos St. Moritz (Hofmann 2017, 3rd Edition) in Forum Paracelsus, St. Moritz, 2020)
Contemporary Painting of the Life at the Paracelsus Mineral spring with a man distributing the water in a cup to the cure guests, entitled: « Les baigneurs à la source Paracelsus. Anno Domini 1820» It shows probably the inside view (Original, exposed at the Mauritius spring capture at Mauritius spa site, Photo W. Balderer (2020)
According to the available sources, this intervention of 1853 was described as a simple cleaning action. However, according to the investigation of Oberhänsli 2017, there is some evidence that the wooden Bronze Age capture tube 2 (Figs. 5 and 6) was shortened and uplifted by this action. This fact agrees with the contemporary people’s impression that “the spring water became less strong and was of less excellence than before.” Probably, through these works, the inflow of local groundwater increased, and therefore, the original famous acidic spring water became “less strong.”
In 1907, the original Bronze Age capture construction of 1411/1410 BC, which prevailed for almost 3317 years was completely removed. This recapture action was aimed to restore the original quality of the outflowing mineral water, and to increase its outflow rate. The new capture headwork was constructed by well-known specialists. They established a containment in concrete corresponding to the current state-of-the-art techniques. The completely removed remains of the bronze age headworks were excavated (Fig. 11). Since 2014 the restored and reassembled prehistoric headworks are exhibited at the “Forum Paracelsus building” in St. Moritz (Fig. 6).
However, the operations of 1907 caused very drastic changes at the spring inflow location. The confining clay layer which separates the mineral spring water aquifer from the overlying local groundwater (Fig. 4) was partially destroyed at the place of the base of the old Bronze Age headworks (Fig. 5).
In 1937, additional engineering works were undertaken to attempt to reinstate the spring conditions that existed before 1907. However, these works caused even greater damage at the intake place as the upper confining clay layer was destroyed. This damage caused a greater admixing of the Mauritius mineral spring water with local groundwater.
In 1938, this unsatisfactory situation was partially corrected by installing an artificial drainage system that lowered the hydraulic head of the local groundwater to about—2.9 m below the actual ground surface by constant pumping.
In the years 1942/1943, the last final last known headworks remediation at the spring site took place. At this time, a more sophisticated drainage system (called the Ring-Leitung/drainage conduit) was installed around the spring to prevent the inflow of groundwater. This complete capture system led to a controlled intake of the mineral water below the confining layer within the altered bedrock into the new sealed capture construction. The emplacement of a surrounding system of horizontal drains further minimizes hydraulically the local groundwater inflow by continuous pumping.
The most recent headworks modifications were undertaken in 2018. This work aimed to provide outside of the original capture building a fountain place accessible to the public within the restored and rearranged old historical building “Forum Paracelsus” (Fig. 15). For this purpose, the original Mauritius spring mineral water is conducted from the still functioning original outflow capture site within the old capture building of 1943 (with technical infrastructure, not accessible to the public (Figs. 12, 13, and 14) to the new exposition place inside the “Forum Paracelsus” building (Fig. 15). For this purpose, a connection by drilling of a horizontal borehole directly through the outcropping crystalline rock was realized. https://www.schenkag.com/wp-content/uploads/2020/04/DitchWitch-3020-AT_2018_Mauritiusquelle-St.-Moritz.pdf.
Main medical/physiological properties and mythological aspects of the Mauritius mineral spring water
Main medical/physiological properties of the Mauritius mineral water
Based on its unique character, the Mauritius mineral water got an early mention of its beneficial effects on humans already by Paracelsus (1534): “who drinks of this water as a real medicament will stay healthy”.
Its main secret is contained in its acidic calcium–sodium–magnesium–bicarbonate composition, joint with free CO2 of more than 2500 mg/l and high iron contents.
Therefore, this water can be denominated (according to German legislation) as ferrous iron-rich sour water (Eisensäuerling or chalybeate water in English) as it fulfills the required threshold values of more than 1000 mg/l of gaseous CO2 and more than 10 mg/l of dissolved Iron (Michel 1997), Deutsche Heilbrunnen im Verband Deutscher Mineralbrunnen e.V,: https://www.vdm-bonn.de/https://www.enzyklo.de/Begriff/Eisens%C3%A4uerling, 01.01.2023.
This high iron content over its solubility in water is maintained in solution at the discharge by the inherently high CO2 concentration. Under such conditions, the iron oxides of the water precipitate only at the discharge area when the excess CO2 escapes. In this area, red deposits are formed at contact surfaces. Such deposits are visible within the historic fountain in Fig. 15. Iron-rich waters are especially recommended for drinking cures in the case of iron deficiency (Eisenmangelanämie). According to the recorded medical effects of Helfft (1867), the intake of iron and the observed therapeutic effects were better in the case of drinking iron-rich mineral water than when available iron medicaments were ingested (Binz Nocco 2007).
[The author Helfft provides information in his “Handbook of Balneotherapie” of 1867 for each health resort. It contains guidelines for each specific mineral spring water about the quantity and temperature that is recommended to drink per day. For the St, Moritz Mauritius mineral water, he recommended a unique dose of about 57–170 g of mineral water and a quantity of 170–1360 g as a ratio per day. In the case of anemia, he recommends higher doses of even several liters per day (Binz Nocco 2007)].
Because of its medical importance, especially in the therapy of heart diseases, the enhanced concentrations of magnesium in the Mauritius mineral spring water are also of importance (Table 1). The highest magnesium ion concentration of 78 mg/l for the Mauritius mineral water was reported in the analysis of Morell in 1788. The lowest value of 12 mg/l was reported in the analysis of Balard in 1824. Magnesium concentrations in the Mauritius mineral spring water since that time have remained in the range of 30 mg/l in 1853 to 36 mg/l in 2022 (Table 1). According to Rosanoff (2013), medical investigations have proven that drinking water and beverages containing moderate to high levels of magnesium (10–100 ppm) could potentially prevent 4.5 million heart disease and stroke deaths per year, worldwide. (This potential was calculated with 2010 global mortality figures, combined with a recent quantification of water-magnesium’s inverse association with heart disease and stroke mortality).
The most spectacular property of the Mauritius mineral spring water is its very high free CO2 content. Its inherent curative properties are described in medical terms by Hartmann, in the Bäderbuch of Käss, W., and H., 2008, on p. 149 as follows: “Gaseous CO2 is enhancing the blood circulation in the human body, stress hormones such as Adrenalin and Noradrenalin were suppressed”. Therefore, it enhances positive effects in the case of low to medium severe cardiac insufficiency and leads to relaxed feeling of well-being, and freshness in the mind without fear or anxiety.
The enhancement of the digestive functions of the stomach and intestines and diuresis as additional curative effects of gaseous CO2 in Mineral water are mentioned in the memorandum of the “Deutsche Heilbrunnen, im Verband Deutscher Bierbrunnen e.V” (2020).
The role of immersion in CO2-rich water in balneotherapy by the effect on free radicals release and total antioxidant status in peripherical arterial occlusive disease was studied by Dogliotti, Galliera, Iorio, De Bernardi di Valserra, Solimene and Corsi (2011). Their result is based on a study with the participation of twenty-five patients with peripheral arterial occlusive disease (stage II of Fontaine) and fifteen healthy individual controls. The result showed positive effects of CO2-enriched water balneotherapy, especially on the microcirculation due to an increase in cutaneous blood flow leading to a decrease in oxidative stress in patients with arterial occlusive disease validated by blood analysis. This aspect is still applied in the therapies of the St. Moritz Heilbad (MTZ Heilbad, Medical wellness KOHLENSÄURE MINERAL BÄDER, http://www.heilbad-stmoritz.ch/#baeder, 02.01.2023.
A more recent medical aspect is the role and importance of CO2 gas in respiratory therapy. According to medical studies by Litchfield (2003), Litchfield (2005), and Litchfield (2006), CO2 plays a special role in the blood circuit of the body.
However, it has also to be mentioned, that gaseous CO2 if present in too high concentrations in closed rooms will be harmful to human health. This aspect is presented in Table 1 according to the published values at: https://learn.kaiterra.com/ (last accessed, 14.3.2022) which provides the limits of effects of gaseous CO2 to the human health (expressed in volume % of CO2 to total normal atmospheric air composition).
Another danger for human health (by the enhanced risk of lung cancer) presents the accumulation of radon 222Rn in higher concentrations in closed rooms. The radon concentration of the water of Mauritius mineral spring is only mentioned in the analysis of Gübeli (1957) by a concentration of 2.27 ME which is equal to 30.5 Bq/l. According to the Swiss Federal Commission on Radioactivity Protection, this value is much lower than the limiting benchmark value of 100 Bq/l for drinking water (Eidgenössische Kommission für Strahlenschutz 2017).
In former periods of spa therapy, radon was practiced as a medical treatment method in waters rich in CO2 and radon. Certain spas were famous throughout Europe for radon cures, e.g., Marienbad in the Czech Republic, Baden-Baden, and Wiesbaden in Germany, Bad Gastein in Austria, and Sirmione and Merano in Italy. According to Kaba (2022), such visits to radon health spas were in Germany even paid for by the health insurance.
Its therapeutic application included especially treatments in the case of bronchial and rheumatic diseases. Such therapies belong to alternative natural medical practices (Erickson 2007; Franke et al. 2007) and are according to the radiation health protection agencies, not recommended to be still applied by spa treatments. [e.g., by the German Federal Office for Radiation Protection https://www.bfs.de/DE/themen/ion/umwelt/radon/wirkungen/heilkuren.html, 21.12.2022].
Furthermore, radon like CO2 is heavier than air and accumulates in closed rooms. Therefore, a spring outflow to open air is most suitable. Today’s accessible Mauritius spring fountain for the public is situated in the renovated Paracelsus building with permanent air circulation. In former times, the spa site was even completely open to the air as in Fig. 8 or with big open doors/windows as in Fig. 10].
Appendix: Significance of the concentration of arsenic in the mineral water of Mauritius spa as drinking water concerning the actual directives of drinking waters
The St. Mauritius water also contains elevated concentrations of arsenic, which originates from the alteration/dissolution processes of iron minerals such as pyrite and hematite which are contained within the mineral water-bearing zone of the crystalline rocks of Corvatsch–Err Crystalline unit (Peters 2005; Rafflenbeul and Balderer 2018).
The amount of arsenic in the Mauritius spring water with values of 24 mg/l (analysis of Bachema, Schlieren, 2015) and 18.8 mg/l (analysis of Official Lab of the state of Graubünden, 2019) became a subject of public health concern because the WHO lowered its limit for drinking water in 2015 to 10 μg/l (According to the WHO Fact Sheet of 15 February 2018).
Although this guideline value is designated as provisional because of practical difficulties in removing arsenic from drinking water, it has also been adopted by the Swiss food legislation, as contained in “Verordnung des EDI über Getränke vom 16. December 2016, Stand am 1. Juli 2020) Nr. (817.022.12)» and specifically the articles: 10 Absatz 4, 12 Absatz 3, 14 and 36 Absätze 3 und 4 der Lebensmittel- und Gebrauchsgegenständeverordnung vom 16. In December 2016, (LGV) stated that the limit for all kinds of drinking water (including mineral water) is 10 μg/L.
As latest news, it is mentioned in the “EAWAG News (2002)” and the newsletter of the Swiss Water Research Institute EAWAG in 2002 that the mineral water distributed to the public in the St. Moritz Mauritius spa site has been limited since 2002 in its Arsenic concentration by technical treatment to the prescribed concentration of 10 mg/l for mineral water use. This is likely to have significantly reduced the potential health risks associated with arsenic in the mineral water.
Mythological aspects of the Mauritius mineral spring water
The discovery of Bronze Age votive treasures at Mauritius mineral spring capture is described in the study of Oberhänsli (2017). The objects found include two bronze swords; one sword fragment; one bronze dagger blade and a treasure needle. These objects were stacked vertically (2 swords) and horizontally (dagger blade and treasure needle) inside at the bottom end of the only functioning capture construction at tube Nr. 2 (Fig. 11) and, therefore, at the place of incoming spring water rich in CO2. These objects date around the construction time of the second headworks construction of 1411 BC. [Another witnessing spring site of cultic attraction besides the Mauritius spring is the Bronze Age spring capture of the Römersprudel at Trier in Germany, also established with wooden tree trunks. It is dated according to Neyses-Eiden (2004), by tree ring and radiocarbon analyses to 1533 BC for capture 1, and to 1969 BC ± 10 years for capture 2].
A further important aspect of consideration to the Bronze Age people presented the red color deposits of iron oxide (ochre) which exist around the Mauritius spring outflow site. They were precious for use for different purposes as coloring objects (bones, tissues, or even the human body (Roebroeks et al. 2012; Wreschner et al. 1980).
Another cultic attraction for the Bronze Age people probably presented uprising CO2-rich water: About antique Greek mythology (cultures of about the same age period) similar occurrences of the uprising water of springs with emanating CO2 were used as cultic places, e.g., at the Oracle place in Delphi (Verros et al. 2007; Jeffery 1976; De Boer and Hale 2000; Stewart and Piccardi 2017) and Thermopylae (Jeffery 1976). These places are situated also in areas with enhanced tectonic activity by crossing active faults with CO2 emanation.
Chemical properties of the Mauritius mineral spring water as covered by analyses during a timeframe of about 234 years
Due to its special character and Europe-wide reputation, the Mauritius spring mineral water was of attraction for well-known analytical chemists from the beginning of the early development of chemical analytical methods in the eighteenth century until 2022, a period of 234 years.
Main composition of the Mauritius mineral spring water as visualized by a Piper diagram
The main chemical composition of the Mauritius mineral spring water is visualized according to the available chemical analysis by the following Piper-type diagram. In the diagram in Fig. 16, the relative proportions of the individual equivalent ion concentrations (meq/l) of the main analyzed anions and cations, expressed as %—proportions of the sum of the total mineralization of the water samples are represented. The following main conclusions that can be drawn from this representation are as follows:
Piper-type diagram of all Analyses of the Mauritius mineral water from 1788 to 2022 and the water sample of the Pro San Gian Geothermal exploration borehole, Aemisegger (1993) and the local groundwater of the St. Moritz Lake area represented by the Surpunt 2020 sample of the municipal water supply pumping station
From the diagram in Fig. 16, it can be recognized that the relative chemical composition of the Mauritius mineral spring water concerning proportions of the dissolved main ions has not changed over the whole period of chemical investigations. It is characterized by a Ca–Na–bicarbonate type by all analyses, except the analysis of Capeller, 1822 which deviates to Ca–Mg–Na bicarbonate type.
For comparison, the water composition of the Geothermal exploration borehole Pro San Gian is also represented. This borehole fluid has a much higher TDS of about 14,000 mg/l and is classified as a sodium-bicarbonate-type water but contains also elevated concentrations of calcium and magnesium ions. According to Aemissegger (1993), the analyzed water sample originates from inflow zones in the depth interval of 750–1150 m depth within the Corvatsch Crystalline Unit. It contains also high amounts of iron, about 100 mg/l.
Also, for comparison, the water composition of the shallow groundwater of the St. Moritz area is represented in Fig. 16 by the water sample of 2020 of the Surpunt pumping station. This local groundwater is characterized as calcium–bicarbonate-type water with a low TDS of about 158 mg/l.
If the three Piper-type composite diagrams are considered (Fig. 16), the following details of properties are revealed:
-
Within the upper diamond representing the relative proportions of the ion pairs of SO42− + Cl− versus Ca2+ + Mg2+, as also of Na+ + K+ versus CO32− + HCO3−, a mixing relation in direction of the ion pairs of Ca2+ + Mg2+ to Na+ + K+ between the local groundwater through the bulk of the Mauritius mineral spring samples up to the Pro San Gian Geothermal exploration borehole water can be defined.
-
By the lower left triangle representing the relative proportions of the cations, the normal local groundwater within the area of St. Moritz is characterized by high Ca2+ proportions, whereas the water of the Pro San Gian Geothermal exploration borehole is situated at the corner of the high Na+ concentration. The Mauritius mineral water samples are characterized by higher Na and fewer Ca2+ concentrations. A special position is revealed for the water sample of the 1822 analysis by Capeller with some higher amounts of Mg2+.
-
Within the lower right triangle, all investigated waters are situated at the lower left sub-triangle, of the bicarbonate-type waters
In conclusion, a mixing relationship between the local groundwater (as the lowest concentrated water) to the Mauritius spring water towards the high mineralized water of the Pro San Gian Geothermal exploration borehole can be deduced: (i) by the Ca2+- to Na+-relationship (lower left triangle), as also (ii) by the Ca2+ Mg2+ to Na+ relationship within the upper diamond diagram.
Main water composition as revealed by a Schoeller diagram
In the Schoeller-type diagram in Fig. 17, the measured equivalent ion concentrations (in meq/l) of the main ion composition of individual water samples are represented in a logarithmic scale. This type of representation allows to reveal of the similarities between the relative ion concentration of individual water samples and to compare the ion composition of groundwater analyses over very large concentration ranges.
According to the concentration patterns of the individual groundwater samples in Fig. 17, the following relationships are revealed:
-
The local groundwater (local GW 2020) is characterized by the lowest concentration for all the major ions (i.e., Mg, Ca, Mg + Ca, Na + K, Cl, HCO3 + CO3, and SO4), with lowest concentration values resulting for chloride.
-
Nearly all samples of the Mauritius mineral water follow the same pattern of concentrations along the x-axis for the main ion components in a very narrow band that overlap each other. These analogous patterns signify a quite good indication for the constancy of the chemical composition of the Mauritius mineral water; and at the same time, a proof of the quite good quality of the chemical analyses, made by different chemists and laboratories during a time frame of 234 years.
-
The two oldest analyses of Morell (1788) and Capeller (1826) resulted in higher chloride concentrations. This may be due to lesser amounts of mixing with local groundwater, which exhibits the lowest chloride content. These analyses show a pattern with closer similarity to the pattern of the San Gian Geothermal exploration well, especially if the concentrations of the analysis reported by Aemisegger, 1993 are considered. This similarity implies the indication that the Mauritius mineral water may contain components of origin within the deeper crystalline bedrock.
Assessing the effects of remediation actions based on the results of the chemical analyses from 1788 to 2022
The results of the main components of all 18 analyses from 1788 to 2022 are represented in Table 2. This whole set also presents a kind of time series of observations that can be compared to reveal the influencing potential impacts caused by hydraulic changes induced by man-made remediation actions on the capture site of the Mauritius mineral spring. In this chapter, all the available analyses from 1788 to 2022 are presented and visualized in various types of diagrams in Figs. 19, 20, 21, and 22.
To detect possible influences, the described capture remediation actions and activities within the period covered by chemical analyses from 1788 to 2022 are presented in Fig. 18 by qualitative estimates of the expected perturbation effects on water quality by a relative scale between 10 and 200. The highest value of perturbation of 200 is attributed to the complete removal works of the spring capture in 1907. In the subsequent diagrams of Figs. 19, 20, 21, and 22 this scale of estimated relative perturbation effects is included to visualize changes in the analyzed chemical parameters of the available analyses of the Mauritius mineral spring.
Concentrations of sodium, magnesium, calcium, chloride, and sulfate according to the available analyses from 1788 to 2022 combined with the events of the restoration works at the capture site with estimated perturbation effect according to the cited historical records. Included are relative errors of 10%
Concentrations of free gaseous CO2 analyzed in mg/l according to the available data from 1788 to 2014 and the events of the restoration works at the capture site with estimated perturbation effect according to the cited historical records. Included are relative errors of 10%. [Some of the original analyses were expressed in Volume of gas/Volume of water. They were converted all into the “per mg/l of Water” unit using the following equation {1}: massCO2 = VCO2[cm.3] /VolMol*molar Mass CO2, with the following values: VolMol = Molar Volume of an ideal gas at STP = 22.4 l/mol, molar Mass of CO2 = 44.0 g]
Dissolved carbonate (expressed as CO3 or HCO3 in mg/l) according to the available analyses from 1788 to 2022. Included are relative errors of 5%. Also shown are the timings of the restoration work at the spring capture site with the estimated perturbation effect according to the cited historical records
Concentrations of sulfate, calcium, sodium, magnesium, and chloride ions
According to the analyzed concentrations represented in Fig. 19, some influences of the individual intervention events with remediation actions can be observed.
The analyzed concentrations of sulfate and sodium follow a quite similar pattern in Fig. 19 with a steady decrease from the first analyses of 1788 to the 1853 capture remediation event. From there on follows a slight increase to the 1873 analysis after which succeeds a drop to the minimum concentrations of 1912. This drop is probably induced by the capture removal works of 1907. With some fluctuations, a later increase can be observed for the values of the analyses up to the 2015 analysis. which reached again the same levels as in 1853. The last analysis result of 2022 is with 199 mg/l remaining at about the same level.
The analyzed concentrations of calcium follow a quite similar pattern in Fig. 19 as sulfate and sodium. Only the value of the first analysis of Morell 1822 presents an uncertainty due to the double meaning of the analyzed substance containing calcium: If the concentration of calcium is derived from the substance CaO it results in a value of 372 mg/l, but if it is derived from CaCO3 it would result in a concentration of 209 mg/l respectively. For the next succeeding analyses, the calcium concentration pattern is quite like the patterns already described for the concentrations of sulfate and sodium.
For the concentrations of magnesium, a value of 78 mg/l is reported for the analysis of Morell (1788). The next followed analysis of 1822 reached the maximum concentration of 93 mg/l, which is followed by a drastic drop to the lowest concentration of 12 mg/l in 1824. After this drop, an increase to 36 mg/l resulted from the analysis in 1853. All later analyses up to 2015 remained at this level of about 30 mg/l and only the last two analyses of 2019 and 2022 showed a small increase to 40.7 mg/l and 36 mg/l, respectively.
The concentrations of chloride show a similar trend over the observed time range as magnesium: the maximum value of 140 mg/l resulted from the analysis of Morell (1788), followed by a concentration of 93 mg/l in 1822. From there, a general trend of lower concentrations of 23.6 mg/l in 1853 ends with 24.6 mg/l in 2022.
Concentrations of free gaseous CO2 of analyzed data from 1788 to 2014
Based on the diagram in Fig. 20, the following observations are revealed:
The highest level of measured free CO2 gas concentration of 3915 mg/l was measured by Morell in 1788. A much lower free CO2 gas concentration of 605 mg/l was measured in 1822, followed by a higher concentration of 2425 mg/l in 1853. Considering the higher values of the next two analyses of 1873 and 1891, an effect of improvement is assumed by the 1853 intervention. The lower concentration of 2200 mg/l measured in 1912 was possibly influenced by the big capture intervention in 1907. In 1950, an increased free CO2 gas concentration of about 3440 mg/l was measured, nearly identical to the 1891 measurement before the extensive headworks construction activities that took place in 1907. The increase measured in 1950 may be due to the construction of the ring drain system in the period 1930 to 1942.
The analyses of 1970 and 2014 resulted in again lower concentrations of free CO2 gas. These lower values may be explained by diverse factors such as other methods of sampling free CO2 gas or by natural fluctuations of the outgassing process along the Engadine Fault Line.
Measured concentrations of dissolved carbonate during the period 1788 to 2022
The measured concentrations of the dissolved carbonate (expressed as CO3 or as HCO3 in mg/l) that were determined from the available analyses of 1788 to 2022 as represented in Fig. 21 show a general increasing trend during the whole period of analyses which is not noticeably influenced by the capture interventions. It just remains the already mentioned uncertainty of the analysis of Morell (1788). However, if it is assumed that the used term “Kalkerde” represents the substance of CaCO3, as interpreted in Wettstein (1824) (1788 b) the result would be consistent with the other analysis results of the whole observation period. The observed increasing trend in concentration for the whole period of analyses is interpreted as being caused by increased outgassing of CO2 fed by gas emissions from the Engadine Fault line.
Variations in the concentrations of dissolved iron (Fe2+) during the period 1822 to 2022
The concentrations of dissolved iron (Fe2+) as represented in Fig. 22 show a decreasing trend from 20 mg/l in 1822 to about 2 mg/l in 2022. The only exceptions to this trend are the two peak values for the analyses of 1824 (29.9 mg/l) and 1998 (15.8 mg/l) which are much higher compared to the preceding and succeeding analyses.
For the 1853 analysis, a lower iron concentration of 11 mg/l was determined just following the maximum value of 30 mg/l of 1824. This decrease in concentration is probably associated with the headworks remediation activities that took place at this time. The next decrease followed in 1912 with a concentration of 9 mg/l. This could be related to the capture removal work of 1907. This concentration level is also reflected by the following analyses which yielded nearly stable concentration values of 8 mg/l (1950 and 1957), and 6 mg/l in 1970 and 1972. After the peak value of 15.8 mg/l in 1998, all later analyses resulted in lower values of about 8 mg/l, to reach finally for 2022 the lowest value of only 2 mg/l.
This trend to lower iron concentrations with progressing time is probably related to an increased mixing with oxidized local shallow groundwater through leaks in the confining seal (layer) as caused by the capture remediation actions, especially in 1853 and 1907. This mixing with oxidized water leads to the precipitation of dissolved iron within the groundwater mixing zone in the spring discharge area. In addition, a loss of CO2 gas overpressure occurred through leaks in the confining layer. Because of these processes, the precipitation of iron oxide occurs already within the mixing zone or in the mineral water storage tank due to increased oxidizing conditions.
Overview of the Mixing History: the struggle for the most “pure and strong” mineral water
Since the beginning of the utilization of the Mauritius spring mineral water by settlers in the Bronze Age time up until today, the challenge has remained the same. That is, how to optimize the strength and quality of the discharging mineral water by preventing excessive mixing with local groundwater. This struggle against the influences of mixing the “pure (and strong) mineral water” with the (low mineralized) local shallow groundwater is a never-ending story that commenced with the construction of the first capture headworks by the Bronze Age settlers in 1412/1411 BC. In this section, the mixing process between mineral water and groundwater and its effects are further elucidated. For this aim, the chemical composition of the analyzed mineral water of the Mauritius spring itself according to the whole set of the 18 analyses is compared with the composition of the local shallow groundwater and the water gained from the San Gian thermal exploration borehole.
Deduced mixing relationships and potential end-members revealed by the calcium and sodium concentrations
Calcium is generally the dominant cation in shallow groundwater in the area due to the dissolution of calcium carbonate which is abundant in alluvial sediments, whereas in the Mauritius mineral spring water the cations of sodium and magnesium in addition to calcium, are much more abundant. This composition of the Mauritius mineral water is due to alteration reactions within the crystalline rocks minerals that comprise the source reservoir for the Mauritius mineral water. In the diagram in Fig. 23, the sodium versus calcium concentrations shows a linear correlation coefficient resulting in R2 of 0.78, (by considering case a) of the analysis of Morell of 1788).
In the diagram of Fig. 24, the same relationship of sodium versus calcium is represented but on a semi-logarithmic scale with a much wider range of concentrations, including also concentrations of the geothermal San Gian exploration borehole (TB) analysis according to Aemisegger (1993). However, despite the goodness of fit of the exponential determination coefficient R2 of 0.9949 in Fig. 24, the concentrations of Ca and Na for the San Gian geothermal exploration borehole (TB) do not directly plot on the curve of best fit. This can be explained by the fact that the analyzed water sample of the San Gian borehole was taken in unsteady hydraulic conditions during the pumping test. It may, therefore, still contain some amounts of drilling fluid. [A further indication of a close relation of origin presents the fact, that this deep water of the San Gian Geothermal Exploration Borehole (TB) is according to analyses of stable isotopes of δ18O and δ2H interpreted to originate from similar infiltration conditions than the St. Mauritius spring water (Aemisegger 1993)].
Concentrations of sodium versus calcium ions for all analyses of the Mauritius mineral spring water and two analyses of local groundwater Surpunt (Sur) and Signuria (Sig) and the San Gian Geothermal Exploration Borehole (TB). Sodium ion concentrations are shown on a logarithmic scale. Error bars refer to a relative error of 5%
Determination of the mixing history of the spring water using magnesium and chloride ions as mixing parameters
In Fig. 25, the ion equivalent concentrations (meq/l) of magnesium and chloride ions of the Mauritius spring mineral water, of the San Gian geothermal exploration borehole, and of the local groundwater are represented in a graph on a double logarithmic scale. Within this graph, a mixing relationship is defined by the line of exponential fit with the resulting equation: y = 36,515 × 0.6003 with a determination coefficient R2 = 0.9503. At the lower end, it includes the two samples of the local groundwater and then crosses the cloud (bulk) of all analyses of the Mauritius mineral spring water up to 2022 and further extends to the highest concentrated water of the San Gian Geothermal exploration borehole. Therefore, this now-defined mixing line extends from the local groundwater, as representative of the low “mineralized end-member” up to the water as representative of the highest mineralized end-member water of the “San Gian Geothermal Exploration” borehole. In this defined mixing relation, the Mauritius spring mineral water analyses are all situated in between the mixing range within the two end-members.
These now-defined opposite end-members A and B which are responsible for the mixed origin Mauritius spring water are represented in Fig. 25 by:
FINAL MIXING ENDMEMBER A : Representing the deep water of origin within the Corvatsch crystalline like the analyzed sample of the “San Gian geothermal exploration” boreholeand,
FINAL MIXING ENDMEMBER B : Representing the local shallow groundwater of the St Moritz area , like the sample of the Surpunt drinking water well
This defined mixing relation in Fig. 25 yields the following semi-quantitative rating of the represented analyses of 1788 to 2022 of the Mauritius spring:
-
The highest concentrations of chloride and magnesium of all analyses of the Mauritius spring mineral water result for the two earliest analyses of Morell (1788), and Capeller (1822). It means that at that period the Mauritius spring water “was strongest,” and, therefore, had much lower levels of mixing with shallow groundwater than any other more recent analysis.
-
Already the analyses from the next group of the nineteenth century, including those of Planta & Kekulé 1853, of Husemann 1873, and Bosshard 1891 (and possibly Balard, 1824) indicate higher mixing levels with local groundwater.
-
The analysis within the next century of Nussberger and His of 1912 shows a further decrease in magnesium and chloride concentration. This is most probably due to damage to the confining layer in the spring discharge zone caused by engineering works and the removal of the Bronze Age headworks in 1907.
-
The later analyses of Högl (1931), Gübeli-Dorta-Treadwell (1950) and Gübeli (1957) were even more diluted showing lower magnesium and chloride concentrations. It seems that the improvements made in the years 1942/1943 had not yet made any effect on these concentrations and, therefore, had not reduced the mixing effect).
-
Concerning the analyses of Höll (1970), Högl and Senften (1972) and VSB (1998) a slight improvement can be recognized, as situated in Fig. 25 close to the 1853 analysis results. In the same group plot also, the analyses of the years 2005, 2009, 2015, and the latest analysis of 2022.
As consequence, it can be stated by this interpretation based on Fig. 25 that the headworks changes that were carried out in 1942/1943 had at least a slight positive effect on reducing the mixing with local groundwater that was caused by the 1907 capture replacement work as evidenced by the 1912 and 1931 samples. The work that was carried out at this time reduced the degree of mixing of spring water with groundwater to about the level shown in 1853 (as represented by the samples of 2005, 2009, 2015, and 2022). According to Grieshaber (1965), several additional remediation actions were undertaken concerning maintaining constant water levels of the local groundwater and the St. Mauritius mineral spring overflow.
Conclusions
Discussion of the observed impacts of human activities on the chemical composition of the Mauritius mineral spring water
Information provided in previous sections indicates that the composition of the Mauritius mineral spring water has been affected by human activities in the discharge zone. Every change to the spring headworks since the Bronze Age has caused hydraulic and water quality changes due to damage to the confining layer at the spring capture site. Already since the installation of the first Bronze Age capture headworks in 1411/1410 BC, there has been an interplay between the upwelling discharging spring water and the local shallow groundwater of meteoric origin within the Upper Engadine valley.
This is based on the very small difference in hydraulic head between the two water types and the missing confining layer directly at the capture site. The degree to which the two water types have mixed over time was largely ruled by the integrity of the confining layer that is present in the spring discharge area and by the hydraulic head difference between the two water types, as mentioned in Grieshaber (1965).
This unstable hydrogeological situation induces the following processes:
First, the mixing between the ascending mineral water and the surficial local groundwater: this main process is due to the different inherent properties of the two water components: as the local groundwater is much less mineralized than the Mauritius mineral spring water, the mixing with the much higher mineralized ascending mineral water leads to a reduction of the concentrations of dissolved ions in the discharging mineral water at the captured spring site, particularly in the concentration of chloride and magnesium ions (refer to Fig. 25).
Second, this mixing process leads to the secondary effects of the loss of free CO2 by escape to local groundwater along leaks of the confining layer at the capture site and, therefore, also to the loss of overpressure. This prevents the spring from discharging in a jet or a geyser-like fashion, and leads further to the loss of dissolved iron: within the mixing front of uprising mineral water to local groundwater, the concentration of dissolved iron is lowered by the loss of CO2 overpressure and the reaction with dissolved oxygen present in the shallow groundwater. In addition, these two processes cause ferric oxyhydroxide phases to precipitate.
Current characteristics of the Mauritius mineral spring
The Mauritius spring mineral water has become well recognized due to its unique, characteristic, main organoleptic taste with the following characteristic attributes:
-
the rusty smell of iron,
-
the sour (acid) taste (carbonic acid),
-
the sparkling water with a high excess of gaseous CO2,
-
and the salty flavor at the end (its finish).
Now, the outflowing Mauritius spring mineral water of today still contains all these properties (but for some connoisseurs of mineral water they may appear less strong).
But if the experience for a visitor is not touching all human senses as the geyser-like outflow fountain/pond with visible uprising CO2 bubbles would do, the attraction remains limited for him.
Therefore, even if all important features of Mauritius mineral spring water, its composition and taste remain unchanged, and it can be tasted as sparkling sour iron-rich water now at a waterspout fountain (Fig. 15), today’s visitor will not be much impressed and excited.
He will not be aware that the medical statement expressed in Käss, W. & H. (2008) is still valid, that the emanating gaseous CO2 will enhance the blood circulation in the human body, nor its effects on suppressing stress hormones such as Adrenalin and Noradrenalin, and that, therefore, it is recommended in case of low to medium severe cardiac insufficiency and to diminish feelings of anxiety.
In other words, he will not be aware that he could grasp for a free, efficient remedy against light forms of anxiety and psychical depression as also to overcome signs of low to medium severe cardiac insufficiency.
Therefore, the still valid suggestion of Paracelsus: “drink Mauritius spring mineral water and stay healthy!” should be stated as brand property more prominently!
Outlook on future aspects of utilization
Today’s Mauritius spring is trapped above the place of the removed original Bronze Age capture (Fig. 11) situated in an underground building that contains all technical installations and at the upper level the spring capture outflow fountain (Figs. 12, 13, and 14). To be spectacular also in the future, the Mauritius mineral spring capture should be restored now, nearly 80 years after the last capture improvements were made!
As a restoration inside the existing technical building containing the spring capture is quite difficult to be realized, there would be as a second option the construction of a completely new spring capture.
Solution for the future: recovered geyser outflow at St. Moritz with a revival of the sparkling Champagne-like water! The re-appearance of the St. Mauritius spring spirit!
This leads to the alternative, at least as a dream, to realize directly a recovered geyser-like outflow, this would be a revival of the sparkling Champagne-like original Mauritius spring water!” as a kind of rebirth of the original St. Mauritius spring spirit!
Such a recovered geyser-like outflow at the soil surface (Fig. 26), accessible to all visitors with realized outside free tasting possibilities would be a new fantastic and famous attraction for St. Moritz as a health resort and mineral spa!
That dream is not too far away as demonstrated for a short period by the San Gian Geothermal exploration borehole, where its outflowing water was described by the drilling supervisor B. Aemissegger (1993) as tasting very strong, sparkling, and salty. “It smelled like the Mauritius mineral spring water, also “sour and rusty” (due to its very high iron content of 100 mg Fe2+/)”.
To fulfill this dream of a completely new recapture of the Mauritius spring water would mean:
-
To establish a new capture by a new deep-drilled borehole (as exemplified by the San Gian thermal exploration borehole),
-
For reducing the risks of failing, to capture the wished high top quality, a geophysical, and geological local site survey must be done including a soil gas survey for localization of the trace of the Engadine Fault Line.
-
In an ideal case, the final accessed resulting water would coincide with the expectations in taste, concentration, water composition, and CO2 gas overpressure.
-
If one does find the idea utopic \(\to\) A similar project was already realized elsewhere as the history of the Andernach Geyser is showing! (https://www.geysir-andernach.de/).
Data availability
The used chemical data as represented in table 2 are included in the referenced publications according to References Part 1 and are included in a not yet published compendium of analyses of the St. Mortiz Mauritius spring by E. Piatti et al.
Change history
27 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12665-023-11074-z
References
References Part 1: Bibliography of the analyses of the Mauritius spring water and some also important notes about the methods applied in early chemical analyses
Amt für Lebensmittelsicherheit und Tiergesundheit Graubünden (2019) Beckmann, M. (Kantonschemiker), Amtlicher Untersuchungsbericht, Amt für Lebensmittelsicherheit und Tiergesundheit, Ringstrasse 10, 7001 Chur
Bachema AG (2009) Probe vom 9.9.2009, Untersuchungsbericht Mauritiusquelle St. Moritz, Analyseergebnis vom 16.9.2009 im Auftrag der Dr. H. Jäckli AG, Zürich (bearb. A. Pfister)
Bachema AG (2015) Probe vom 6.7.2015, A. Rust, Untersuchungsbericht der Bachema AG Mineralquelle Mauritius, Trinwasserkontrolle im Auftrag Bauamt St. Moritz, via Maistra 12, 17. Juli 2015, Bachema, Schlieren/ZH
Balard, Antoine, Jérôme (1824) Montpellier, Analyse in (b) Heinz, B (1824) Notice sur les eaux minérales de St-Maurice dans la Haute Engadine. Imprimerie X. Jullien, Montpellier
Balard, Antoine, Jérôme (1830) Montpellier, Analyse in (a) Barbezat, J, (1830) Les Bains les plus fréquentés de la Suisse. Tome second, Imprimerie Auguste Mie, Paris, Genève, S. 324–326
Böhm Ch (2005) Wasseruntersuchung Gemeinde St. Moritz, Probe Mineralquelle Mauritius, Probe vom 26.01.2005, Labor Christian Böhm, Geologie/Hydrogeologie, Obere Gasse 40, 700 Chur
Burggravens DJP (1752) Chur-Manntzischen Leib Medici, Medici Ordinarii zu Franckfurt am Mayn, der Kayserl. Acad. Nat. curiol. Mitglieds, dann derer Med. Doct. & Phys. Christoph. Le Cerf und Joh. Christian Senkenbergs daselbst, Bedencken, Von dem Gehalt und denen Kräften des Fachinger Sauer-Wassers, ohnfern der Stadt Dietz. Samt beigefügten einigen andern kurzen Gutachten, 1752, Certified by Christophorus le Cerf, Doct. und Physicus primarius und Johann Christian Senckenberg, M. Dr. und Physicus Extraordinarius. Franckfurt am Mayn den 15. Iulii, 1747
Capeller GW (1826) Die Mineralquellen zu St. Moritz, Schuls, Tarasp, Fideris, St. Bernhardin, Peiden, Vals und Belvedere. Chemisch untersucht von G. W. Capeller, historisch – topographisch und therapeutisch dargestellt von J. A. Kaiser, Druckerei U. T. Otto, Chur, 1826, S 24–25
Encyclopädisches Wörterbuch der medicinischen Wissenschaften (1840) Herausgegeben von den Professoren der medicinischen Facultät, D. W. A. Busch, C. F. v. Gräfe, E. Horn, H. F. Link, J. Müller, E. Osann, 24. Band, (Moos veneris Natrium), Berlin, Verlag von Veit et Comp., 1840
Grieshaber G (1965) St. Moritz – Die Mauritiusquelle, Buchdruckerei Walter Gammeter, St. Moritz
Gübeli O (1957) Bericht über die Totalanalyse des Mineralwassers der Mauritiusquelle von St. Moritz, Zürich, 1. April. 1958
Gübeli O and Dorta-Schäppi Y (1950) Bericht über die Analyse des Mineralwassers von St. Moritz
Högl O (1931) Analyse in: A. Hartmann, 1944, Bericht über die Mineralquellen von Sankt. Moritz, Engadin, zu Handen des Amtes für Verkehr, Bern, & des schweizerischen Bäderverbandes im Dezember 1944
Högl O and Senften H (1972) Analyse in: Högl O., 1973, Bericht über die eingehende Analyse des natürlichen Mineralwassers der Mauritiusquelle oder Ova Cotschna (Rotes Wasser) von St. Moritz (Kanton Graubünden) Anhang zum Bericht, Wassrprobe erhoben am 2.9. 1972, Bern 26. Juli 1973
Höll K (1970) Analyse in: Kasper, P., 1976, Die Entwicklung von St- Moritz. 3000 Jahre Heilbad St. Moritz. Festschrift, anlässlich der Eröffnung des neuen Heilbad-Zentrums, vom 10.-11. Juli 1976, Hrsg. Gemeinde St. Moritz, 104 S., Verlag Gammeter, St. Moritz, 1976
Husemann, August (1874) Der Curort St. Moritz und seine Eisensäuerlinge, Orell Füssli Verlag, Zürich, 1874; Analyse in: Biermann, A., 1881, St. Moritz und Das Oberengadin. Sein Clima und seine Quellen als Heilwerthe, 2. Veränderte und vermehrte Auflage, Verlag Otto Wiegand, Leipzig, 1881
Morell CF (1788) Chemische Untersuchung einiger der bekannten und besuchtern Gesundbrunnen und Bäder der Schweiz, insbesonders des Kantons Bern (nebst einer Beschreibung der neuesten UntersuchugsMethoden). Verlag Emanuel Haller, Bern, 1788, S. 360–361
Mosmann G (1856) Die Bestandteile, Wirkung und Gebrauch der Mineralquellen von St. Moritz im Oberengadin: Kanton Graubünden, Buchdruckerei Pargätzi und Felix, Chur, 1856. (Analysenangaben in metrischem Mass und Gewicht sowie in preussischen (rheinländischem) Mass und Gewicht)
Mosmann G (1858) Die Regeln der Bildung chemischer Namen, Zeichen und Formeln: Zur leichten Uebersicht und Repetition besonders für Anfänger bearbeitet, Schaffhausen: Brodtmann, Zweite vermehrte Auflage – 1858
Nussberger G and His H (1912) In: Nussberger, G. Heilquellen und Bäder im Kanton Graubünde; Verkehrsverein Graubünden, Verlag Bischofberger und Hotzenköcherle, 1914, 135 S., Chur
Planta von Reichenau A and Kekulé A (1854) Chemische Untersuchung der -Heilquellen zu St. Moritz im Kanton Graubünden. Chur: Druck der Offizin von Fr. Wassali, 1854
Planta von Reichenau A and Kekulé A (1855) Analyse chimique des eaux de Saint-Moritz (canton des Grisons). Coire, Impr. de F. Wassali
Scheuchzer, Johann Jacob (1708) Ouresiphoites (Graece) Helveticus, sive Itinera Alpina Tria, In: Quibus Incolae, Animalia, Plantae, Montium Altitudinis Barometricae, Coeli & Soli Temperies, Aquae Medicatae, Mineralia, Metalla, Lapides Figurati, aliaque Fossilia; et quicquid insuper in Natura, Artibus, & Antiquitate, per Alpes Helveticas & Rhaeticas, rarum sit, & notatu dignum, exponitur, & Iconibus illustratur. 3 Teile in 1 Band. Mit 3 (1 gefalt.) Titelkupfern, 1 gest. Portrait als Frontispiz u. 41 (2 gefalt.) Kupfertafeln von J. Nutting, nach M. Füssli u. A. Waser., London, H. Clements, 1708. Gr.-8°. [3] Bll., 72 S., [1] Bl., 58 S., [1] Bl., 22 S
TIBIO Nr.-924082022 (2022) Jährliche Analyse der Mauritiusquelle Z.H. Bauamt St. Moritz, date of sampling: 05.09.2022, analysis report Nr. 22–4260 by SCITEC RESEARCH SA, Av. de Provence 18–20, CH-1000 Lausanne 20, Switzerland
Veraguth C (1894) St. Moritz und seine Eisenquellen, 2. Auflage, Chur
VSB (1998) Wasseranalyse der Mauritiusquelle vom 14.12.1998, VSB Vertrauenslabor für Spezialanalytik und Betriebshygiene (Spezialabteilung der Versuchsstation schweizerischer Brauereien), Zürich
Wettstein JU (1824) Sulla sorgente acidula di san Maurizio nel cantone dei Grigioni con norme ragionate di pratica direzione per fare la cura, con ordine e con intelligenza, Del Dottor J. U. Wettstein già Medico Chirurgo nell Armata Francese cra medjco esercente in Samaden, E Membro del Collegio Medico Dei Grigioni, Traduzione Dal Tedesco., Milano, Dalla Tipografia d' Omobono Manini, Contrada de' Tre Re, n.° 4085. 1824
References Part 2: References to the text of the publication
Aemissegger B (1993) Geothermie-Bohrung Pro San Gian, St. Moritz Bulletin der Vereinigung Schweiz. Petroleum-Geologen und Ingenieure, Bd 60/1993, Heft 136
Aldefeld CK (1835) Die älteren und neuen Maasse und Gewichte der Königlich Preussischen Rheinprovinz, Achen und Leipzig, 1835, bei Jacob Anton Mayer
Binz Nocco PE (2007) Mineralwasser als Heilmittel medizinisch-pharmazeutische Aspekte im 19. und frühen 20. Jahrhundert unter besonderer Berücksichtigung des Kantons Tessin. Dissertation ETH Zürich
Braunschweig OFJ (1840) Lehrbuch der rationellen Praxis der landwirthschaftlichen Gewerbe, Das Kalk- und Gypsbrennen. ETH-Bibliothek Zürich, Shelf Mark: Rar 37239, Persistent Link: https://doi.org/10.3931/e-rara-81786
Butcher T, Cook S, Crown L, Rick Harshman R, Hockert C, Eds (2012) NIST Handbook 44 2012, Specifications, Tolerances, and Other Technical Requirements for Weighing and Measuring Devices as adopted by the 96th National Conference on Weights and Measures 2011, Chief Office of Weights and Measures National Institute of Standards and Technology Office of Weights and Measures Gaithersburg, MD 20899–2600 U. S. Department of Commerce Dr. Rebecca Blank, Acting Secretary National Institute of Standards and Technology Dr. Patrick D. Gallagher, Director NIST Handbook 44
CRC, Handbook of Chemistry and Physics, 60th Edition, 1979–1980, Weast, R.C. Astle: Conversion Factors
De Boer JZ, Hale JR (2000) The geological origins of the oracle at Delphi. Greece Geol Soc Lond 171:399–412. https://doi.org/10.1144/GSL.SP.2000.171.01.29. (Special Publications)
Deutsche Heilbrunnen im Verband Deutscher Mineralbrunnen e.V., 2020, https://www.heilwasser.com/deutsch/inhaltsstoffe_die_gut_tun/kohlensaeure.html
Earth System Research Laboratories (2020) Trends in Atmospheric Carbon Dioxide, CO2 actual atmospheric concentration on 4.9.2020, according to https://www.esrl.noaa.gov/
EAWAG NEWS (2002) Risikofaktoren im Wasser, Arsen im Trinkwasser-auch ein Schweizer Problem? April 2002, 53D
Eberhard R (2017) The history of the St. Moritz Heilbad, https://www.eberhard-stmoritz.ch/wp-content/uploads/2017/03/REB_history_of_the_spa_02.pdf
Eidgenössische Kommission für Strahlenschutz (2017) 2.1 Radioaktivität im Trinkwasser, CPR / KSR approuvé / genehmigt am 05.12.2017
Eisensäuerling, Definition: Quelle mit einem Gehalt an freiem, gelöstem Kohlendioxid von > 1000 mg u. an Eisen von > 10 mg pro kg Wasser. https://www.enzyklo.de/Begriff/Eisens%C3%A4uerling, https://www.vdm-bonn.de/
Engadin St. Moritz Tourismus AG (2020) St. Moritz CULTURAL TRAVEL GUIDE, Publisher: Engadin St. Moritz Tourismus AG, Marijana Jakic, marijana.jakic@stmoritz.com — Copyright: all rights reserved. Engadin St. Moritz Tourismus AG, visited 2.1.2022 at https://www.estm.ch/fileadmin/user_upload/St_Moritz_Kulturguide.pdf
Erickson BE (2007) The therapeutic use of radon: a biomedical treatment in Europe; an “alternative” remedy in the United States. Dose Response 5(1):48–62
European Food Safety Authority (2014) Dietary exposure to inorganic arsenic in the European population EFSA 2014; https://www.efsa.europa.eu/fr/efsajournal/pub/3597
Fanger L (2013) Mauritiusquelle Geologischer Bericht im Auftrag der Gemeinde St. Moritz, unpublished report of CSD Engineers AG, cited text in Oberhänsli, M, 2017, p. 30
Forum Paracelsus (2022) Engadin St. Moritz Tourismus AG, Via San Gian 30, 7500 St. Moritz Switzerland, visited on 17.2.2022 at https://www.engadin.ch/en/sightseeing/forum-paracelsus/
Franke A, Reiner L, Resch KL (2007) Long-term benefit of radon spa therapy in the rehabilitation of rheumatoid arthritis: a randomized, double-blinded trial. Rheumatol Int 27(8):703–713
Fricke, K (1977) Gutachtliche Stellungsnahme zur Frage der Erschliessung von Mineral-, Heil- und Thermalwasser im Heilquellengebiet von St. Moritz. unpublished report, Bad Driburg, BRD, cited in Aemissegger, B. (1993)
Froitzheim N, Manatschal G (1996) Kinematics of Jurassic rifting, mantle exhumation, and passive margin formation in the Austroalpine and Penninic nappes (eastern Switzerland). Geol Soc Am Bull. https://doi.org/10.1130/0016-7606(1996)108%3c1120:KOJRME%3e2.3.CO;2
Furrer A (1889) Volkwirtschafts-Lexikon der Schweiz, II Band, Bern, Seite 386
(Geoscience Reference In-Depth Information. http://what-when-how.com/Tutorial/topic-65u94bp/Hydrogeologie-165.html (20.12.2022)
German Federal Office for Radiation Protection: https://www.bfs.de/DE/themen/ion/umwelt/radon/wirkungen/heilkuren.html, visited 21.12.2022
Gesundheits – Mythos St. Moritz (2017, 3rd Edition), Dr Oscar Bernhard Foundation, Publishing house: Gammeter Media AG St. Moritz (ISBN 978-3-9524798-0-3)
Grieshaber, G (1965) St. Moritz, Die Mauritiusquelle, Buchdruckerei Gammeter, St. Moritz, 1965.
Hartmann B (2008) Kohlenstoffdioxidhaltige Wässer und Mofetten: Evidente Kur- und Heilmittel. In: Käss, W. und H. Deutsches Bäderbuch, Stuttgart, 2008, pp 146–151
Hartmann B, Hartmann M (2008) Pharmakologische Wirkungen des Badens in Heilwässern, in Käss, W. und H. (Hrsg.) Deutsches Bäderbuch. Stuttgart 2008:92–95
Heierli J (1907) Die bronzezeitliche Quellfassung von St. Moritz. Anzeiger für schweizerische Altertumskunde : Neue Folge = Indicateur d'antiquités suisses : Nouvelle série, 9 (1907), Heft 4
Heilbad MTZ, Medical wellness, 22.12.2022, http://www.heilbad-stmoritz.ch/#baeder
Helfft H (1867) Handbuch der Balneotherapie mit einer Heilquellenkarte. Berlin 1867
Hoffmann, Camill, Pfr (1895) St. Moritz - Bad. Europäische Wanderbilder. Mit 15 Illustrationen von J. Weber und einer Karte, 236 u. 237, p. 15, cited in Heierli, 1907, Page 265.
Hofmann H (2014) Das Heilbad darf nicht sterben! -Publisher: Paracelsus Foundation St. Moritz. Publishing house, MTZ Heilbad St. Moritz (978-3-033-04575-0)
Hofmann H (2017, 3rd Edition) Gesundheits-Mythos St. Moritz; Sauerwasser, Gebirgssonne Höhenklima. Publisher: Dr Oscar Bernhard Foundation, Publishing house: Gammeter Media AG St. Moritz (ISBN 978-3-9524798-0-3)
Jeffery LH (ed) (1976) Archaic Greece: The City States c. 700–500 BC. Ernest Benn Ltd., London, p 73 (ISBN 0-510-03271-0)
Käss W und H (2008) Deutsches Bäderbuch. Stuttgart
Kissling E, Schlunegger F (2018) Rollback orogeny model for the evolution of the Swiss Alps. Tectonics 37:1097–1115. https://doi.org/10.1002//2017TC004762
Leuzinger U (2014) Ur- und Frühgeschichte - von der Altsteinzeit bis zu den Römern. In: Kreis G (ed) Die Geschichte der Schweiz. Schwabe Verlag, Basel, pp 6–29
Liniger MH (1992) Der ostalpin-penninische Grenzbereich im Gebiet der nördlichen Margna-Decke (Graubünden, Schweiz), Dissertation ETH Zürich, Nr. 9769
Litchfield PM (2003) A brief overview of the chemistry of respiration and the breathing heart wave. California Biofeedback 19(1)
Litchfield PM (2005) Capno Breath Training. California Biofeedback 21 (3)
Litchfield PM (2006) Good Breathing, Bad Breathing, Breathing is behavior, a unique behavior that regulates body chemistry, pH http://www.breatheon.com/static/media/docs/Peter-Litchfield-on-goodbad-breathing-CapnoTrainer8.pdf
Lupine publishers (2018) Open access, archives of organic and inorganic chemical sciences, ISSN: 2637–4609, https://doi.org/10.32474/AOICS.2018.02.000142
Margadant S, Maier M, Lütscher M (1993, 2022) St. Moritz, eine aussergewöhnliche Geschichte. Gammeter Media AG, St. Moritz, Scuol
Michel G (1997) Mineral- und Thermalwässer, Allgemeine Balneogeologie. Lehrbuch der Hydrogeologie 7, Berlin, Stuttgart
Naturforschende Gesellschaft in Zürich (1988) Erste Ergebnisse der Alpentraversen von NFP-20, «Geologische Tiefenstruktur der Schweiz», Schweizerische Arbeitsgruppe für Reflexionsseismik, Vierteljahrsschrift 133/2: 61–98
Neyses-Eiden M (2004) Der Trierer "Römersprudel» Von der Quellfassung der Bronzezeit zur römischen Brunnenstube. Funde Und Ausgrabungen Im Bezirk Trier 36(2004):7–14
Oberhänsli M (2014) Sakrales aus der Tiefe. Die mittelbronzezeitliche Quellfassung von St. Moritz GR, University of Zurich, Master Thesis.
Oberhänsli M (2015) Citation of CO2 Content of Mauritius spring water of 2500 mg/l of CO2, Personal communication of H. Hofmann to M. Oberhänsli
Oberhänsli M (2017) St. Moritz, Mauritiusquelle, Die Bronzezeitliche Quellfassung- Archeologie Graubünden, Sonderheft 6, Archeologischer Dienst Graubünden, ISBN: 978–3906064–92–5, Somedia Buchverlag, Glarus, Chur, Switzerland
Ohlendorf C (1998) High Alpine Lake sediments as chronicles for regional glacier and climate history in the Upper Engadine, southeastern Switzerland, Dissertation ETH Zürich No. 12705
Okaya N, Freeman R, Kissling E, St M (1996) A lithospheric cross-section through the Swiss Alps I. Thermokinematic modeling of the Neoalpine orogeny. Geophys J Int 125:504518
OSHA (Occupational Safety and Health Administration/ US Department of Labor); Sampling and Analytical Methods / Carbon Dioxide in Workplace Atmospheres, https://www.osha.gov/ (Last accessed 4.1.2023)
Paracelsus [Theophrastus von Hohenheim] (1562) Baderbuchlin: sechs kostliche tractat, armen vnd reychen, nutzlich vnd notwendig, von wasserbadern. Mülhausen: Peter Schmidt, 1562. 62 pp., 19 cm. Signatures A-H4, New York, New York Society Library, Win 189
Paracelsus A, Theophrastus, von Bodenstein A (1563) von Tatarjschen kranckheiten nach dem alten nammen, vom griess sand unnd stein, as cited in Oberhänsli, M. 2017.
Pattison M (1907) A history of chemical theories and laws, 1st edn. Wiley, London
Pattison M (1975) A history of chemical theories and laws, 1st edn. Arno Press, New York
Peters T (2005a) Blatt 1257 SI. Moritz.-Geol. Atlas Schweiz I: 25000, Karte 118. Blatt 1257 St. Moritz
Peters T (2005b) Geol. Atlas Schweiz 1:25 000, Erläuterungen. 118
Piatti E (2018) The Mauritiusquelle St. Moritz, analyses from 1788 to 2022 with the conversion of original chemical expressions and units. Overview of the historical chemical analyses of the mineral water, unpublished document in Excel format
Querfurth H (1977) C02-Messungen St. Moritz: Ergebnisse der geophysikalischen Bodenluftuntersuchungen in St. Moritz-Bad, unpublished report, cited in Fricke, K. (1977) and Aemissegger, B. (1993)
Rafflenbeul J and Balderer W (2018) Enhanced Arsenic Contents in Spring Water within the Engadine Valley (South-eastern Switzerland; Canton of Graubünden)
Roebroeks, W., Sier, M. J., Kellberg Nielsen T., De Loecker, D., Maria Parés, J.E.S., Arps, C. J., Mücher H (2012) Use of red ochre by early Neandertals. Proc Natl Acad Sci USA 109, Nr. 6, 7. Februar 2012, S. 1889–1894.
Rosanoff A (2013) The high heart health value of drinking-water magnesium. Med Hypotheses 81(2013):1063–1106 (published by Elsevier Ltd)
Schenk P, Schenk U, Schenk B (2018) Mauritiusquelle, Horizontalbohrung.pdf. https://www.schenkag.com/wp-content/uploads/2020/04/DitchWitch-3020-AT_2018_Mauritiusquelle-St.-Moritz.pdf
Schmid SM, Fügenschuh B, Kissling E, Schuster R (2004) Tectonic map and overall architecture of the Alpine orogen. Eclogae Geol Helv 97(2004):93–117. https://doi.org/10.1007/s00015-004-1113-x. (0012-9402/04/010093-25, Birkhäuser Verlag, Basel, 2004)
Stewart S, Luigi Piccardi L (2017) Seismic faults, and sacred sanctuaries. Aegean antiquity Iain, July 2017. Seismic faults and sacred sanctuaries in Aegean antiquity. Proc Geologists’ Assoc 128(5–6):711–721
USGS (2020) GW-Chart Piper type Diagram, in GW_Chart: A Program for Creating Specialized Graphs Used in Groundwater Studies, USGS MODFLOW Team, Email: modflow@usgs.gov, https://www.usgs.gov/s
Verordnung des EDI über Getränke vom 16. Dezember 2016, Stand am (1. Juli 2020): Swiss Federal Department of the Interior, Federal Office of Health; Eidgenössisches Departement des Innern EDI, Bundesamt für Gesundheit BAG Direktionsbereich Verbraucherschutz 1/7 Oktober 2020 Arsen
Verros G, Latsos T, Anagnostou K, Αυλακιωτησ Π, Chaikalis C, Liolios C, Antoniou D, Kotsopoulos S, Arsenos P (2007) Physicochemical Characteristics of Thermopylae Natural Hot Water Springs in Central Greece. Chem Geothermometry AIP CoNf Proc 963:412–415. https://doi.org/10.1063/1.2836100
Wexteen P, Jaffe F, Mazor E (1988) Geochemistry of cold´ CO-rich springs of the Scuol-Tarasp region, lower Engadine, Swiss Alps. J Hydrol 104:77–92
WHO (2018) Fact Sheet of 15 February 2018, World Health Organization, 2017, Guidelines for Drinking-water Quality Fourth Edition Incorporating the First Addendum. World Health Organization, p 2017
Wreschner EE, Bolton R, Butzer KW, Delporte H, Häusler A, Heinrich A, Jacobson-Widding A, Malinowski T, Masset C, Miller SF, Ronen A, Solecki R, Stephenson PH, Thomas LL, Zollinger H (1980) Red Ochre and human evolution: a case for discussion [and comments and reply]. Source 21(5):631–644
Wyss R, Greber E, Freimoser M, Schenker F, Haarpaintner RT (2002) Grundlagen zur Charakterisierung der Erdgasgefährdung bei Untertagentertagbauten. Arbeitsgruppe Gasführung Im Untergrund Bull Angewandte Geol 7(Nr 1):45–65
Acknowledgements
The authors would like to thank the collaborators of the City of St. Moritz, especially Mr. H. Denoth, as also Mr. B. Lüscher of the construction department and municipal water supply of St. Moritz for their support of the Mauritius spring facilities and site properties. Great thanks are expressed to Dr. Heini Hofmann for the permission to use figures and photos from his book publications. Also, our expression of gratitude goes to Mrs. Simone Wölfli of the Swiss topographical survey (Figs. 1 and 2), to Dr. Roger Heinz, editor of the Swiss Bulletin für Angewandte Geologie (Fig. 3), to Mrs. Dr. Monika Oberhänsli for the permission of publication of Figs. 4 and 5, and Mr. Michael Stünzi of Fig. 7, to Mrs. Prisca Binkert of the St. Moritz Library and Documentation center for Fig. 11. We thank also the technical staff of the forum Paracelsus for the access to the Museum and exhibition. Especially we thank Dr. Fanny Leuenberger and Matthäus Balderer for the scientific and English corrections, the important suggestions, the scientific review, and the comments on the manuscript.
Funding
The authors declare that they made this publication free of any outside interests and financial support.
Author information
Authors and Affiliations
Contributions
The first author designed the aspects of the study in conception, historical aspects, chemical and hydrogeological interpretation as presented, and editing of the whole manuscript. All drafts and the final version of the manuscript were written by Werner Balderer. The merit of the second author (Enrico Piatti) consists of the collection of all old chemical analyses in the literature of the eighteenth and nineteenth centuries up to the present day in retrieving libraries and archives. He was also building a consistent database including the transformation into the appropriate chemical units.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Werner Paul Balderer: Retired.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balderer, W.P., Piatti, E. The St. Moritz Mauritius mineral spring (Upper Engadine Valley, SE Switzerland): review of its importance by the joint facts of geological occurrence, archeology, health effects, chemical properties, and long-term chemical stability. Environ Earth Sci 82, 233 (2023). https://doi.org/10.1007/s12665-023-10800-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-10800-x