Abstract
This research demonstrated the application of hydrochemical data and stable water isotopes of δ18O and δD (or delta 2H) for evaluating the relationship between surface water in Lake Bosumtwi and the underlying groundwater system. It aimed at determining the presence or absence of a hydraulic relationship and for evaluating the possible direction of flow at the interface between the two reservoirs. The study also estimated evaporative losses of infiltrating rainwater as it transits the unsaturated zone and provided important information on the hydrological processes prevailing in the area. The results of Q-Mode hierarchical cluster analysis (HCA) clearly differentiate the lake water from the groundwater based on their spatial relationship. These results indicated that groundwater recharge occurs on the hilltops of the crater, where it is slightly acidic with low levels of dissolved ions, characterised by short residence time and rapid unrestricted vertical infiltration and recharge. The groundwater becomes more mineralized with longer contact times and deeper circulation with the host rock, while it flows from the recharge areas towards the lake at lower elevations. Analyses of δ18O and δD showed a high evaporation rate on the lake surface (90%) with a significant evaporative enrichment, whereas groundwater showed no significant isotopic variations. Thus suggesting that the aquifers have been recharged by recent meteoric water that has undergone some evaporative enrichment since the study established an evaporation rate of water infiltrating the unsaturated zone ranging from 54 to 60%. Both reservoirs do not appear to be hydraulically connected, and where such a connection exists, it is expected to favour the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the wake of the recent report of the Intergovernmental Panel on Climate Change (IPCC) on climate projections, there have been conscious efforts at building resilience against the unremitting impacts of climate change, especially on vulnerable communities. One of the most important strategies for enhancing resilience and improving upon the adaptive capacities of vulnerable communities is the development of groundwater resources. Globally, there have been various strategies for improving the knowledge base on groundwater resources to facilitate effective development and sustainable management (eg., Yira et al. 2017; Mustafa et al. 2019; Perera et al. 2019; Yu et al. 2019). Most of these investigations have assessed the relationship between groundwater and other hydrological systems and have proffered strategies for improving resource resilience. For instance, Mustafa et al (2019) used a 3D numerical groundwater flow model to evaluate the relationship between surface and groundwater resources and suggested essential recommendations for improving upon effective groundwater resources sustainably. There are many other studies which have applied numerical process-based approaches which are data-intensive and are not effective in data-poor regions such as most parts of sub-Saharan Africa. In such regions, the development of novel techniques, involving the use of tracers (natural and artificial) is critically important for improving understanding of the relationship between hydrological systems.
Hydrochemical and isotope tracers have been copiously used to study various aspects of hydrogeology and hydrological systems. They have been used to evaluate sources of variation in water quality and for delineation of groundwater flow paths (e.g. Cloutier et al. 2008; Banoeng-Yakubo et al. 2010; Loh et al. 2012, 2016; Yidana et al. 2012a, b). However, there are few studies that have effectively used hydrochemical and isotope studies to characterize the relationship between surface and groundwater reservoirs to provide the basis for developing conceptual models on the relationship between the two reservoirs (e.g., Nuan et. al. 2021). An accurate depiction of the conceptual relationship between surface and groundwater resources is critical in the development of useful decision-support systems for managing both resources. Thus, large-scale basin-wide investigations must first begin with an adequate conceptual understanding of the beneficial relationship between surface and groundwater systems so that it will be possible to evaluate the effect of stresses on one reservoir on the other.
This research demonstrates the application of hydrochemical and isotope tracers for evaluating the relationship between a lake and the underlying groundwater system. It is aimed at determining the presence or absence of a hydraulic relationship and for evaluating the possible direction of flow at the interface between the two reservoirs. This will serve the purpose of managing both resources effectively. In the context of Lake Bosumtwi, a natural crater and a subject of many global research endeavours, this research provides important information on the hydrological processes prevailing in the area. Although similar techniques have been applied in other parts of the world (e.g., Abid et al. 2010; Dogramaci et al. 2012; Pu et al. 2020; Zhang et al. 2020; Zhou et al. 2021; Carreira et al. 2021; Kebede et al. 2021), very few have quantitatively evaluated surface–groundwater relationships in the manner demonstrated in this study using stable isotopes and hydrochemical datasets. The objective of this study is to demonstrate the use of hydrochemical and isotopic tracers to facilitate an understanding of the hydraulic relationship between two major reservoirs and for estimating evaporative losses of infiltrating rainwater. The processing of the hydrochemical datasets provides insights into the net direction of groundwater flow, which is relevant for the designation of groundwater recharge and discharge areas.
Study area
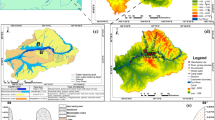
The study area, Lake Bosumtwi and its environs, is one of the major tourist sites in Ghana, which attracts both leisure and educational visits. The Lake is close to Kumasi, the second largest city in Ghana (Fig. 1). The study area is situated within the deciduous forest zone and is strongly influenced by the West Africa monsoon winds (Balek 2011). The climate is generally warm with variable temperatures masked by seasons and elevation and characterised by two rainy seasons; major and minor rainy seasons. The major rainy season begins in March, peaks in June and ends in July, while the minor season spans from September to November with a peak in October. Rainfall amounts range between 1.6 m and 1.8 m per annum. On average, there are about 150 rainy days.
Temperatures range between 32 ℃ in March and 20 ℃ in August, with relative humidity ranging between 70 and 80% (Turner et al. 1996a). Details of the hydrological characteristics of the area are copiously discussed by other researchers (e.g., Turner et al. 1996a; Amu-Mensah et al. 2019).
Extensive farming, illegal mining and lumbering have changed the vegetation cover in the study area from semi-deciduous and rainforest to mosaic secondary forest. Dendritic drainage pattern is the drainage type pertaining to the area. However, around Lake Bosumtwi, there is internal drainage where the streams flow from the surrounding highlands into the Lake, forming a dense network due to the double maxima rainfall regime.
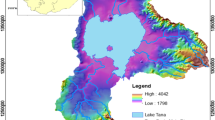
Lake Bosumtwi falls within the Birimian Supergroup of West Africa and made up of two major lithostratigraphic units of the early Proterozoic age, which are the Birimian Sedimentary Basins and Birimian Volcanic Belts (Kesse 1985; Karikari et al. 2007) (Fig. 1). Extensive studies have established that these rocks host most of Ghana’s mineral deposits such as gold and diamond (Leube et al. 1990; Hirdes et al. 1992; Fougerouse et al. 2017; Kazapoe & Arhin 2021). These studies showed that the Eburnean tectonothermal event, which stabilised the West African Craton, caused the Birimian Supracrustal rocks to become folded and metamorphosed under greenschist-facies conditions, and intruded by granitoids. The impact crater has a steep rim rising up to 300 m above the present lake level. It is surrounded by an irregular circular depression with a rim-to-rim diameter of 10.5 km, as well as an outer ring of minor topographic elevations with a diameter of about 20 km (Jones et al. 1981; Reimold et al. 1998). Rocks at and around Lake Bosumtwi are dominated by meta-sandstones, shales, phyllites, and schist (Leube et al. 1990; Hirdes et al. 1992). These metasediments are rich in quartz, feldspars and micas (biotite, muscovite).
The hydrogeology of the study area is characterised by the Birimian Province. Here the hydrogeology is controlled by secondary porosity and permeability and the extent of weathering. These secondary porosity and permeability in the rocks of the study area were created during the Eburnean orogeny that occurred about 2.1 Ga ago (Kesse 1985) and impact cratering (Reimold et al. 1998). Where there are tectonic lines of weakness as a result of the Eburnean orogenic event, deep weathering of the rocks occurs in the study area. Weathering is enhanced where the fractures, joints, faults, folds and quartz veins are extensive. Thick weathering zones are known to be reservoirs for delivering huge volumes of water (Banoeng-Yakubo et al. 2010). In some places, the weathered zones can be as thick as 137 m (Kesse 1985; Dapaah-Siakwan and Gyau-Boakye 2000). In the Birimian Province, the lower part of the saprolite and the upper part of the saprock forms the most productive section with regard to groundwater. These complement each other in terms of transmissivity and storage (Carrier et al. 2008). A conceptual model developed by Carrier et al. (2008) shows that the hydrostratigraphy of the Birimian Province starts with topsoil/laterite, followed by the saprolite then the saprock and eventually the competent bedrock. Borehole depths completed within the weathered and fractured units ranged between 26 and 64 m with an average of 43 m (Loh et al. 2016) and static water levels ranging between 3 and 43 m below ground level (Banoeng-Yakubo et al. 2010). Average aquifer transmissivity value in this Province is 7.4 m2/d (Banoeng-Yakubo et al. 2010). Successful boreholes that have been drilled in this Province suggest there is an average borehole yield of 12.7m3/hr (Banoeng-Yakubo et al. 2010; Dapaah-Siakwan and Gyau-Boakye 2000). Loh et al. (2016) established that drilled and developed boreholes within the study area tap water under semi-confining conditions.
Materials and methods
Water sampling was carried out over a generally cold and dry period from late July to early August 2012. Thirty-four groundwater samples and seven lake water samples were collected in triplicate into 50 mL sterilised polypropylene bottles. Out of the three samples, two were filtered via a 0.45-micron membrane. One of the filtered samples was subsequently acidified with nitric acid (HNO3−) to pH less than 2 for cation analysis, and the other was used for anion analysis. Duplicate samples were taken as a quality control measure for the obtained analytical results. The concentrations of δ18O and δD were determined using the third unfiltered sample. Physical parameters such as total dissolved solids (TDS), water temperature (T), salinity (Sal), electrical conductivity (EC), pH, dissolved oxygen (DO) and oxidation–reduction potential (ORP) were measured in situ for all samples collected with a HANNA Instruments HI 98,280. In the field, the alkalinity (Alk) (as HCO3−) was measured using a Hach digital titrator. Major cation and trace element concentrations were determined using an inductively coupled plasma-mass spectrometer (ICP-MS) at Activation Laboratories Ltd in Canada. Inductively coupled plasma optical emission spectrometry (ICP-OES) was then used to reanalyse samples that were over the required range (i.e., > 25 ppm of Na+, K+, and Sr2+; and > 100 ppm of Ca2+, Mg2+, and Si4+). The analytical precision for both ICP-MS and ICP-OES was estimated to be within 5% based on repetitive sample analysis and duplicate sample results. Anions of chloride (Cl−), sulphate (SO42−), and nitrate (NO3−), fluoride (F–), nitrite (NO2−), and phosphate (PO43−) were measured in milligram per litre (mg/l) using a Dionex DX 120 ion chromatograph (I.C) with an analytical precision of 5%. Additionally, the standard error for the obtained results of individual elements was calculated by dividing the sample standard deviation by the square root of the total number of samples.
Ten rainfall samples collected in the months of April, May, June and July were analysed for isotopes of δ18O and δD. The Los Gatos Research Liquid Water Isotope Analyzer was used to analyse these samples at the University of Rochester's Department of Earth and Environmental Sciences. The findings were standardised (Coplen 1994) on scales so that the δ18O and δD of the Vienna Standard Mean Ocean Water (V-SMOW) values were 0‰ and 0‰, respectively, whereas the δ18O and δD values of Standard Light Antarctic Precipitation (SLAP) were, respectively, − 428‰ and − 55.5‰. Unless otherwise stated, the 2-sigma uncertainties for δ18O and δD results were 2.0‰ and 0.20‰, respectively. This suggests that the result of isotopic analysis of the same sample would fall 95% of the time within the uncertainty bounds if it were reanalysed.
Microsoft Excel and the statistical package for social sciences (SPSS) version 25.0 were used to conduct univariate, bivariate and multivariate statistical analyses of the data sets obtained. The multivariate statistical analyses were performed to constrain relationships among multiple variables in the entire data set with an emphasis on revealing the intrinsic clusters/ groupings within the entire data, which on the contrary would not have been noticed. To achieve optimal results from the statistical analysis, the normality of each parameter was tested and those parameters which did not meet normality were transformed accordingly. Log transformation was employed and then standardised to their respective z-scores. In producing the spatial distribution maps, kriging was performed using different semi-variogram models to determine the best model fit for the data set. Continuous adjustment was made to the variogram parameters until all the criteria for an optimal model was arrived at. The Gaussian model was the most accurate predictor for pH and EC data sets. The obtained parameters (Co = 0.00021; Co + C = 0.00654: Ao = 0.03: r2 = 0.786: Residual Sum Square = 1.056E-05) from the variogram model were then used to generate the spatial distribution maps of pH and EC in ArcGIS version 10.7.
Following the model developed by Craig and Gordon (1965) and applied by Yidana et al., (2013), the surface evaporation rate from the Lake was estimated. This model determines the fraction of water lost by evaporation (f) (Eq. 1).
which is determined from Eq. 2:
h is the average relative humidity of the study area, δA ambient air vapour isotope signature and ɛ isotopic enrichment factor (Gat and Gonfiantini 1981).
δp and δL represent the isotope signatures of the initial source water for the lake and sampled lake water, respectively, while the index (m) is given by Eq. 3
The kinetic isotopic enrichment factor, \({\varepsilon }_{k}\), is linked to the relative humidity between water (w) and water vapour (v) with regards to δ18O (Eq. 4) (Gonfiantini 1986).
The \(\varepsilon_{k}^{18} O_{w - v}\) value of 4.26‰ was estimated at a local humidity of 70% (Turner et al. 1996a) for the lake water.
ε provides the sum of both equilibrium and non-equilibrium (kinetic) enrichment factors in Eq. 5.
\(\varepsilon_{{{\text{eq}}}} \, = \,{10}^{3} (1 - \alpha_{w - v}^{ - 1}\)= and \(\alpha_{w - v}^{ - 1} ,\) which is temperature (T measured in Kelvin) dependent is expressed for 18O by Eq. 6 (Faure 1998);
For a mean annual T of 298.15 K (25 ℃), \({\varepsilon }_{\mathrm{eq}}\) returned a value of 9.11‰. The overall enrichment under the prevailing conditions was \(-13.37\permil\). Peng et al. (2012) proposed that a relationship between initial precipitation (\({\delta }_{IP}\)) and ambient air vapour isotope signature (δA) presumes an isotopic equilibrium (Eq. 7).
Results and discussions
Major ion chemistry
The summary statistics and distribution of hydrochemical parameters from the lake and groundwater (GW) are presented in Fig. 2 and Table 1. The relative abundance of the major cations in the lake water occur in the order Na+ > > Mg2+ > K+ > Ca2+, while in the groundwater, the cations occur as Ca2+ ≥ Mg2+ ≈Na+ > > K+. The anions on the other hand occur in the order; HCO3− > Cl− ≈ SO42− > > NO3− for groundwater and HCO3− > Cl− > SO42− > NO3− for lake water (Fig. 3a). The dominance of \({\mathrm{HCO}}_{3}^{-}\) suggests both the lake water and groundwater are dominated by meteoric waters (Freeze and Cherry 1979). The \({\mathrm{HCO}}_{3}^{-}\) may have resulted largely from the dissociation of carbonic acid (H2CO3) when falling precipitation dissolved CO2 in the atmosphere, which process is well described by Freeze and Cherry (1979).
Hierarchical cluster analysis (HCA) performed on the hydrochemical parameters using Q-mode, to discriminate samples based on their spatial relations resulted in four clusters, based on a phenon line drawn at a linkage distance of 3 on the resultant dendrogram (Fig. 4). The clusters are ordered from left to right, with clusters 1–3 being groundwater, and showing an increasing trend in ionic enrichment, whereas cluster 4 is composed of lake water samples. Cluster 1 (C1) members, generally located in the southwest, on the top of the crater rim, in higher elevations (about 308 m above sea level), exhibit relatively lower ionic content (median EC = 188 µS/cm), dominated by acidic waters (median pH = 5.6); characteristic of recharge zones in the evolutionary sequence of groundwater flow regimes (Table 1; Fig. 5a and b) (Chebotarev 1955; Freeze and Cherry 1979). The low mineralization also suggests a short residence time of infiltrated and rapidly recharged groundwater (Table 2).
The relatively acidic waters observed in C1 members may have resulted from recharge from CO2-charged rainwater, and the dissolution of CO2 in the root zone as a result of plant and animal respiration (Hem 1985; Hasan and Tewari 2020). Indeed, comprehensive research by Akoto et al. (2011) on the chemical composition of rainwater sampled in the Pra River Basin suggests acidic rainwater with a pH range of 4.0–5.6 averaging 4.76 ± 0.47. Turner et al. (1996a) also observed that the decomposition of organic matter within the root zone lowers the pressure of CO2−, resulting in low pH within lake Bosumtwi. Even though there are fault zones distributed near the metavolcanic rocks, extensive research by various authors (e.g., Koeberl et al. 1997, 1998; Reimold et al. 1998; Boamah and Koeberl 2002; Karikari et al. 2007) on the rocks of the area do not indicate an abundance of CO2− so the migration and dissolution of deep mantle-derived CO2− with groundwater may not be an important reason for the low pH values. ‘Hard’ rock and associated silicate mineral dissolution is aided by acidic conditions as a result of low pH, which explains the relatively high levels of Si in C1 members as compared to the other clusters (Table 1). These processes cause the incongruent dissolution of mainly plagioclase, biotite and potassium feldspar, which are common minerals identified in the underlying geology of the study area (Garrels and Mackenzie 1967; Dedzo et al. 2017).
Average concentration of the major ions used to plot a Schoeller diagram (Fig. 3b) corroborated the evolutionary sequence observed above; Na+ level is higher than Mg2+ and Ca2+ for C1 members. The dissolution of Si is enhanced under conditions of high CO2 and low pH. Under such conditions, the groundwater gains high Si content over relatively short residence time. And where the dissolution of plagioclase is present, a 2:1 Si to Na ratio is expected (Garrels and Mackenzie 1967; La Plante et al. 2021). This is consistent with the assertion that the high levels of Si in C1 members resulted from the aforementioned processes while CO2-charged meteoric waters recharged groundwater, and in the process dissolved a sodium-rich plagioclase feldspar such as albite, which is common among the rocks of the study area. The weathering of silicate minerals such as albite is probably the cause of the high levels of Na+ in C1 members. Samples from C1 are most likely groundwater collected from boreholes located in areas designated as potential recharge zones. The groundwater evolved from Cluster 1 to Clusters 2 & 3 as one moves down the gradient towards relatively lower elevations in the northeast (NE). Cluster 2 (C2) members occur at elevations around 251 m above sea level (asl), characterised by a high ionic content and a slightly acidic (pH = 6.55) system. Cluster 2 appears to be the transmission zone members, which evolved into a more mineralized group (EC = 888.5 µS/cm) in Cluster 3 (C3), located in a much lower elevation (163 m asl) with average pH of about 6.72 (Table 1 and Fig. 5 a and b). The relatively high mineralization in Cluster 3 members is attributable to the relatively longer residence time and increased interaction with aquifer materials as the groundwater transits and evolves from C1 to C3 (Freeze and Cherry 1979; Stuyfzand 1999; Appelo and Postma, 2005; Cochand et al. 2019; Ren et al. 2021). Although C2 and C3 appear to be equally balanced on the cations, with no particular cation dominating, Ca2+ appears to be the most abundant cation in most of the C2 and C3 members. While various studies (e.g. Hendry and Schwartz 1990; Herczeg et al. 1993; Hiscock et al. 1996; Zhou et al. 2020; Mirzavand et al. 2020; Kumar et al. 2021) have employed major ion chemistry to ascertain the main sources of solutes and to better evaluate and understand groundwater evolution, several other studies (Panno et al. 1994; Gosselin et al. 2001; Stimson et al. 2001; Pazand et al. 2018; Jeen et al. 2021) have demonstrated that the study of regional hydrogeochemistry is valuable to the management of regional aquifers, tracing flow and salinisation of groundwater.
It can be observed from Table 1 that the Si levels decrease along the flow path as the groundwater evolves, in the order; C1 > C2 > C3, suggesting possible crystallisation of some secondary mineral other than kaolinite that may have substituted silica in the groundwater (Garrels and Mackenzie 1967). Various factors such as aquifer mineralogy and characteristics, climate and topography and composition of recharge, (e.g., Güler et al. 2002; Loh et al. 2016, 2020) have been identified to influence the chemistry of groundwater, and collectively influence the diverse water types that change in time and space. The analyses and observations corroborate the assertion that hydrochemical facies analysis can be applied to adequately delineate and trace potential groundwater recharge source(s). Hence it is cogent to conclude that the observed pattern is in tandem with the general hydrogeological principle that groundwater becomes relatively more mineralized, with increased residence and contact time with the aquifer material along its flow paths. Detailed work on the hydrochemistry and quality of groundwater and lake water in the study area for various uses are presented by Loh et al. (2016) and Loh et al., (2021).
Stable isotopes
Figure 6 presents summary results of δ18O and δ2H for rainwater (RW), lake water (LW) and groundwater (GW) from the study area. The results revealed that δ18O and δ2H values varied from − 3.6 to − 2.3 ‰ for δ18O, and − 16.5 ‰ to − 7.6 ‰ for δ2H for the groundwater in the study area. Average δ18O values for the RW, LW and GW in the area are − 4.47 ‰, − 3.06 ‰ and 4.74 ‰ respectively, whereas their respective average δ2H are − 25.49 ‰, − 12.46 ‰ and 23.98 ‰, although samples from the lake and rainfall were limited in number. Since water from natural water bodies such as rivers, lakes, dams, groundwater etc. is derived from precipitation, its characteristic signatures in 18O and2H are expected to be evident in such water bodies in the study area. However, comparing the isotopic signatures of δ18O and δ2H for GW and LW to the precipitation in the study area, a progressive enrichment was observed in their isotope compositions, suggesting that the rainwater may have undergone some modification (Clark and Fritz 1997).
A biplot of δ2H against δ18O for the various water sources sampled in this study is presented in Fig. 7. Rainfall event RW04, which was sampled at the beginning of the rainy season, around April is relatively isotopically enriched, compared to latter events in May to July, and exhibit similar signature as groundwater in the areas. RW04 was particularly an early and light rainfall event that commenced the rainy season. The enrichment of RW04 is attributable to the harsh weather conditions associated with the onset of the rainy season; characterised by high temperatures and evaporation rates (Fig. 7). The harsh weather conditions in the study area lessens once the rainy season begins, leading to a much cooler environment since the dry air column and the ground gets saturated with water. This minimises the evaporation rate and subsequent depletion of rainfall events (Fig. 7) (Clark and Fritz 1997).
To further understand the relative isotopic enrichment or depletion of the various water sources in this study, the regression line established by Akiti (1986) for the Accra plains was adopted as the local meteoric water line (LMWL) due to the lack of sufficient precipitation data. The LMWL established by Akiti (1986) as: \(\delta\)2H = 7.87\(\delta\)18O + 13.6 is comparable as a result of the proximity and similar geographic and climatic conditions of Accra Plains to this current study area. The intersection of the LMWL and the LEL produced an initial isotope content (Clark and Fritz 1997; Marfia et al., 2004; Dogramaci et al. 2012) of − 29.76‰ δ2H and − 5.51‰ δ18O that is more negative than the mean isotope composition (δ2H = − 25.49‰ and δ18O = − 4.47‰) of rainwater but comparable to the isotopic composition (δ2H = − 30.07‰ and δ18O = − 4.88‰) of the heaviest rainfall event collected in May. This agrees with the findings of Turner et al. (1996a) that the lake water input budget is dominated by heavy and intense rainfall events who estimated that about 80% of the water that enters the lake annually is sourced from rainfall on its surface.
It can be observed in Fig. 7 that most of the samples from the rain and groundwater plot between the LMWL (Akiti 1986) and the global meteoric water line (GMWL), except for the LW samples, which plot far below both MWLs. The level of departure of the samples from the meteoric water lines (MWLs) reveals the degree of relative enrichment. Samples with little or no enrichment plot more closely to the MWLs as compared to more enriched samples. Significantly enriched samples fall to the right of the regression line generating larger intercept value (Alley 1993; Mazor 1997). Hence GW samples that are plotted close to the MWLs have not undergone any significant evaporative enrichment. This is probably due to the fact that groundwater is generally protected from the impacts of high temperature and evaporation on the land surface. On the other hand, the lake water, due to its exposure to harsh weather conditions is prone to high evaporation rates and subsequently relatively high enrichment. This explains the divergence of the lake water samples from the MWLs (Fig. 7).
A model proposed by Craig and Gordon (1965) has been adopted to estimate the rate of evaporation from the lake surface. This model, which has been used in several studies (Dogramaci et al. 2012; Yidana 2013; Gonfiantini et al. 2018; Dar et al. 2021) employed average temperature in the area, slope of the local evaporation and the initial isotopic signature of the precipitation to estimate evaporation rate for the lake. The isotopic signature of RW04 sampled in April was considered as the initial precipitation, and the ambient air vapour isotope signature (δA) estimation resulted in − 12.40 ‰ for δ18O using Eq. 7. The observed ~ 90% evaporation rate from the lake surface is in tandem with the harsh climatic conditions that characterise the study area. This further corroborates the assertion that water from the lake has undergone some intense evaporation, and as a result it is isotopically enriched.
The GW samples from the study area, fit nicely with a least square regression line, given as: \({\delta }^{2}H=5.49{\delta }^{18}O+4.33\) with a strong correlation (r = 0.7). The slope and intercept, 5.49 and 4.33, respectively, suggest that the rainwater, prior to infiltration and recharge, may have undergone relative evaporative enrichment within the unsaturated zone, especially when compared with the LMWL and GMWL. This agrees with what has been reported in the literature about d-excess values of less than 10 ‰ (Dogramaci et al. 2012; Sreedevi et al. 2021). The evaporation rate for groundwater was estimated to range from 54% to 59.5%, using − 17.03 ‰ and − 3.89 ‰, respectively, as initial δ2H and δ18O isotope contents. This suggests that in the study area, more than half of the rainwater that begins transit through the unsaturated zone is lost to evaporation and aquifer recharge probably resulted from vertical infiltration or passage along flow paths in fractures. Thus, rainwater available for groundwater recharge is reduced at most locations within the area. This corroborates the assertion that evaporation is an active process in the unsaturated zone prior to recharge as observed in some studies in Ghana (Addai et al. 2016; Akurugu et al. 2020). The observed groundwater evaporation rate could be attributed to the matrix of flow mechanisms within the unsaturated zone before recharge. In a system where the piston and preferential flow are characteristic, a mix of old and new waters could impact the isotopic signature of the final recharging water (Cheng et al. 2014). Fynn et al., (2016) in an attempt to characterize the unsaturated zone of the Nabogo basin in Ghana, established the effect of the flow mechanism on isotopic signature of recharged groundwater. The estimated initial value of δ18O (− 3.89 ‰) is comparable to the average δ18O values obtained for the rainwater (− 4.47 ‰), Akiti (1986) (− 3.20 ‰) and groundwater (− 3.10 ‰) in the study area, which are characteristic of recent meteoric waters. This supports the claim that the groundwater may have been recharged through fissures and fractures by recently evaporated meteoric water.
Furthermore, it is evident in Fig. 8 that the groundwater EC (138 – 1746 μS/cm) shows a generally increasing trend whereas the isotopic composition remains the same. This suggests that one of the main factors influencing the hydrochemistry of the groundwater is rock mineralization and not evaporation. Therefore, the isotopic signature as observed in the groundwater could only have resulted from direct precipitation (Gibrilla et al. 2017).
Depletion gradients in stable water isotopes have been observed per 100 m rise in elevation, ranging from − 1 to − 4 ‰ for δ2H and − 0.15 to − 0.6 ‰ for δ18O (Clark and Fritz 1997; Dogramaci et al. 2012). Groundwater samples in lower elevations and close to the lake were relatively lighter in isotopic signatures than the lake water but with isotopic content close to that of the samples located around the crater rim with an elevation difference of about ~ 300 m. This shows that the isotopic contents of the various groundwater samples had no relationship with elevation in the study area (Fig. 8a) suggesting no seepage of water from the lake towards groundwater. However, this does not exclude a possible hydraulic connection between the lake and shallow aquifers in the study area.
Figure 8c shows the relationship between elevation and EC. It can be deduced that the EC (138–1746 μS/cm) of the groundwater increases as water moves towards the lake in lower elevations, suggesting that the direction of groundwater flow is from the crater rim into the lake. Hence the claim that the crater rim areas are the plausible recharge zones is in tandem with results from the cluster analysis (Fig. 4). Since the groundwater flows towards the lake, a detailed study may be required to establish the possible connection between the lake and groundwater if any. This is important to establish the impacts of low or high groundwater levels on the lake and vice versa, in the wake of climate change, land cover change, population growth and urbanisation.
Conclusions
This study has successfully demonstrated the utility of hydrochemical and stable isotopic tracers to determine the possible hydraulic connection between surface and groundwater reservoirs. The study finds that there is no obvious hydraulic connection that benefits the underlying aquifers through seepage from the Lake; the reverse situation whereby groundwater discharges into the Lake is more plausible, based on the stable isotopic data and the hydrochemistry. The data suggest that large volumes of evaporative losses of water from both the Lake and infiltrating rainwater in transit into the groundwater system. A conceptual framework inspired by the processed hydrochemical data suggests net groundwater discharge into the Lake. Evaporation rate of precipitation, which is thought to occur during infiltration prior to recharging the groundwater is estimated at 54–59.5%, whereas evaporation over the Lake Bosumtwi surface is ~ 90% based on δ18O and δD analysis, resulting in increased levels of univalent cations in the groundwater system. The results also suggest that any hydraulic connection between the lake and groundwater may not be benefitting aquifers in the study area. The significantly high levels of evaporative losses of water from both the infiltrating groundwater system and surface water in the Lake may even be exacerbated by the predictions of regional and global climate models which indicate rising temperatures and evapotranspiration rates in the West African sub-region. Prudent management strategies are therefore recommended to safeguard water resources for sustainable development.
Data Availability Statement
The data employed in this research would be made available on request.
References
Abid K, Zouari K, Abidi B (2010) Identification and characterisation of hydrogeological relays of continental intercalaire aquifer of southern Tunisia. Carbonates Evaporites 25(1):65–75
Addai MO, Yidana SM, Chegbeleh L, Adomako D, Banoeng-Yakubo B (2016) Groundwater recharge processes in the Nasia sub-catchment of the White Volta Basin: analysis of pore water characteristics in the unsaturated zone. J Afr Earth Sc 22:4–14
Akiti TT (1986) Environmental isotope study of ground water in crystalline rocks of the Accra plains (Ghana). In: 4th Working Meeting on Isotopes in Nature, Proceedings of an Advisory Group Meeting, IAEA, Vienna.
Akoto O, Darko G, Nkansah MA (2011) Chemical composition of rainwater over a mining area in Ghana. Int J Environ Res 5(4):847–854
Akurugu BA, Chegbeleh LP, Yidana SM (2020) Characterisation of groundwater flow and recharge in crystalline basement rocks in the Talensi district, Northern Ghana. J Afr Earth Sc 161:103–665
Alley WM (1993) Regional ground-water quality. Wiley, Hoboken
Amu-Mensah FK, Amu-Mensah MA, Akrong MO, Addico G, Darko H (2019) Hydrology of the major water sources of Lake Bosomtwe in Ghana. West Afr J Appl Ecol 27(1):42–51
Appelo CAJ, Postma D (2004) Geochemistry, groundwater and pollution. CRC Press, New York
Balek J (2011) Hydrology and water resources in tropical Africa. Elsevier, Amsterdam
Banoeng-Yakubo B, Yidana SM, Ajayi JO, Loh Y, Asiedu D (2010) Hydrogeology and groundwater resources of Ghana: a review of the hydrogeology and hydrochemistry of Ghana. In: Mcmann JM (ed) Potable water and sanitation. Nova Science Publishers Inc, New York, pp 77–114
Boamah D, Koeberl C (2002) Geochemistry of soils from the Bosumtwi impact structure, Ghana, and relationship to radiometric airborne geophysical data. In: Plado J, Pesonen L (eds) Impacts in Precambrian shields. Springer, Berlin Heidelberg, pp 211–255
Carreira PM, Marques JM, Guerra A, Nunes D, Marques JE, Teixeira J, Chaminé HI (2021) Caldelas and Gerês hydrothermal systems (NW Portugal): a comparative study based on geochemical and isotopic signatures. Environ Earth Sci 80(3):1–20
Carrier MA, Lefebvre R, Racicot J, Asare E (2008) Northern Ghana Hydrogeological Assessment Project
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering—1. Geochim Cosmochim Acta 8(1–2):22–48
Cheng L, Liu W, Li Z, Chen J (2014) Study of soil water movement and groundwater recharge for the loess tableland using environmental tracers. Trans ASABE 57(1):23–30
Clark ID, Fritz P (1997) Environmental isotopes in hydrology. Lewis Publishers, Boca Raton New York
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(3–4):294–313
Cochand M, Christe P, Ornstein P, Hunkeler D (2019) Groundwater storage in high alpine catchments and its contribution to streamflow. Water Resour Res 55(4):2613–2630
Coplen TB (1994) Reporting of stable hydrogen, carbon, and oxygen isotopic abundances (technical report). Pure Appl Chem 66(2):273–276
Dapaah-Siakwan S, Gyau-Boakye P (2000) Hydrogeologic framework and borehole yields in Ghana. Hydrogeol J 8(4):405–416
Dar FA, Jeelani G, Perrin J, Ahmed S (2021) Groundwater recharge in semi-arid karst context using chloride and stable water isotopes. Groundw Sustain Dev 14:100634
Dedzo MG, Tsozué D, Mimba ME, Teddy F, Nembungwe RM, Linida S (2017) Importance of rocks and their weathering products on groundwater quality in Central-East Cameroon. Hydrology 4(2):23
Dogramaci S, Skrzypek G, Dodson W, Grierson PF (2012) Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J Hydrol 475:281–293
Faure B (1998) The red thread: Buddhist approaches to sexuality, vol 1. Princeton University Press
Fougerouse D, Micklethwaite S, Ulrich S, Miller J, Godel B, Adams DT, McCuaig TC (2017) Evidence for two stages of mineralization in West Africa’s largest gold deposit: Obuasi Ghana. Econ Geol 112(1):3–22
Freeze RA, Cherry JA (1979) Groundwater. New Jersey: Prentice-Hall geochemical parameter distribution in a ground water system contaminated with petroleum hydrocarbons. J Environ Qual 30:1548–1563
Fynn OF, Yidana SM, Chegbeleh LP, Yiran GB (2016) Evaluating groundwater recharge processes using stable isotope signatures—the Nabogo catchment of the White Volta Ghana. Arab J Geosci 9(4):1–15
Garrels RM, Mackenzie FT (1967) Origin of the chemical composition of springs and lakes. In: Equilibrium concepts in natural water systems. Advances in Chemistry Series. American Chemical Society, no. 67, pp 222–242
Gat JR, Gonfiantini R (eds) (1981) Stable isotope hydrology Deuterium and oxygen-18 in the water cycle. International Atomic Energy Agency (IAEA): IAEA
Gibrilla A, Adomako D, Anornu G, Ganyaglo S, Stigter T, Fianko JR et al (2017) δ18O and δ2H characteristics of rainwater, groundwater and springs in a mountainous region of Ghana: implication with respect to groundwater recharge and circulation. Sustain Water Resour Manage 3(4):413–429
Gonfiantini R (1986) Environmental isotopes in lake studies. Handb Environ Isot Geochem 2:113–168
Gonfiantini R, Wassenaar LI, Araguas-Araguas L, Aggarwal PK (2018) A unified Craig-Gordon isotope model of stable hydrogen and oxygen isotope fractionation during fresh or saltwater evaporation. Geochim Cosmochim Acta 235:224–236
Gosselin DC, Harvey FE, Frost CD (2001) Geochemical evolution of ground water in the Great Plains (Dakota) Aquifer of Nebraska: Implications for the management of a regional aquifer system. Groundwater 39(1):98–108
Güler C, Thyne GD, McCray JE, Turner KA (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10(4):455–474
Hasan Z, Tewari DD (2020) Characteristics of Groundwater Quality in the Aquifer of Indo-Nepal Border of Balrampur City. Int J Plant Environ 6(02):146–151
Hem JD (1985) Study and interpretation of the chemical characteristics of natural waters. US Geol Survey Water Supply Paper 2254:1473
Hendry MJ, Schwartz FW (1990) The chemical evolution of ground water in the Milk River aquifer Canada. Groundwater 28(2):253–261
Herczeg AL, Simpson HJ, Mazor E (1993) Transport of soluble salts in a large semiarid basin: River Murray Australia. J Hydrol 144(1–4):59–84
Hirdes W, Davis DW, Eisenlohr BN (1992) Reassessment of Proterozoic granitoid ages in Ghana on the basis of U/Pb zircon and monazite dating. Precambr Res 56(1–2):89–96
Hiscock KM, Dennis PF, Saynor PR, Thomas MO (1996) Hydrochemical and stable isotope evidence for the extent and nature of the effective Chalk aquifer of north Norfolk UK. J Hydrol 180(1–4):79–107
Jeen SW, Kang J, Jung H, Lee J (2021) Review of seawater intrusion in western coastal regions of South Korea. Water 13(6):761
Jones WB, Bacon M, Hastings DA (1981) The Lake Bosumtwi impact crater, Ghana. Geol Soc Am Bull 92(6):342–349
Karikari F, Ferriere L, Koeberl C, Reimold WU, Mader D (2007) Petrography, geochemistry, and alteration of country rocks from the Bosumtwi impact structure Ghana. Meteorit Planet Sci 42(4–5):513–540
Kazapoe R, Arhin E (2021) Determination of local background and baseline values of elements within the soils of the Birimian Terrain of the Wassa Area of Southwest Ghana. Geol Ecol Landsc 5(3):199–208
Kebede S, Charles K, Godfrey S, MacDonald A, Taylor RG (2021) Regional-scale interactions between groundwater and surface water under changing aridity: evidence from the River Awash Basin Ethiopia. Hydrol Sci J 66(3):450–463
Kesse GO (1985) The mineral and rock resources of Ghana. A.A. Balkema, Boston, Rotterdam, United States, p 610
Koeberl C, Bottomley R, Glass BP, Storzer D (1997) Geochemistry and age of Ivory Coast tektites and microtektites. Geochim Cosmochim Acta 61(8):1745–1772
Koeberl C, Reimold W, Blum J, Chamberlain CP (1998) Petrology and geochemistry of target rocks from the Bosumtwi impact structure, Ghana, and comparison with Ivory Coast tektites. Geochim Cosmochim Acta 62(12):2179–2196
Kumar S, Joshi SK, Pant N, Singh S, Chakravorty B, Saini RK, Kumar V, Singh A, Ghosh NC, Mukherjee A, Rai P (2021) Hydrogeochemical evolution and groundwater recharge processes in arsenic enriched area in central Gangetic plain India. Appl Geochem 131:105044
La Plante EC, Simonetti DA, Wang J, Al-Turki A, Chen X, Jassby D, Sant GN (2021) Saline water-based mineralization pathway for Gigatonne-Scale CO2 Management. ACS Sustain Chem Eng 9(3):1073–1089
Leube A, Hirdes W, Mauer R, Kesse GO (1990) The early Proterozoic Birimian Supergroup of Ghana and some aspects of its associated gold mineralization. Precambr Res 46(1–2):139–165
Loh YS, Banoeng-Yakubo B, Yidana SM, Asiedu D, Akabzaa T, Jørgensen NO (2012) Hydrochemical characterisation of groundwater in parts of the Volta Basin, Northern Ghana. Ghana Mining J 13:24–32
Loh YSA, Yidana SM, Banoeng-Yakubo B, Sakyi PA, Addai MO, Asiedu DK (2016) Determination of the mineral stability field of evolving groundwater in the Lake Bosumtwi impact crater and surrounding areas. J Afr Earth Sc 121:286–300
Loh YSA, Akurugu BA, Manu E, Aliou AS (2020) Assessment of groundwater quality and the main controls on its hydrochemistry in some Voltaian and basement aquifers, northern Ghana. Groundw Sustain Dev 10:100296
Loh YSA, Addai MO, Fynn OF, Manu E (2021) Characterisation and quality assessment of surface and groundwater in and around lake Bosumtwi impact craton (Ghana). Sustain Water Resour Manag 7(5):1–18
Marfia AM, Krishnamurthy RV, Atekwana EA, Panton WF (2004) Isotopic and geochemical evolution of ground and surface waters in a karst dominated geological setting: a case study from Belize, Central America. Appl Geochem 19(6):937–946
Mazor E (1997) Chemical and isotopic groundwater hydrology: the applied approach, 2nd edn. Marcel Dekker Inc, New York
Mirzavand M, Ghasemieh H, Sadatinejad SJ, Bagheri R (2020) An overview on source, mechanism and investigation approaches in groundwater salinization studies. Int J Environ Sci Technol 17(4):2463–2476
Mustafa SMT, Hasan MM, Saha AK, Rannu RP, Uytven EV, Willems P, Huysmans M (2019) Multi-model approach to quantity groundwater-level prediction uncertainty using an ensemble of global climate models and multiple abstraction scenarios. Hydrol Earth Syst Sci 23:2279–2303
Nuan Y, Zhou P, Wang G, Zhang B, Shi Z, Fu L, Li B, Chen X, Liang G, Dang X, Gu X (2021) Hydrochemical and isotopic interpretation of interactions between surface water and groundwater in Delingha, Northwest China. J Hydrol 598:126243. https://doi.org/10.1016/j.jhydrol.2021.126243
Panno SV, Hackley KC, Cartwright K, Liu CL (1994) Hydrochemistry of the Mahomet bedrock valley aquifer, east-central Illinois: indicators of recharge and ground-water flow. Groundwater 32(4):591–604
Pazand K, Khosravi D, Ghaderi MR, Rezvanianzadeh MR (2018) Identification of the hydrogeochemical processes and assessment of groundwater in a semi-arid region using major ion chemistry: a case study of Ardestan basin in Central Iran. Groundw Sustain Dev 6:245–254
Peng TR, Huang CC, Wang CH, Liu TK, Lu WC, Chen KY (2012) Using oxygen, hydrogen, and tritium isotopes to assess pond water’s contribution to groundwater and local precipitation in the pediment tableland areas of northwestern Taiwan. J Hydrol 450:105–116
Perera E, Zhu F, Dodd N, Briganti R, Blenkinsopp C, Turner IL (2019) Surface-groundwater flow numerical model for barrier beach with exfiltration incorporated bottom boundary layer model. Coast Eng 146:47–64
Pu H, Song W, Wu J (2020) Using soil water stable isotopes to investigate soil water movement in a water conservation forest in Hani Terrace. Water 12(12):3520
Reimold WU, Brandt D, Koeberl C (1998) Detailed structural analysis of the rim of a large, complex impact crater: Bosumtwi crater Ghana. Geology 26(6):543–546
Ren X, Li P, He X, Su F, Elumalai V (2021) Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch Environ Contam Toxicol 80(1):74–91
Sreedevi PD, Sreekanth PD, Reddy DV (2021) Deuterium excess of groundwater as a proxy for recharge in an evaporative environment of a Granitic Aquifer, South India. J Geol Soc India 97(6):649–655
Stimson J, Frape S, Drimmie R, Rudolph D (2001) Isotopic and geochemical evidence of regional-scale anisotropy and interconnectivity of an alluvial fan system, Cochabamba Valley Bolivia. Appl Geochem 16(9–10):1097–1114
Stuyfzand PJ (1999) Patterns in groundwater chemistry resulting from groundwater flow. Hydrogeol J 7(1):15–27
Turner BF, Gardner LR, Sharp WE, Blood ER (1996) The geochemistry of Lake Bosumtwi, a hydrologically closed basin in the humid zone of tropical Ghana. Limnol Oceanogr 41(7):1415–1424
Yidana SM (2013) The stable isotope characteristics of groundwater in the Voltaian Basin–an evaluation of the role of meteoric recharge in the basin. J Hydrogeol Hydrol Eng 2:2
Yidana SM, Banoeng-Yakubo B, Sakyi PA (2012a) Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J Earth Syst Sci 121(2):491–507
Yidana SM, Ophori D, Banoeng-Yakubo B Samed AA (2012b) A factor model to explain the hydrochemistry and causes of fluoride enrichment in groundwater from the middle Voltaian sedimentary aquifers in the northern region, Ghana. ARPN J Eng Appl Sci 7(1):50–68
Yira Y, Diekkruger B, Steup G, Bossa AY (2017) Impact of climate change on hydrological conditions in a tropical West African catchment using an ensemble of climate simulations. Hydrol Earth Syst Sci 21:2143–2161
Yu X, Moraetis D, Nikolaidis NP, Li B, Duffy C, Liu B (2019) A coupled surface-subsurface hydrologic model to assess groundwater flood risk spatially and temporally. Environ Model Softw 114:129–139
Zhang Q, Luo Z, Lu W, Harald Z, Zhao Y, Tang J (2020) Using water isotopes and hydrogeochemical evidences to characterize groundwater age and recharge rate in the Zhangjiakou area, North China. J Geog Sci 30:935–948
Zhou Y, Li P, Xue L, Dong Z, Li D (2020) Solute geochemistry and groundwater quality for drinking and irrigation purposes: a case study in Xinle City North China. Geochem 80(4):125609
Zhou J, Liu G, Meng Y, Xia C, Chen K, Chen Y (2021) Using stable isotopes as tracer to investigate hydrological condition and estimate water residence time in a plain region, Chengdu China. Sci Rep 11(1):1–12
Acknowledgements
We are grateful to the Office of Research, Innovation and Development (ORID), the Department of Earth Science Capacity Building Project, the University of Ghana and the University of Rochester, New York. We are also grateful to the Ghana Geological Survey Authority for availing us of the accommodation facilities at Mmrontuo during the fieldwork. We are greatly indebted to Profs. Asish Basu and Carmala Garzione and Dr. Penny Higgins, all of the Department of Earth and Environmental Science, University of Rochester, for allowing us to use their laboratories and helping with the analysis of the samples.
Funding
This work has been partly supported by ORID, through the Faculty Development Grant Award, the Department of Earth Science Capacity Building Project, the University of Ghana and the University of Rochester, New York. We are also grateful to the Ghana Geological Survey Authority for availing their accommodation facilities at Mmrontuo during the fieldwork. We are greatly indebted to Profs. Asish Basu and Carmala Garzione and Dr. Penny Higgins, all of the Department of Earth and Environmental Science, University of Rochester, for allowing us to use their laboratories and helping with the analysis of the samples. This work has been partly supported by the Office of Research, Innovation and Development (ORID), through the Faculty Development Grant Award of the University of Ghana, Ghana and the Rochester International Council Fellowship for Doctoral Thesis Completion, University of Rochester, New York.
Author information
Authors and Affiliations
Contributions
YSAL: conceptualization, methodology, data curation, writing–original draft, visualization, funding acquisition. OFF: formal analysis, investigation, visualization. BAA: methodology, writing–review and editing. GYA: writing–review and editing, investigation, resources. MOA: investigation, validation. EM: writing–review and editing, investigation, resources. SMY: conceptualization, writing–review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loh, Y.S.A., Fynn, O.F., Manu, E. et al. Groundwater - surface water interactions: application of hydrochemical and stable isotope tracers to the lake bosumtwi area in Ghana. Environ Earth Sci 81, 518 (2022). https://doi.org/10.1007/s12665-022-10644-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10644-x