Abstract
Essentuki mineral groundwater (EMGW) basin is characterized by unique and complex geological and hydrogeological settings due to monoclinal character of the geological structure, breaking by north-eastern faults, significant stratum of clays, overlaying major aquifers, volcanic laccolite peaks. EMGW area involves the unique wide variety of mineral waters with different TDS, pH, chemical, and gas composition. TDS changes from fresh (0.5–0.9 g/L) to high salinity (10–13 g/L) and strongly depends on the chemical composition of water. The region is characterized by a wide spread of high pCO2 sparkling Ca–Na–HCO3–Cl waters, which are the trademark of “Essentuki 4” and “Essentuki 17”. Such waters have strong therapeutic properties. Geothermal conditions, major- and micro-elements, isotopic characteristics of water (δ18O and δ2H) and gas (δ13C in CO2 and CH4, 3He/4He) phases of carbon dioxide mineral waters of EMGW basin were analyzed in this paper. The CO2free gas is probably a mixture of gas from deep (volcanic) and biogenic sources, although methane has the marine microbial origin and evolves from the degradation of dispersed organic matter in the host rocks. 3He/4He values prove that the free associated gas in mineral groundwaters of the region is a mixture of several sources of gas (mantle, crustal, and biogenic).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Caucasian mineral water (CMW) region is the unique spa area in the Russian Federation, known by therapeutic mineral springs. Essentuki mineral groundwater basin is located in the central part of the CMW region. The study of mineral groundwater has more than 200 years of history (Ivanov 1972). The first documents about saline springs were published in 1810 when doctor F.P. Gaas found and depicted three springs. In 1823, professor A. P. Nelyubin described 28 springs (Potapov and Danilov 2012).
The Essentuki mineral groundwater (EMGW) area involves the unique wide variety of mineral waters with different TDS, pH, chemical, and gas composition. TDS changes from fresh (0.5–0.9 g/L) to high-salinity (10–13 g/L) and strongly depends on the chemical composition of water. According to the main anions, the chemical type of groundwater varies from HCO3 to Cl–HCO3 and SO4–HCO3. Depending on the gas content, the mineral groundwater within the Essentuki basin can be still water, high-pCO2 sparkling water, or H2S-rich water. The springs # 4 and # 17, so-called “Essentuki-4” and “Essentuki-17”, were found to be the most valuable in their properties and composition, and are currently known all over the world.
This paper presents: (1) the geological structure of the Essentuki mineral groundwater basin and its hydrogeological conditions, (2) the main aspects of the chemical composition of mineral groundwater, including water phase, gas phase, and balneological components, and (3) the isotopic characteristics of water (δ18O and δ2H) and gas (\(\delta^{13}{\text{C}}_{({\text{CO}}_2)}\), \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\), and 3He/4He) phase.
Study area
Meteorology and landscape
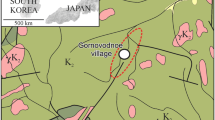
The investigated area is located in the Russian Federation, in the southern part of the Stavropol region, near the northern branches of Greater Caucasus, between the Black and Caspian seas (Fig. 1). The main object of the study is a part of Mineralovodsky artesian basin (10.2 thousand km2), located in the center of the CMW region (5.2 km2), called Essentuki mineral water area. Essentuki is one of the spas of the CMW region, which also includes Mineralnye Vody, Zheleznovodsk, Kislovodsk, and others.
There are two large orographic regions:
the northern slope of the Greater Caucasus on the south (2000–2400 m)
the gently sloping Mineralovodskaya plain on the north (400–650 m), which borders with the system of cuestas forming ridges of 1300–1500 m height.
Spa Essentuki occurs within the quite flat area on the height of 600–700 m, near the boundary of mountain terrain and steppe plain. In addition to the cuesta relief forms, there are laccolite peaks within the CMW region. The laccolite peaks also disturb the calm tectonic structure of the monocline of Neogene, Paleogene, and the underlying Cretaceous rocks, breaking through their igneous cores. There are 17 different volcanic structures, partly eroded to crystalline nuclei, represented by trachytes and trachiliparites. Some of them were not exposed yet to crystalline nuclei but raised the sedimentary rocks to form the dome-shaped piercing structures. Nature and the originality of those domes are potentially similar to the tectonics salt domes. These mountains are northeastern of Elbrus, the central volcanic mountain of the Caucasus. The primary northeastern line is Elbrus (5642 m)–Beshtau (1400 m)–Zmejka (Snake, 994 m). There are two lines on both sides of the main one, Dzhuca-2 (1190 m)–Dzhuca-1 (or Yutsa, 912 m)–Mashuk (993 m) and Sheludivaya (874 m)–Ostraya (Sharp, 881 m)–Kabanka (Tupaya, 772 m)–Medovaya (Honey, 721 m)–Zheleznaya (Iron, 851 m)–Razvalka (926 m). Those lanes form 10 km wide zone (Fig. 2). To the north, the domain extends for 30 km along the northwestern direction to Verblud (Camel, 885 m)–Byk (Bull, 817 m)–Lysaya (739 m). Such straight-line relationship of volcanic mountains is entirely consistent with the magmotectonic structure of the region and the total Central Caucasus with predominantly northeastern and northwestern trends (Grekov 2003). The area of volcanic mountains distribution is named Mineralovodsky intrusive region.
The Essentuki mineral water area of 130.75 km2 is located in the northwest of the CMW region, which involves the rivers Bugunta and Podkumok. The main water stream is a mountain river Podkumok, which flows from southwest to northeast. Its average flow rate is 2.8 m3/s and the maximal flow is 235 m3/s. Both Bugunta and Podkumok rivers are fed by precipitation and by the melting of snowfields and glaciers of the mountains outside of the CMW region. The low-water season lasts from January to March (winter) and from July to September (summer). During the low-water periods, the majority of the small streams in the area dry out.
Various high-altitude positions, different types of aquifers, the combination of the open and closed hydrogeological settings, and the laccolite-peaks existence create microclimatic zones inside the CMW. The climate of EMGW basin is continental, mountain plain. Winter is mild; the average January temperature is − 4.2 °C, due to many positive temperature days. The lowest frost peak is − 33 °C. Fogs are frequent in November and December (13–14 days per month). Spring is very short, and the last night frost usually occurs in mid-April. Summer is warm, with lots of hot and dry days. The average July temperature is + 20.4 °C; the maximal temperature reaches + 37 °C. The average annual precipitation is 536 mm, about 45% of which occurs from May to July (Savelieva and Magomedov 1987). The total potential evapotranspiration is 400–450 mm. Area’s aridity index (ratio of precipitation to evaporation) varies from 0.7 in the flat part to 1 or more in the mountainous area. This makes the basin territory a good-rainfall zone.
Tectonics and geology
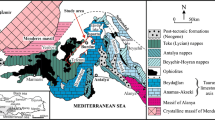
The geological structure of the CMW region and its hydrogeological conditions are incredibly complicated and unique due to its localization on the joint of the northern part of the Greater Caucasus mega-anticlinorium and the Scythian (Pre-Caucasian) epi-Hercynian plate. The boundary between structures passes along the Cherkesskaya flexure-fractured zone on the north, the regional Armavir–Nevinnomyssk depth fault on the northeast, the Nagutsko-Lysogorskaya flexure-fractured zone on the east.
From north to south, the Mineralovodsky Offset with the Nagutsky uplift, the North Caucasian monocline, the Labino-Malkinsky zone of the intermittent folding are distinguished in the tectonic structure of the CMW region (Fig. 2). The Essentuki mineral water area (EMWA) is located within the North Caucasian monocline, complicated by the large basement structure of the Transcaucasian uplift. This uplift of the basal complex produces a wide (up to 60 km) and flat anticline in the sedimentary cover. The EMWA is disposed to the crown of this anticline, which is accompanied by the Kislovodsk-Kumagorsky fracturing zone. The majority of the mineral water basins are associated with the area of fast-breaking, such as Kislovodskaya, Essentuksko-Novo-Blagodarnenskaya, Zheleznovodskaya, and others (Fig. 2). The Essentuksko-Novo-Blagodarnenskaya zone contains linear narrow horsts and grabens and the northeastern faults. The surrounding faults are another type of tectonic structure ring, distributed in the CMW region. The low-amplitude semi-circular fault zones and the associated zones of increased tectonic fracture in sedimentary rocks, surrounding the volcanic mountains from the south and west, are the most hydrogeologically active as water-conducting channels and ways for CO2 transport.
The depth of basal complex within Transcaucasian uplift is shallow (1–2 km) unlike the surrounding depressions, where the basement lies at depths of 3–8 km. The Proterozoic–Palaeozoic basement has a block structure and is composed of sedimentary-metamorphic and erupted rocks (granites, granodiorites, shales, gneisses, amphibolites, tuffs, sandstones, conglomerates, etc.). Rocks are greatly crumpled and fractured. The basal complex slowly dives to the north and northeast (Fig. 3).
The sedimentary stratum consists of Jurassic, Cretaceous, Palaeogenic, and Miocenic marine carbonate-terrigenous rocks and Pliocene–Quaternary continental sediments.
Hydrogeological setting
The hydrogeology section of the CMW region is represented by the monoclinal layers of Meso-Cenozoic aquifers going down towards the aquitards Proterozoic–Palaeozoic basement (Fig. 3). The recharge occurs in the southern and southwestern mountainous parts of the region, represented by the system of the Upper Jurassic and Cretaceous cuestas. The rest of the area, covered primarily by waterproof Maikop sediments, are represented by Upper Jurassic and Cretaceous sediments, which are the main domains of the water transit and discharge. Mainly, water permeability and the diverse lithological composition of sedimentary rocks define the heterogeneity of the aquifer hydraulic properties. The principal aquifers of EMWA are following (Fig. 3):
The Danish-Zealand aquifer \(({\text P\!\!\!\!-}_{1}{{\text{d-sl}}})\)
The Upper Cretaceous aquifer (K2)
The Lower Cretaceous aquifer (K1a-al1)
The Titon-Valanginian aquifer (J3tt-K1v).
Their brief hydrogeological description is presented below.
The Danish-Zealand aquifer \(({P\!\!\!\!-}_{\it 1}{d}{\text{-}}{sl})\) is one of the major pumped aquifers. The depth of the aquifer varies from 10 to 16 m in the central part of EMGW basin to 350–400 m on the north of the basin; with a total thickness of 50–150 m. The palaeogenic malms with interlayers of sandstones, argillites, and aleurolites lay on the surface of the Upper Cretaceous sediments. The aquifer permeability is low. The shallow groundwater are characterized by low TDS and the prevalence of HCO3 and SO4–HCO3 anions. The deep groundwaters have higher TDS 17–28 g/L and belongs to Na–Cl water type. Danish-Zealand aquifer is overlaid by Palaeogenic thick clay layer.
The Upper Cretaceous aquifer (К2) is widely distributed in the CMW region; it crops out on the south, where it recharges. There are numerous springs in the outcropping area, usually associated with the fracture and karst zones. The spring flow rate varies a lot from < 0.1 to 200 L/s. The aquifer lies from 30 m on the south to 770 m on the north of the EMGW basin. The total thickness of the Upper Cretaceous aquifer, changes from 300 to 350 m. The sediments contain limestones and malms. Hydrodynamic condition of the Upper Cretaceous aquifer is complicated. The recharge flow from the outcropping domain to the diving parts of the hydrogeological section occurs mainly through the tectonic fracturing zones. The hydraulic heads decrease from south to north, according to the direction of groundwater flow. There hydrodynamics anomalies due to discharge high-confined groundwater from the Lower Cretaceous and Titon-Valanginian aquifers. The sediments permeability depends on the degree of their fracturing, which causes variation of wells flow rates: from 0.01 L/s with groundwater drawdown of 100 m for low-fracturing rocks and up to 22–100 L/s with lowering of 330 m for high tectonically disturbed zones. According to the chemical composition, the Upper Cretaceous aquifer has Na–HCO3 type in springs, while being predominantly Na–Cl type in most of the wells. There is a high heterogeneity of hydrodynamic and hydrochemical parameters within the Upper Cretaceous aquifer and its adjacent areas. The exception is the Essentuki mineral water area. In the EMGW area, Na–HCO3–Cl water type was formed as a result of mixing its own (Upper Cretaceous) gas-free saltish waters, gas-free fresh waters of the Lower Cretaceous aquifer, and high-pCO2 mineral waters of the Titon-Valanginian aquifer.
The Lower Cretaceous aquifer (K1) consists of sandstones and aleurolites with shell limestones interlayers in the low part of strata. The depth of the aquifer varies from 400 m on the south to 900 m on the north of the basin; with a total thickness of 350–400 m. Groundwater is highly confined; the excess hydraulic heads reach 150–400 m over the layer top. According to the well rates of 2–20 L/s, the hydraulic conductivity is high. The aquifer provides high water supplies. Groundwater is warm, has nitrogen, and its TDS varies from 0.5 to 6 g/L. Lower Aptian clays underlie this aquifer. The overlaying Middle-Upper Albian sediments consist of argillites with a total thickness of 70–90 m. The sediments are the regional aquitard for the Caucasus Artesian basin even for the majority of the territory affected by the intrusions that have burned and raised these sediments without the hydrogeologically active zones formation.
The Titon-Valanginian aquifer (J3tt-K1v) contains Lower Cretaceous limestones and Upper Jurassic red terrigenous rocks with a total thickness up to 200 m. The aquifer lays at a depth of 850 m of the subsurface on the south and 1600 m on the north of the EMGW basin. The TDS of groundwater in the CMW region significantly varies from low (0.5 g/L) to high-salinity (10–11 g/L). According to the chemical composition, EMGW basin aquifer is usually salinity, belongs to Cl–HCO3 (SO4–Cl–HCO3) Na–Ca type, and has a high gaseous factor (15–30 L/L). The chemical composition of the aquifer potentially originates from the contact of the meteoric waters with the basement and carbon dioxide fluid coming from depth.
Detailed chemical composition of the mentioned aquifers is presented in the part 3.
Sampling and analytical methods
The natural (surface and mineral waters) and dissolved and associated free gases of the EMGW basin were studied in 2003–2017. The groundwater samples were pumped from wells and collected in acid-washed, high-density polyethylene sample bottles. Before sampling, all water samples were filtered through 0.45 μm cellulose filters to remove particulates. Only those aliquots that were used for cation determination were acidified with HNO3 to pH of 2–3. The measurements of water temperature, pH, Eh, alkalinity, and conductivity were performed directly in the field during sampling. Waters for cation analysis were acidified to pH < 2 with ultrapure HNO3.
Major cations and anions were analyzed by ion chromatography (Shimadzu LC-20) at the Far East Geological Institute. Dissolved carbonate species were determined by titration with reagent grade HCl (0.1 mol) and NaOH (0.02 mol) with phenolphthalein and methyl orange immediately after sampling. Trace element and rare earth elements (REE) concentrations in groundwater were determined by ICP-MS (Agilent 7500) at the Far East Geological Institute. Relative standard deviations (RSD) are better than 5% for elements at mg/l levels and better than 4% for elements at µg/l levels. The quality of sample analysis was assessed from ionic balance: all samples indicate imbalances below ± 5%.
Unfiltered water samples for H- and O-isotope analysis were collected into glass tubes for later measurement of D/H and 18O/16O ratios (‰ SMOW) using a Finnigan MAT253 gas-source mass spectrometer, working in continuous He-flow mode, after on-line pyrolysis with a Thermo Finnigan TC/EA (Far East Geological Institute). The high-temperature converter provides quantitative transformation of oxygen and hydrogen of water into CO and H2 in reducing conditions at 1450 °C and following chromatographic gas separation. Results are reported in standard delta notation relative to SMOW. Analytical precision was ± 0.8‰ for δ2H and ± 0.2‰ for δ 18O.
Samples of free associated gas were collected in glass tubes by the replacement method. The gas composition was determined by gas chromatography (Crystal-2000) using using using standard gas mixtures for the calibration (Lavrushin 2012). The concentrations of CH4 and CO2 were determined on a flame-ionization detector with a methanator, and hydrogen and oxygen on a thermal conductivity detector. The determination error for each component did not exceed 0.5 vol%. The composition of dissolved gasses (O2, N2, CO2 and H2S) was determined (also at Geological Institute of RAS) following the procedures described in Lavrushin (2012).
Carbon (\(\delta^{13}{\text{C}}_{({\text{CO}}_2)}\) and δ13C(DIC)) were carried out by mass spectrometry at the Geological Institute RAS (Moscow). The analytical results of (\(\delta^{13}{\text{C}}_{({\text{CO}}_2)}\) and δ13C(DIC)) were given in ‰ relative to the PDB International Standard. The accuracy of the analysis was better than 0.25‰.
CH4 gases isotopes ratios (13C/12C) were determined by isotope-ratio mass spectrometry MAT-253 using Trace-GC gas chromatograph at the Geologica Institute of RAS. The precisions of the analyses were 0.1‰ for \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\). A portion of the noble gas analysis was conducted by a static noble gas mass spectrometer (Micromass MM5400) at the Geological Institute of RAS. The analytical error, including reproducibility, was ~ 3%.
Chemical composition of mineral water
Water phase
Chemical composition
There is a wide variety of mineral waters with different temperatures, TDS, pH, chemical, and gas composition within the Essentuki mineral groundwater basin (Filimonova et al. 2019; Ivanov 1972; Lavrushin 2012).
The temperature of mineral waters range from 10 to ~ 70 °C and strongly depends on the depth and position of aquifers. Temperatures of waters increase in the northerly direction, reflecting the descend of water-bearing sediments. In the near-surface zone, there is shallow cold mineral water with low TDS and Na–HCO3–Cl chemical type (Fig. 4). That is why the local geothermal gradient is approximately 40–42 °C per km. There is a direct correlation between water temperatures and the aquifer depth (Fig. 4). The water Tmin is close to the mean annual air temperature. The data points above the trend line can be explained by the possible mixture of groundwater from multiple aquifers.
The pH of mineral waters from all wells is weakly alkaline and varies from 6.3 to 7.2 (Table 1). TDS changes from fresh (0.5–0.9 g/L) to high-salinity (10–13 g/L) and is strongly influenced by the chemical composition of water. The chemical composition of mineral waters mainly depends on the lithological composition of water-bearing rocks and the constituents of the gas phase. Besides, the interfacial overflows and the degree of hydrogeological openness of the horizon influence the geochemical feature of the waters. All these factors lead to the formation of mineral waters with various chemical compositions within the same aquifer complex.
According to the main anions, the chemical type of groundwater varies from HCO3 to Cl–HCO3 or SO4–HCO3 (Fig. 5).
In the Lower Paleocene (Danish-Zeland, Elburgan) aquifer \(({P\!\!\!\!-}_{\it 1}{d}{\text{-}}{sl})\), the predominantly cold sparkling waters with TDS from 7 to 10 g/L are widely spread. According to the contents of major ions, these water are preferably Na–HCO3–Cl type. Escaped free gas presented mainly by CO2 60–98%; CH4 3–22%; N2 1–36%, and O2 0.1–2.0%.
The mineral groundwaters in the Upper Cretaceous aquifer (K2) are rather warm with TDS varied from 0.5 to 6.3 g/L and mainly belong to Na–HCO3–Cl type. TDS of these waters is strongly reflected in the pumping rate and increases with the rate. In escaped free gas, CO2 dominants (varies from 28 to 80 vol%), the second most common gas is CH4 (up to 34 vol%). The content of CO2dis. ranges from 0.2 to 1.9 g/L and H2Sdis. from 0.012 to 0.019 g/L. The gas factor is low (< 0.85 L/L). The decrease in the mineral water pumping rate results in a decrease in CO2 content.
The Lower Cretaceous aquifer (K1a-al1) is bound warm mineral waters with TDS varied from 0.5 to 6 g/L. These geochemical water types are changed from Ca–Mg–Na–SO4–Cl–HCO3 to Na–SO4–Cl–HCO3. Groundwaters contain dissolved hydrogen sulfide (up to 2.2 mg/L), and the increased amounts of silica (H2SiO3 up to 45.5 mg/L) and iron (up to 5.2 mg/L). The composition of spontaneous gas mainly contains nitrogen, and also a smaller amount of helium, oxygen, methane, and carbon dioxide. The temperature of the groundwaters at the surface is significantly lower than the subsurface reservoir. We have noticed that it rises with an increasing water pumping rate and reaches + 43.0 °C.
The Titon-Valanginian aquifer (J3tt-K1v) contents thermally moderately mineral water with Cl–HCO3 (SO4–Cl–HCO3) Ca–Mg–Na (Na–Ca) types. The groundwater salinity varies from 7.0 to 8.5 g/L. Groundwaters contain an elevated quantity of carbon dioxide (CO2 0.5–1.6 g/L) and silicic acid (H2SiO3 up to 91 mg/L) and are characterized by high gas saturation. The gas factor varies from 15 to 30 L/L. Spontaneous free gases are mainly carbon dioxide, nitrogen, methane, and oxygen.
Based on the gas content, the mineral groundwaters within the Essentuki basin could be still water or high-pCO2 sparkling water, or, sometimes, H2S-rich water. This study was undertaken to identify the source of water and gas phases in mineral groundwaters from EMGW.
Mineral waters are characterized by a wide range of TDS from 0.6 to 15.2 g/L. The mineral waters that interact with meteoric infiltration waters have a lower TDS (< 2 g/L). In general, there is a trend towards an increase in TDS in the northerly direction.
The inversion of TDS is clearly visible in the Upper Cretaceous aquifer (K2) in the Essentuki area. Mineral waters with the brand names “Essentuki 4” and “Essentuki-17” are collected from this aquifer. According to our data, the average TDS here is ~ 10 g/L, while the lower TDS (~ 4.5 g/L) is detected in the underlying aquifers.
HCO3 is the primary anion of mineral waters within the Essentuki area (Fig. 5). The amount of this ion ranges from 35 to 81 mg-eq%. The presence of other anions and cations strongly depends on the aquifer type where mineral waters are located.
The most contrasting differences in the composition of aquifers are observed in sulfate ion concentration. For example, the proportion of sulfate ion among anions varies from ~ 20 to 40 mg-eq% in J3tt-K1v aquifer. Due to the presence of gypsum and sandstones lays in the late Jurassic sediments, sulfate ion concentration reaches 1.5–2 g/L.
Aquifer systems of the study area are quite different in concentrations of HCO3 and Cl-ions. The maximal concentration of HCO3 ion (up to 5.6 g/L) is typical for groundwaters of the late Cretaceous aquifer.
The most significant differences in the composition of groundwaters associated with the Upper Cretaceous (K2) and Titon-Valanginian (J3tt-K1v) aquifers are observed in the concentration of chlorine ion. For example, the concentration of chlorine ion significantly below 1 g/L in the Titon-Valanginian aquifer (J3tt-K1v), while it reaches nearly 2 g/L in Upper Cretaceous aquifer (K2) of Essentuki area. Probably, the increased concentration of chlorides in the groundwaters of the Upper Cretaceous aquifer (K2) is caused by the presence of halite in sediments, which is partially preserved in poorly permeable blocks of massive limestone of K2. The correlation between the concentrations of chlorides and hydrocarbonates is probably reflected by the processes of dilution of high chlorides and hydrocarbonates mineral waters with low-salt meteoric water. These processes also reflect the dependence of the chlorine–ion concentration in the water on the depth of the well.
Lower calcium and higher sodium (> 90 mg-eq%) concentrations are typical for waters of the Upper Cretaceous aquifer (K2).
In general, the concentrations of main components vary a lot. The J3tt-K1v aquifer composition of anions is characterized by the presence of high concentrations of sulfate ion. According to the cation content, there are waters of different types (Ca–Mg–Na, Ca–Na, and Na–Ca), but a significant role of Ca2+ ions is typical for all of them. On the other hand, groundwaters of the Upper Cretaceous aquifer (K2) of the Essentuki area are characterized by HCO3–Cl–Na-type, in which the sulfate ion concentration is analytically undetectable by ICP–MS (< 17 µg/L by S). However, they contain a higher concentration of HCO3 and Cl-ions than in the underlying aquifers.
The mineral waters located in the J3tt-K1v aquifer have significantly higher contents of K, Si, Rb, Cs, Mn, Fe, Zn, and Sr relative to groundwaters from K2 sediments; although the latter are enriched by B, Ba, and Br.
Isotopic composition
The isotopic composition of water oxygen (δ18O) varies in the range from − 14.3 to − 7.4 ‰, and hydrogen—from − 100 to − 63‰. On the diagram δ2H–δ18O (Fig. 6) it can be seen that the figurative points of mineral waters are mainly dropped along the line of meteor waters (Craig’s line). This is consistent with the previously obtained results of studies of the isotopic characteristics of mineral waters of Essentuki area, which showed that the main part of the water balance of mineral water source is infiltration meteor waters. We found a good correlation for δ18O and δ2H with concentrations of Cl, HCO3, Ca, Mg, Na, which reflect the process of mixing isotope-light oxygen fresh meteoric waters and high-salt waters (possible sedimentogenic) with higher values of δ18O and δ2H. The latter can probably have a sedimentary genesis and, obviously, their mixing and causes an “oxygen” shift in the diagram δ2H–δ18O.
Gas phase
Chemical composition
In the Essentuki area, dominated by carbon dioxide groundwaters, the share of CO2 in the free gas phase, which reaches 98%. However, there are also groundwaters with high concentrations of methane (up to 44%) and nitrogen (up to 70–90%).
Methane in gases of the mineral water has a significant prevalence (from 4 to 44%) in the central and southern parts of the Essentuki area. It is present in all gas samples of the Essentuki area (from 4 to 20%), with a higher concentration in the Apt-Albian and Upper Cretaceous aquifers. The maximal concentration of CH4 is detected in the associated free gases of the Essentuki well #17.
The high nitrogen concentrations (up to 90 vol%) are typical for low-salt waters (TDS < 2 g/L). Here its appearance can be associated with shallow waters enriched with nitrogen from the atmosphere. The higher gas concentrations (2–32.6 vol%) are detected in the carbon dioxide–methane gas mix of the Essentuki and Nagutsky areas (Lavrushin 2012). There is a direct relationship between methane concentrations and nitrogen in the es “caped free gas of all studied wells. On the one hand, it can reflect the dilution of these gases with CO2, and, on the other hand, it indicates the non-atmogenic genesis of nitrogen in the gases of the northern part of Essentuki area. Similar to methane, nitrogen can be formed during the decomposition of organic matter.
Helium is present in gases at the concentrations from < 0.01 to 1.3 vol%. Helium concentration is continuously increasing with the increasing depth and the degree of hydrogeological closure of aquifers that can explain the correlation between helium and methane concentrations.
The data described above explain the paragenetic relationship of the concentrations of the detected gases. Their accumulation depends on geological conditions and explains the similarities between gases with similar genesis. These conditions are represented by hydrogeological closure of aquifers, elevated temperatures, and the time of the water storage in a reservoir. In addition, this correlation can be explained by the dilution of gases by other gas with deficient concentrations of nitrogen, methane, or and helium. For example, gases can be diluted by magmatogenic CO2.
Isotopic composition
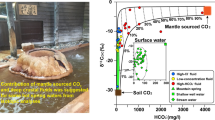
The value of δ13C in methane varies from − 61.7 to − 29.5‰. According to the δ13C value, gases collected within EMGW can be separated into three groups (Fig. 7a). The first group (J3tt-K1v aquifer) is characterized by the maximal values \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\) with minimal concentrations of methane (0.4–0.5 vol%). The second group (K1a-al aquifer) is characterized by the narrow range of the values \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\) from − 42.6 to − 40.8‰, while the third one (K2 aquifer) has the narrow range of values \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\) − 61.7 to − 59.6‰. The second group of gas samples was exclusively found in the Apt-Albian aquifer. The third group of samples was found only in the wells of the Essentuki area (“Essentuki 4” and “Essentuki 17”). We had detected only one sample with the intermediate value \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\) = − 48.4‰.
The isotopic composition of carbon dioxide in the gas phase of mineral groundwaters varies from – 16.7 to – 4.3‰ (Fig. 7b). We have detected an inverse relationship between the value of \(\delta^{13}{\text{C}}_{({\text{CO}}_2)}\) of the gas and the fraction of HCO3 in the aquatic phase of Essentuki groundwater. Therefore, Essentuki groundwaters (HCO3–Cl–Na type) are characterized by the presence of carbon dioxide with an admixture of isotope-light (biogenic) CO2, formed by the oxidation of organic matter. This emphasizes the critical role of biogenic processes in the formation of “salt-alkaline” type of the waters, such as Essentuki.
The carbon dioxide with lower values δ13C (< − 8‰) was detected in gas samples that are rich in methane (concentration = 4–44%).
The isotopic composition of helium in the gas phase in EMGW basin was studied in detail earlier (Polyak et al. 2005; Lavrushin 2012). The 3He/4He values, detected for more than 40 samples, ranged from 30 to 160 (× 10−8). Such data suggest that the spontaneous gases of EMGW mineral groundwaters have an insignificant amount of mantle helium (3He/4Hemant = 1200 × 10−8). This explains the genetic relationship of CO2 phase with the Pliocene–Quaternary volcanoes of Great Caucus (Elbrus volcano).
Balneological components
As we mentioned above, EMGW basin is characterized by a wide variety of mineral water types. From medical aspects, the most interesting of them are carbon dioxide bicarbonate–chloride sodium waters, known as “Essentuki 4” and “Essentuki 17”, which are widely spread in the upper Cretaceous and Paleogene sediments. They have been used in balneology as well as for bottling of mineral table water. Due to the therapeutic properties of these carbon dioxide waters, Essentuki resort quickly became widely known in Russia. Moreover, groundwaters of the similar composition, found in other places, became known as the “Essentuki” type mineral waters.
The main therapeutic property of these waters is the high concentration of soda (60%). Such mineral waters are used externally and internally, providing a wide range of medical effects. Patients with diseases of the circulatory system are prescribed to have carbon dioxide baths, while patients with issues in the digestive tract, such as stomach, liver, and kidneys, are prescribed to drink Essentuki mineral waters.
The presence of organic matters in therapeutic mineral waters is extremely interesting for balneologists. The nature of organic substances in the waters of Essentuki area varies from bituminous–humus with a clear predominance of bituminous substances (resins, acid bitumen, petroleum hydrocarbons) in the waters of bedrock to humus–bituminous in the waters of Quaternary deposits.
Groundwaters of Essentuki area are deficient in radioelements. On the contrary, the water from the nearby Pyatigorsk resort, with the radon content reaching 600–900 units, is valuable for radon treatment. Some Essentuki mineral water wells contain a high concentration of fluorine, iodine, bromine, and boric acid.
Gas composition Gases represent an essential component of the EMGW mineral waters. They are mainly composed of carbon dioxide and, to a lesser extent, nitrogen, hydrogen sulfide, and methane. The content of CO2 in the carbon dioxide waters for external use is normalized to 1.4 g/L. The composition of carbon dioxide mineral water is HCO3–Cl Ca–Na and SO4–Cl–HCO3 Mg–Na–Ca with TDS of 5.7–7.8 g/L, and the concentration of free carbonic acid is 1.1–1.4 g/L. The thermal waters with such parametres were found in the Titon-Valanginian aquifer (J3tt-K1v) at a depth of 1378 m. These waters as well as carbon-free waters, from the Lower Cretaceous aquifer, are mixed in 1:2 ratio to fill up the therapeutic baths. In recent years, this water mixture was called “Essentuki-new”.
Conclusions
-
1.
The CMW region is characterized by an active geodynamic regime and a high seismic intensity. The zones of heating, deconsolidation, and partial rock melting are located at the depths of 15–20 and 35–45 km and catalyze the production of carbon dioxide.
-
2.
The geological settings of the two-stage structure are the basal complex and carbonate-terrigenous sedimentary cover with a total thickness of 1500–1800 m, which declines in the northeastern direction. The monoclinal character of the geological structure and a slow depression of the lower age rocks under the upper age ones determine the area division into two parts: the recharge zone and the transport zone. The increased depth of layers and removal of the recharge area consequently change the chemical and gas compositions.
-
3.
EMGW area is located in the extension structure, which consists of narrow horsts and grabens, and is restricted by north-eastern faults. Faults and their surrounding fractured rocks serve as vertical channels to fluid migration between the layers and from the basal complex to the sedimentary cover and as lateral channels for speeding migration inside the aquifers that is how the hydrochemical inversion appeared in the past, and it currently exists in the cross-section of EMGW area.
-
4.
The volcanic laccolite-peak set in the northeastern direction creates thermal, hydrochemical, and gaseous anomalies and generates CO2. Laccolites play an important role in the discharge of deep aquifers. The local linear, semi-circular, and circular tectonic faults formed near the mountains serve as the discharge for large volume of deep groundwater to the surface.
-
5.
A significant stratum of Neogene clays overlays the Palaeogenic and Upper-Cretaceous carbonate sediments, preserving high-salinity groundwaters. In the southern part of monocline, the subjacent Lower-Cretaceous aquifer appears on the surface without overlaying the sediments and recharge by precipitation. The constant freshwater flow causes stepwise freshening in the lateral migration in northeastern direction.
-
6.
The obtained isotope data (δ18O and δ2H), plotted along the GLMW, proved the meteoric origin of the mineral waters within EMGW basin. The chemical composition of mineral waters mainly depends on the lithological composition of water-bearing rocks and the constituents of the gas phase. Besides, the interfacial overflows and the degree of hydrogeological openness of the horizon influence the geochemical feature of the waters.
-
7.
The temperature of mineral waters widely varies (10–70 °C) and strongly depends on the subsurface depth of aquifers and the local geothermal gradient (40–42 °C per km). The temperatures of waters increase in the northern direction according to the depth of the water-bearing sediment.
-
8.
The Essentuki mineral water basin is characterized by a wide spread of high pCO2 sparkling Ca–Na–HCO3–Cl waters, which are known as “Essentuki-4” and “Essentuki-17”, and have high therapeutic properties. These waters are confined to the upper Cretaceous and Paleogene sediments. The main therapeutic properties of these waters are dictated by the high concentrations of Na+ and pCO2. They are characterized by a high content of fluorine, iodine, bromine, and boric acid.
-
9.
The dominated free escaped gas in the majority of mineral waters within the EMGW basin is CO2 (up to 98 vol%), which is probably a mixture gas from deep (volcanic) and biogenic sources. However, in some mineral waters located in the central and southern parts of EMGW basin, a high concentration of methane (up to 44 vol%) and nitrogen (up to 70–90 vol%) were found. Isotopic data \(\delta^{13}{\text{C}}_{({\text{CH}}_4)}\) indicate the marine microbial origin of methane phase and prove that the high methane content in the water is caused by the degradation of dispersed organic matter in the host rocks. The detected values of 3He/4He suggest that the bubbling gas in mineral waters is a mixture of several sources of gas (mantle, crustal, and biogenic).
References
Craig H (1961) Isotopic variations in meteoric waters. Science 133(3465):1702–1703. https://doi.org/10.1126/science.133.3465.1702
Filimonova E et al (2019) Withdrawal and chemical composition of mineral water spa Essentuki (Caucasus, Russia). In: Geophysical research abstract EGU General Assembly 2019 https://meetingorganizer.copernicus.org/EGU2019/EGU2019-11134-1.pdf. Accessed 20 May 2019
Grekov II et al (2003) Geologicheskiy atlas severnogo Priel’brus’ya i Kavkazskih Mineral’nykh Vod (Atlas-KMV) (in Russian). Geological atlas of northern vicinity of the Mt. Elbrus and the Caucasian mineral water region (Atlas of CMW)
Ivanov VV (1972) Kavkazskie mineral’nye vody. Izd. Tsentr. in-ta kurortologii i fizioterapii. T. 21 (in Russian) Caucasian mineral water Moscow, pub. Cent. Ins. balneology and physiotherapy, p 158
Lavrushin VY (2012) Podzemnyye flyuidy Bol’shogo Kavkaza i ego obramleniya (in Russian). In: Polyak BG (ed) Subsurface fluids of the Greater Caucasus and its surrounding. GEOS, Moscow, p 348
Polyak BG et al (2005) Heat and mass transfer from the mantle: heat flow and He-isotope constraints. Ann Geophys 48:9–17
Potapov EG, Danilov SR (2012) Istoriya izucheniya uglekislykh mineral’nykh vod Essentukskogo mestorozhdeniya. Zhurnal Kurortnaya meditsina (in Russian). History of Essentuki carbonic mineral water deposits studies (in Russian). J Resort Med 3:9–12
Savelieva VV, Magomedov KA (1987) Geografiya Stavropol’skogo kraya. Stavropol’skoe knizhnoe izdatel’stvo (in Russian) Geography of Stavropol Territory. Stavropol Book Publishing House, Stavropol, pp 134–135
Acknowledgements
These investigations were supported by the Russian Science Foundation no. 18-17-00245. We want to thank Dr.Tatiana Velivetskaya, Mrs. Natalia Zarubina, and Olga Sukhanova for carrying out the chemical and isotopic analyses. Also, the authors gratefully acknowledge the editor, James W. LaMoreaux, and the anonymous reviewers for critical comments on this manuscript which helped to improve it remarkably.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a part of the Topical Collection in Environmental Earth Sciences on “Mineral and Thermal Waters” guest edited by Drs. Adam Porowski, Nina Rman and Istvan Forizs, with James LaMoreaux as the Editor-in-Chief.
Rights and permissions
About this article
Cite this article
Elena, F., Vasiliy, L., Natalia, K. et al. Hydrogeology and hydrogeochemistry of mineral sparkling groundwater within Essentuki area (Caucasian mineral water region). Environ Earth Sci 79, 15 (2020). https://doi.org/10.1007/s12665-019-8721-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8721-2