Abstract
The largest CO2-rich mineral water resource in the Sikhote-Alin ridge of Eastern Russia at Gornovodnoe village was studied. The high-pCO2 groundwaters are cold (5.8–10 °C) with pH 5.9–6.5, TDS varied from 1.2 to 2.7 g/l and belong to Ca–Mg (Ca–Na)–HCO3 type. New data on geology, mineralogy, hydrogeology and hydrogeochemistry, in conjunction with isotope data of water and gas phases, have provided a much better understanding of the origin of this distinctive groundwater. It was found that this water is of meteoric origin, but its unusual chemical composition is controlled by interactions of CO2-rich groundwater and the aquifer materials. The dissolved CO2 gas makes the water slightly acidic (at about pH 6.2) which increased the leaching of many trace elements from host volcanic rocks typically considered immobile at these pH values. 3He/4He ratios and δ13C indicate that mantle degassing is important as a source of deep exogenic fluids. The cold CO2-rich groundwater of the Eastern Sikhote-Alin ridge is the result of interactions between fresh groundwater of meteoric origin, mantle gases and the host volcanic rocks. It thus highlights connectivity between deep and shallow fluids along with deep fractures related to ancient terrane boundaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medical specialists have devoted much attention to CO2-rich mineral waters, which are considered a unique therapeutic natural resource. Within the former USSR major areas of these waters were identified: Carpathian, Caucasian, Pamir, Tien Shan, Sayan, Transbaikalia, as well as Far East territories: Primorye, Sakhalin, Kamchatka and Chukotka (Krainov et al. 2004; Dubinina et al. 2005; Mityusheva and Simakova 2007; Tchepkaia et al. 2006; Kharitonova et al. 2007, 2017, 2019; Kopylova et al. 2009, 2011; Zamana 2015; Shvartsev et al. 2017; Chelnokov et al. 2018 and others). As the preference within the Russian Far East, sparkling CO2-rich mineral waters are on the second place after thermal springs. Based on numerous occurrences resorts, sanatoriums, hospitals, and bottling plants for bottling of mineral water have been established.
Gornovodnoe spa is one of the mineral water sources, and it is located on the largest aquifer on the eastern slopes of the Sikhote-Alin ridge, in the Primorye region (Maritime), 60 km south-westward of the town of Olga (Fig. 1). The aquifer forms part of the Eastern Sikhote-Alin volcanic belt in the valley of the small River Solontsovaya. The aquifer is divided into four interlinked areas: Central, Northern, Southern and Yurganovsky. The chemical composition of the mineral waters in those regions is variable in calcium, magnesium and sodium. A key feature of the waters is a high concentration of dissolved carbon dioxide and a rather high content of iron and silicon.
Geological and hydrogeological studies of the aquifer have been undertaken for more than 60 years, including large-scale drilling works, hydrogeochemical and balneological studies. These have provided a much better understanding of the geology and hydrogeology, as well as spatial distribution of the aquifer (Shand et al. 1995; Chudaeva et al. 1999; Chelnokov and Chelnokov 2003; Chudaev 2003). However, the groundwater flow system is somewhat complicated in a tectonically active region, such as the Sikhote-Alin area, because several blocks split the area via faults which promote the mixing between shallow and deep-seated groundwaters.
The full-scale hydrogeochemical works on understanding the sources of the solutes in this area have not been conducted yet. In this paper, we present new data on the geology, hydrogeology, and mineralogical composition of bedrock as well as hydrochemistry and isotopic composition for aquatic (δ18O, δD) and gas (δ13C, 3He/4He, 4He/20Ne, 20Ne/22Ne, 38Ar/36Ar, 84Kr, 132Xe) phases of high-pCO2 groundwater circulated within volcanic aquifer system of Eastern Sikhote-Alin ridge. These data contribute to identifying the genesis of the CO2-rich groundwater and estimate the rate of transformation of the chemical composition of these groundwaters during the water–bedrock–gas interaction. Moreover, we try to estimate the groundwater residence time.
Sampling and analytical methods
In this study of the chemical and isotopic composition of CO2-rich water within Gornovodnoe spa, we used our analytical data of samples taken from 2004 to 2018 yrs. Sampling was carried out once a year, in the summer season; moreover, we compared our results with published data (Chudaev 2003). In general, more than 100 chemical analyses of water for 18 wells were taken into account.
Unstable chemical parameters (pH, Eh, ToC, DO, HCO3−, CO2) were measured directly in the field during the sampling procedure. To remove the particles from the “real” solute ions, we carried out filtration through membranes with a nominal pore size of 0.45 μm. Cations were analyzed from samples which were filtered and acidified with nitric acid (pH 2). The samples after filtration without acidification were collected in plastic bottles for further anion analyses. Major cations and anions were analyzed by ion chromatography (Shimadzu LC-20); trace element and REE concentrations were determined by ICP-MS analysis (Agilent 7500c) at the Far East Geological Institute.
Samples collected for analysis of stable isotopes (δ2H(H2O), δ18O(H2O)) were processed without filtration and stored in glass bottles. The collected water samples were stored in a dark and cold container before analysis. In the laboratory, the water samples were measured by mass spectrometry, with a Finnigan MAT253, working in continuous He-flow mode, after on-line pyrolysis with a Thermo Finnigan TC/EA. The high-temperature converter provides quantitative transformation of oxygen and hydrogen of water into CO and H2 in reducing conditions at 1450 °C and following chromatographic gas separation. Water sample injection (0.5–1 mcL) into the reactor of thermoconverter was made automatically using autosampler Combi PAL. The analytical precision was ± 0.8‰ for δ2H(H2O and ± 0.2‰ for δ18O(H2O). The stable isotope values were measured relative to internal standards that were calibrated using international standards: VSMOW (Vienna Standard Mean Ocean Water)—δ18O(H2O) = (0.0)‰; δ2H(H2O = (0.0)‰;SLAP (Standard Light Antarctic Precipitation)—δ18O(H2O) = (− 55.50)‰; δ2H(H2O = (− 427.5)‰; GISP (Greenland Ice Sheet Precipitation)—δ18O(H2O) = (− 24.76)‰; δ2H(H2O = (− 89.5)‰. Results are reported in standard delta notation relative to SMOW.
Tritium has been measured at the Pacific Ocean Institute FEB RAS by counting β-decay in a liquid scintillation counter (QUANTULUS-1220). The preliminary enrichment in two stages was carried out before the measurement. This allowed decreasing the detection limit to 0.03 TU.
Samples of gas were collected in glass tubes by the replacement method. The gas composition was determined by gas chromatography using thermal conductivity detectors and molecular sieve columns with He and Ar as carrier gases for the determination of He, H2, N2, and O2. Carbon (δ13C(CO2) and δ13C(DIC)) and noble gas isotopes (3He/4He, 20Ne/22Ne, 38Ar/36Ar, 84Kr, 132Xe) were carried out by mass spectrometry. The analytical results of (δ13C(CO2) and δ13C(DIC)) were given in ‰ relative to the PDB International Standard. The accuracy of the analysis was better than 0.25‰.
The samples of host rocks obtained during drilling of wells in the Northern, Central and Southern sections were studied in detail at interval from 1 to 5 m. In total, 29 samples of rocks from different depths were investigated. A detailed petrographical, mineralogical and chemical study of the samples was carried out. Optical microscopy, classical chemical analysis, X-ray diffractometry (Dron III), electron microprobe chemical analysis (Jeol, JXA-8100) with EDS INCAx-sight (Oxford, Instruments) and inductively coupled plasma-mass spectrometry (Agilent 7500c) analysis were used to analyze the rock samples and mineral phase. The detection limit of trace elements is 0.01 µm and the error of measurement was better than 5% RSD.
Simulation of the reaction in the water–rock system, as well as the calculation of saturation indices of minerals in the waters, was performed using the program Aquachem 5.1 (User Guide 2005).
To assess the aqua mobility of the chemical elements during groundwater–volcanic rock processes we used the coefficient of aqua migration Kam (Perel’man 1982), which is expressed as follows:
where mx is the concentration of the element in groundwater (g/l), M total dissolved solid (g/l) of groundwater and nx is the concentration of the element in bedrock (g/t), respectively.
Geological and hydrogeological setting
The CO2-rich mineral waters of the Gornovodnoe area were discovered in 1902, but a hydrogeological study begun only in the 1960. Significant contributions to this study were done by E.Yushakin (1963–1964) and the Federal State Unitary Enterprise “Primorsky hydrogeological expedition” (1989–1992) (Chelnokov and Kharitonova 2008). Since 1989, 44 wells have been drilled with depth from 30 to 302 m in Gornovodnoe area, and hydrogeochemical studies were carried out. The first comprehensive study comprising a wide range of chemical elements was completed within the framework of project INTAS No. A2-075 led by Dr. W.M. Edmunds and the British Geological Survey (Edmunds et al. 1996; Shand et al. 1995). Total storage of CO2-rich mineral water within Gornovodnoe volcanic rock area is 144 m3/day (Chelnokov and Chelnokov 2003). The maximum depth of high pCO2 groundwater circulation is 300 m. Currently, the CO2-rich groundwater is actively utilized by private companies for bottling and spa procedures.
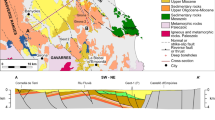
The studied area is located within the Eastern Sikhote-Alin volcanic belt and is confined to a system of faults of northwestern direction, the Solontsov volcano-magmatic structure. Submeridional and northeastern faults control the valley of the River Solontsovaya (Fig. 2). These faults probably experienced postmagmatic reactivation, which led to the formation of a decompaction zone observed along the river valley (Chudaev 2003). In hydrogeological terms, the area is located in the volcanogenic province of the Sikhote-Alin hydrogeological massif (Markov 1988).
Fresh groundwaters, widely distributed within the area are in hydraulic continuity with mineral waters (Fig. 2). The aquifer of alluvial Upper Quaternary sediments is present along the river valley. Water-bearing rocks have a thickness of 7–10 m and are represented by sands, gravels, pebbles, and boulders. Upper Cretaceous volcanic rock aquifers are common in this area. Water bearing rocks have 350 m thickness and comprise ignimbrites and tuffs of acid composition penetrated by intrusions of granites and dykes of andesite, andesite–basalt, rhyolite, and rhyodacite (Chudaeva et al. 1999).
The Solontsovaya volcano-magmatic structure refers to tectonic disturbances of the northwestern strike and is manifested on the surface by a central-type dome with two major ring faults. The inner ring of faults has a diameter of 3.5 km, and the outer ring about 10 km. The most fragmented and significant is the peripheral fault system. Submeridional and northeastern faults with lengths of about 15 km control the location of the valley of the River Solontsovaya.
The maximum width of the fracturing zone is about 300 m and is noted in the central part of the valley near the deposit of CO2-rich water. To the north and south, the fracturing zone decreases to a few meters (Chudaev 2003). A major tectonic fault crossing the Solontsovaya volcano-tectonic structure serves as a channel for the entry of carbon dioxide into the weakened stretching zones. The active geological processes in this area were presumably completed with the extinction of volcanic activity (Upper Cretaceous), which produces a 2–4 km of eruptive sediments and magma intrusions (Fig. 2).
The upper fissured zone of water-bearing rocks (in the river valley) contains the leached and decomposed products of volcanic glass and feldspars. As a result, the permeability of rocks in this part of the section is reduced, which leads to the formation of upward pressure of groundwater.
The maximum productivity (5.0–6.2 L/s) comes from wells with a depth of more than 50 m, which revealed fracturing zones associated with faults. The interval of depths from 15 to 30 m is generally represented by a relatively impermeable layer of rocks, with an absence of discharge of mineral waters into alluvial deposits across most of the area. Hydrodynamic features of the territory where the high-pCO2 groundwater is widely spread are associated with extended fracturing zones. Wells in the faults have an abnormally high production rate with high permeability.
Results and discussion
Chemical composition of fresh and mineral waters
The surface waters of the studied area are represented by the River Solontsovaya, with mountain stream characteristics: it dries up in the summer, freezes in the winter, and strongly depends on the amount of precipitation in the region (Table 1). Fresh groundwater is widespread in Quaternary alluvial deposits and eruptive rocks of the Primorsky series of Upper Cretaceous. We analyzed fresh groundwater from three wells, as well as published data (Chudaev 2003). The studies showed that fresh groundwater is cold (the temperature in the estuary is 7.5 °C), circum neutral (from 6.50 to 7.30), and contains a mixed cation composition of water–calcium and sodium preferably; TDS does not exceed 129 mg/L (Table 1, Fig. 1). Mg and K are a relatively minor species in all studied samples. The comparison of our data on fresh groundwaters with those already published shows good agreement; however, the physico-chemical parameters of water in one of the wells were slightly different (high TDS up to 2302 mg/L, high HCO3 content up to 1546 mg/L, up to 421 mg/L calcium, and up to 87 mg/L sodium. The high concentrations of cations and bicarbonate ion are most likely caused by the fact that mineral water is discharged into the well and mixed with fresh water. Minor enrichment of fresh groundwater with silica (up to 23 mg/L as H2SiO3), as well as strontium and barium, in comparison with surface waters, was observed.
Investigation of the chemical composition of the mineral waters showed that they are cold (the water temperature is + 7.0 to 7.5 °C), slightly acidic (pH 6.0–6.6), with TDS up to 2700 mg/L and are characterized by significant heterogeneity in their chemical–physical features. Within the aquifer, four sections of the CO2-rich mineral waters were outlined (Fig. 2), based on differences in the chemical composition of the waters (Table 1, Fig. 3). The cation composition of bicarbonate water is calcium, magnesium–calcium and sodium–calcium with high iron content (Fe2 + concentration reaches 40 mg/L) and silica (the content of H2SiO3 reaches 130 mg/L). The degree of saturation with carbon dioxide of the mineral waters is heterogeneous and varies in individual sections and wells (Table 2); the highest value of CO2 was recorded in the wells of the Yurganov area (the well #8 in Table 1). The TDS of waters also varies greatly in areas: the maximum TDS up to 2700 mg/L is in the water of the well #66 (the Southern section), and the minimum is less than 1400 mg/L in the wells #1 and #37b (the Northern section).
The chemical composition of mineral waters is constant and does not vary seasonally or on the amount of atmospheric precipitation. It is, therefore, likely that the mineral waters have a reasonably long residence time. Analysis of the data shows that the increase in the proportion of bicarbonate, sodium and calcium (and also TDS) occurs in the direction from surface water to fresh groundwater to mineral water, reaching the highest concentrations in the mineral waters of the Central and Southern sections (Fig. 4). All investigated mineral waters (Table 1) are characterized by elevated lithium concentrations (up to 1.75 mg/L), strontium (up to 3.16 mg/L) and manganese (up to 9.2 mg/L). In the Southern section, elevated concentrations of iron (up to 130 mg/L) and manganese (up to 5.13 mg/L) were noted.
As indicated in Table 1, there are clear contrasts in the chemical composition of groundwater from the well #8 obtained in the previous study (Chudaev 2003) and our data. This may reflect a difference in the sampling procedure or the well production period. Perhaps, well #8 was out of pumping during the 2003 year, and the presence of some amount of stagnant water in the well gave the different chemical composition.
In volcanic aquifer such as Gornovodoe area, the sources of solute components in groundwaters are problematic to interpret because the mineralogical and chemical properties of the bedrock are highly heterogeneous. Moreover, heat flux, stress regime, infiltration depth, along with residence time in the reservoir, are likely to be different and specific to each well (spring).
To establish correlations between the chemical elements in mineral waters, a statistical analysis was carried out both on the whole area and on individual sections. Analysis of the results showed that there are no significant correlations between the components, if we calculate them in the whole area. Correlation dependence is observed only between the contents of the bicarbonate ion and calcium, bicarbonate and silica, as well as iron and magnesium. However, the separation of mineral waters along the sampling sites shown in Fig. 4 revealed another picture; in the mineral waters of the Northern section, there is a distinct direct dependence of the contents of calcium and sodium on the content of bicarbonate ion and magnesium on dissolved CO2. In the Southern section, the only correlation between TDS and HCO3 content was traced; no other dependencies have been identified.
At the Gornovodnoe deposit, the total REE concentration is quite high—6.58–9.85 μg/L—similar to that found by Shand et al. (2004). The water of the deposit is enriched with heavy REE (HREE) in comparison with light (LREE); the sum of heavy REE is approximately 82%. Distribution profiles smoothed out with stable enrichment towards HREE; the ratio La*/Yb*, which indicates enrichment LREE in comparison to HREE, is stable for the entire observation period and is 0.01 (Fig. 5). There is a slight fluctuation in the concentrations of REE, depending on the year of sampling, but the distribution profile of REE does not change (Kharitonova et al. 2016). A comparison of the REE content in filtered and unfiltered water samples showed enrichment of unfiltered REE samples. This allowed us to conclude that a sufficient amount of REE is carried in the suspension on particles larger than 0.45 microns. Our previous study indicated that total REYsus in high-pCO2 groundwater is less than 10% although in the other geochemical types of groundwater REYsus form is prevalent (Chelnokov et al. 2014; Chudaev et al. 2015; Kharitonova et al. 2017).
Modeling of the REE solution complexity was carried out for the actual composition of groundwater under atmospheric pressure and the measured temperature. The main parameters on which the model was based were the pH and Eh, as well as the chemical composition of water. If the physico-chemical parameters of the model solutions did not coincide with the actual measurements, the model was calibrated to the correspondence of the calculated and measured values of pH and Eh. Accordingly, the forms of finding REE with variable valence (2, 4) were regulated in the system by their oxidation–reduction parameters.
The results of calculations show that the main form of REE migration in all types of water in the region is a complex with the carbonate ion REE [CO3]+, but their quantity (%) varies from place to place. The complex enrichment with carbonate ion is clearly expressed moving from LREE towards HREE. The second most common form is REE3+ (up to 10%). The amount of this form decreases in the direction towards HREE. The third most common complex is the sulfate complex (REE-[SO4]+) in the case of lanthanum and gadolinium, and the fluorine complex (REE-[F]2+) in the case of ytterbium. The remaining complexes are in a subordinate amount, and their total values do not exceed 1%.
Isotope composition of waters
To solve the problem of the origin of high-pCO2 mineral and fresh groundwaters, stable isotopes of oxygen and hydrogen were used. The results of the measurement are given in Table 1 and Fig. 6.
The relationship between deuterium and oxygen-18 in the natural water samples collected along Sikhote-Alin mountain systems. 1—meteoric water (Chudaev et al. 2005); 2—surface water of Primorye region (Chelnokov et al. 2013), 3–9 CO2-bearing groundwaters: 3—Gornovodnoe; 4—Lastochka (Kharitonova et al. 2007); 5—Shmakovka; 6—Nizhnie Luzhki; 7—Mukhen (HCO3-Ca–Mg); 8—Nerobinskiy; 9—Fadeevskoe (data for 5–6 taken from Chelnokov et al. 2013; Kharitonova et al. 2019)
The hydrogen and oxygen stable isotopes of all analyzed water samples are parallel with the global meteoric water line, indicating that the recharge source of the fresh groundwater and CO2-rich mineral water of the Gornovodnoe aquifer is mainly meteoric precipitation. Obtained data are very similar to the value of δ18O and δD in the other springs of Sikhote-Alin region (Chelnokov et al. 2013; Bragin et al. 2016; Chelnokov et al. 2015; Kharitonova et al. 2019). Furthermore, the range of hydrogen and oxygen isotope of the fresh (δD ranges from −74.5‰ to −77.0‰, and δ18O ranges from −11.1‰ to − 12.5‰) and high-pCO2 (δD ranges from −79.1‰ to −84.0‰, and δ18O ranges from −11.5‰ to −12.3‰) groundwaters are covered by the range of surface water samples (Fig. 6), suggesting that CO2-bearing groundwater is recharged by local precipitation. Our calculation indicates that for this area, each increase of 100 m in elevation will reflect 2‰ and 0.2‰ reduction in the value of δD and δ18O, respectively. According to the topography of Gornovodnoe area, the main recharge area should be at the mountain peak (elevation of 1574 m a.s.l.) located about 70 km away from studied wells.
In addition, we assume that the winter precipitation prevails in the groundwater recharge within the Gornovodnoe area so δ18O and δD in the meteoric water attain maximum values in summer (δD ranges from −24.5‰ to −44.0‰, and δ18O ranges from −3.3‰ to − 6.5‰).
The absence of a CO2 oxygen shift in CO2-rich groundwaters is probably due to the insignificant amount of CO2 gas in these waters and the short period of interaction between gas and water. It should also be noted that the similarity of the isotope composition of two types of waters confirms the hydrogeological data.
To determine the residence time of groundwater, we used the tritium method, which is widely used to describe the water cycle. The data indicate that the surface waters of the deposit contain tritium within the regional background which does not exceed 10 TU (Chelnokov et al. 2013), while the groundwater and CO2-rich groundwaters have very low isotope concentrations (0.4–0.8 TU), i.e., the waters have residence times greater than 60 years. No correlation was found linking 3H concentration with either the altitude of the sampling sites or with the TDS of groundwater.
Gas phase
The mineral waters of the aquifer contain high concentrations of dissolved carbon dioxide (from 1 to 2.1 g/L), and the gas factor sometimes exceeds 1. The calculated partial pressure of carbon dioxide varies from 0.3 to 1.8 bar in different sections. The chemical analysis of the spontaneously evolved gas showed that its main component is carbon dioxide, and its content varies from site to site ranging from 94.75 to 99.51% (Table 3). The remaining gases have low concentrations: nitrogen up to 5.25%, oxygen and methane—less than 0.05%. The content of noble gases in associated free gases in CO2-bearing groundwaters located within Sikhote-Alin ridge is presented in Table 3. Our data indicate that He concentrations in free gas samples and in corresponding dissolved gas samples are principally in good agreement. The ratio of isotopes 38Ar/36Ar, 40Ar/36Ar, 20Ne/22Ne, 21Ne/22Ne is very close to the air values that indicate a predominantly atmospheric genesis of the components. The minor (accessory) gases, such as N2 and O2, have an atmospheric character, and their concentrations vary due to technogenic factors (e.g., spa exploitation). The chemical composition of the associated free gas phase from the CO2-rich groundwater of Gornovodnoe area is very close to that of gases from other CO2-bearing spas (Table 3).
Unfortunately, there is no quantitative data on the volumes of carbon dioxide supplied to underground waters, and they can be judged only qualitatively. An indirect indicator of the volume of carbon dioxide can be estimated from the area distribution of mineral waters (see Table 2). The maximum area of groundwater saturated with carbon dioxide was recorded in the Southern section of the field.
To constrain the sources of the CO2 free phase into groundwaters, analyses of the helium and carbon isotopes were conducted. Also we used prior studies of other mineral springs located within Sikote-Alin ridge (Table 3). The obtained values of carbon isotopes (− 5.19‰) indicate the closeness of the values of δ13C between − 8‰ and − 4‰ mantle carbon (Deines 1970) and glasses of oceanic basalts (− 9‰ to − 4‰) (Marty and Jambon 1987). Geological studies of the territory testify in favor of mantle source of carbon dioxide because numerous faults of the northwestern strike have been found, on which carbon dioxide emissions are often recorded. Using only isotope data on carbon, it is impossible to accurately establish the nature of carbon dioxide, because of CO2 mixture formed during the metamorphism of carbonate rocks and strata rich in organic matter, which can provide δ13C similar to δ13C values of mid-oceanic basalts. However, there are no carbonate rocks within the Gornovodnoe area.
Helium isotopes are often used as tracers to determine the sources of free and dissolved gas into high-pCO2 springs (Lavrushin 2012) because it is inert for chemical reaction. In the mantle gas, the 3He/4He ratios are commonly higher (up to 10−5), although the crust gas has low value (at about 10−8). All of the CO2-rich groundwaters from Sikhote-Alin ridge show high 3He/4He ratios (up to 7.2 × 10−6), which are above the atmospheric ratio (Table 4). These values are an intermediate position between two types of geological settings: stable platform and a tectonically active volcanic arc.
Ratios of R/Ra for cold high pCO2 spring of the Sikhote-Alin ridge range from 2.3 to 5.1 and can generally be considered as a mixture of mantle-derived He and radiogenic He. Using the equation proposed by Pinneker et al. 1995, we roughly estimated the mantle source of He :
where A, M, and S, respectively, represent 3He/4He ratio of the air, mantle and samples.
Our calculation indicates that more than 46% of the CO2-rich spring helium comes from mantle sources, which suggests that CO2-rich fluids infiltrating the Gornovodnoe system may also be partially derived from the mantle.
Moreover, we calculated the proportions of helium sources (atmosphere, mantle and crust) based on the equations proposed by Sano and Wakita (1985):
where A, M, and C represent atmospherics, mantle and crust He, respectively. The (3He/4He)S and (4He/20Ne)S are the isotope ratios of samples.
Taking values (3He/4He)A = 1.4 × 10−6, (3He/4He)M = 1.1 × 10−5, (3He/4He)C = 1.5 × 10−8/(4He/20Ne)A = 0.318, (4He/20Ne)M = 1000, and (4He/20Ne)C = 1000, respectively, we can estimate the amount of helium sources in the sample.
Our results presented in Table 4 indicate that more than 50% of the CO2-rich groundwater helium in Gornovodnoe area comes from mantle sources, about 40% helium is crustal and less than 2% helium has an atmospheric origin.
Some scientists (Sano and Marty 1995; Umeda et al. 2006) proposed using the ratio of 13C/12C and CO2/3He to determine the genesis of CO2. According to previous data (Marty and Jambon 1987; Tolstikhin et al. 1996; Karaka et al. 1999), a C/3He value in the mantle ranges from 107 to 1010. Our data show the variations in the CO2/3He ratios in the various CO2-bearing groundwaters located within the Sikhote-Alin region. These values that ranged from 4.7 (× 107) to 13 (× 107) prove the preferable mantle source of CO2-free gas.
The diagram in Fig. 7 illustrates the ratio of isotopes δ13C and CO2/3He at some CO2-bearing groundwaters of Sikhote-Alin mountain system. The associated free gas of all the studied cold CO2-bearing groundwaters of the Far East Russia falls into the same area as mid-oceanic basalts. The dissolved gas has a small difference in the ratio of CO2/3He and falls into the gray area where CO2 from the hot springs of Japan are located. Thus, it can be clearly stated that the CO2 of the Gornovodnoe groundwater, as well as of the other Sikhote-Alin groundwaters have mantle origin (Chudaev et al. 2001).
Correlation between CO2/3He and δ13C in the gas phase of the CO2-rich mineral waters of Sikhote-Alin. 1—Volcanic gases of thermal springs of Japan (Sano and Wakita 1985; Umeda et al. 2006); 2—volcanic gases of mid-ocean basalts (Sano and Wakita 1985); 3, 4—gases of cold mineral springs at Russian Far East (Chudaev et al. 2001): 3—associated free gas; 4—dissolved gas
The evidence for mantle helium in CO2-rich groundwaters from Gornovodnoe area suggests that the fault system of Sikhote-Alin ridge is, in some manner, linked to the mantle. It is possible that fluid derived from the mantle wedge (particularly CO2) penetrated the groundwaters, such that the original volatiles become contaminated with atmospheric and crustal components during the migration process. The geological setting of the area suggests that numerous regional faults can act as channels for the upward movement of deep-seated gas, while the local faults present across the region provide the possible pathways for CO2 movement to the surface.
Chemical and mineral composition of the host rocks
The mineral and chemical composition of the water-bearing rocks of the Gornovodnoe area was studied in three sections: the Northern, Central and Southern. In the Northern section, rock samples were obtained from the well #10, in the Central section from the well # 1 and in the Southern section from the well # 8. All these wells were in the aquifer with CO2-rich mineral waters. Rock samples from wells with fresh ground water were investigated outside of the Northern Section (the well #20). The upper fissured zone in the river valley is comprised of leached and decomposed products of volcanic glass and feldspar. The composition of aquifer rocks in the area does not display significant differences. Minor changes in the rocks composition do not distort the general geochemical background in the area. In general, the rocks are represented by lithocrystalloclastic tuffs of rhyolite with crystals of sulfide minerals detected as pyrite (5–7%). The main mass of tuff is ash recrystallized into an illite–quartz aggregate. Tuffs have the following mineralogical composition including fragments of rocks: 45%, plagioclase; 20%; quartz 10%; potassium feldspar 8%; rhyolite 8%.
Accessory minerals are mainly represented by zircon, xenotime, apatite, monazite, leucoxene and rutile, and pyrite grains are also encountered (Fig. 8). Secondary minerals include: sericite 10–15%; quartz 20–25%; illite 8%; and iron hydroxides 3%. The dimensions of fragments vary from 0.2 to 2.8 mm, with an average of 0.5–1.0 mm. In some places, the aquifer rocks are carbonated; the carbonate forms round, irregular grains (0.1–0.8 mm) along the binding mass. X-ray microprobe analysis of aquifer rocks showed that they are mainly composed of potassium feldspar (orthoclase), while sodium-containing plagioclases are present in insignificant amounts. Samples of rocks of the upper fractured zone often contain iron hydroxides and clay minerals.
In chemical composition, SiO2 predominates (58–64 wt%), with other major elements being: Al2O3 16–18 wt%; CaO2 4 wt%; Na2O 1.0–2.9 wt%, and MgO 1.1–2.3 wt% (Table 5). The rocks contain a significant amount of iron, from 3 to 5% by weight. In general, the aquifer rocks are enriched in Li, Mn, Fe, Zn and Pb, but the concentration of trace elements with depth varies considerably. Thus, the concentrations of Li, Ca, K, and Zn decrease with depth, while Al, Si, Na, Mg, and Fe increase.
The aquifer rocks characteristics were obtained by pumping (Chelnokov and Chelnokov 2003). Effective filtration parameters of a productive aquifer zone at different sites of the area have similar values (Table 2), and the water conductivity coefficient is in the range of 24–39 m2/day. The obtained data indicate that the filtration permeability of the water-bearing rocks of the Gornovodnoe area depends mainly on the tectonic fragmentation of the rocks and the degree of their weathering.
The contents of REE in sulfidized tuffs of rhyolites from the Gornovodnoe aquifer are low and fluctuate in the range of 115–134 ppm (Table 6). There is considerable enrichment of LREE (89–92%) in comparison with HREEs. The comparison of REE contents in fresh and altered aquifer rocks shows that in the hypergenesis zone of the aquifer units there is a substantial depletion of REE, while the ratio between heavy and light REE remains practically unchanged and remains constant in both fresh and altered water-bearing rocks. These data show that during migration or accumulation of REE in the waters of the hypergenesis zone, there are no significant changes in the overall balance of ratios between light and heavy REE. The high concentrations of LREE in the water-bearing rocks of the deposit are caused, most likely, by the presence of a large number of minerals concentrating the LREE (feldspar, biotite, monazite and clay minerals), and a much less number of HREE, which are typically enriched in the minerals zircon, pyroxene, amphibole).
Origin of CO2-rich groundwater in the Gornovodnoe area
To understand how the chemical composition of the CO2-rich mineral waters of the Gornovodnoe area is formed, it is necessary to assess the equilibrium of waters with minerals of water-bearing rocks. Based on a large body of data on the chemical composition (more than 300 analyzes), modeling of their equilibrium with minerals at the outlet temperature has been carried out. The results of the simulation (Table 7) indicate that the CO2-rich waters are in equilibrium with respect to calcite, or even slightly supersaturated (the saturation index (SI) of the mineral lies within − 0.5 to 0.5), supersaturated with respect to quartz (SI 0.7–1.35), clay minerals kaolinite (SI 1.25–5.38), and montmorillonite (SI − 0.60 to 4.10), but rather undersaturated with respect to the main rock-forming minerals: plagioclase (SIanorthite (− 7.5 to − 7.1), SIalbite (− 3.08 to − 0.48)), and potassium feldspar (SIFsp − 1.68 to − 0.74).
The fresh water of the studied area is even more unsatisfied with the primary rock-forming silicate, as well as clay minerals. Analysis of the obtained data shows that the concentrations of the principal chemical elements in the waters are insufficient to reach equilibrium with the primary aluminosilicates. The formation of the chemical composition of the waters in the areas occurs due to the interaction of meteoric water with aluminosilicates of the aquifer rocks. The source of both calcium and sodium is mainly feldspar, as well as silica, which subsequently precipitates as quartz or chalcedony.
The flow of carbon dioxide through the fractures from the depths changes the pH of fresh waters to a more acidic level (pH 6.2) and, as a result, this increases the capability of leaching elements from rocks. Since the amount of carbon dioxide in mineral waters is on the verge of its solubility the excess gas is released as a free gas phase.
The regime of water flow during pumping of the wells of the Gornovodnoe area is determined not only by the filtration characteristics of the water-supply system but also by the degree of openness of the discharge system. This factor controls the time of interaction of water with rock and carbon dioxide, which is determined by the degree of openness of the geological structure.
In general, the groundwater has a sufficiently long residence (more than 60 years) for significant interactions to occur. The central part of the area is mostly open, and the upper fractured zone is weaker than in other areas (see Table 2). Besides, studies show that the CO2-rich mineral waters of each section within Gornovodnoe area do not have a hydrodynamic connection with each other and are formed separately in local areas within local flow systems when water-bearing rocks (tuffs) interact with meteoric waters and fluid (preferably CO2).
Thus, in the conditions of the Gornovodnoe area, the permeability of the aquifer units depends mainly on tectonic influences and is also determined by the rate of weathering in the upper part of the section. These data reflect the ability of the water to pass through the host rocks and can indirectly cause a change in TDS and chemical composition.
The calculation of water migrations coefficient made it possible to evaluate the mobility of chemical elements relative to each other, and also to track how it varies with depth. Calculations showed (Table 8) that up to a depth of 75 m the mobility of most elements remains constant (exceptions being sodium and lead). The most mobile element is Sr and least mobile Al. In general, the mobility of the elements is Al > Pb > Si > K > Cu > Zn > Cr ≫ Mg > Na > Ca > Ni > Be > Li > Sr. Thus, groundwater dissolves primary non-equilibrium mineral phases and keep the most mobile elements in aquatic form before the solution reaches the saturation with respect to secondary mineral phases (mainly Al-, Si-, K-, Mg-bearing and others). After that, these elements move away from the solution and form new phases.
Even though the mineral waters of the Gornovodnoe area are oversaturated with calcite, careful study of the water-bearing rocks has not revealed an abundance of cracks filled with carbonate. Moreover, even in the places where mineral waters discharge, travertines are not formed. A distinctive feature of the area where mineral waters discharge is the formation of colloids of iron hydroxides, which is natural, since the waters are strongly supersaturated with respect to iron hydroxides (the saturation index of goethite lies in the range 5.5–5.0). Clay minerals (kaolinite and montmorillonite) release potassium and magnesium from the water. The consequence of the secondary mineral formation, which controls water’s composition, is the following: Fe hydroxides → illite → montmorillonite → calcite. The leaching elements from rocks increase their porosity (the lower part of wells) and clayeness (the upper part of section).
Conclusions
The chemical composition of CO2-rich mineral waters in studied area is the result of interaction processes in the water–rock-carbon dioxide system. The initial waters are of meteoric origin, and their chemical composition depends on the composition of the water-bearing rocks. The transition of elements into water is caused by interphase interactions in the water–gas–rock system. Helium, neon and carbon isotope systematics prove the predominantly mantle origin for associated free gas in CO2-bearing groundwaters. Our results indicate that more than 50% of helium in the CO2-rich groundwater of Gornovodnoe area comes from mantle sources, at about 40% helium is crustal and less than 2% helium has atmospheric origin. Geological setting of the territory confirms the mantle source of carbon dioxide, because numerous faults of the northwestern strike are present, on which CO2 emissions are often observed. We can conclude that dissolution of primary silicate and aluminosilicate phases plays a key control on chemical composition of high-pCO2 groundwaters. In the formation of the bicarbonate sodium–calcium type of water, HCO3− component is formed under the influence of an excess amount of CO2 of deep origin dissolved in rather shallow ground water; the dominant calcium is due to leaching of Ca-containing plagioclases (anorthite) with sodium derived from the leaching of albite. One of the important distinctive features of this water is high contents of silicon and iron. The total REE concentration in the CO2-rich groundwater is rather high. Distribution profiles smoothed out with stable enrichment towards HREE. The results of calculations show that the main form of REE migration in all types of water in the region is a complex with the carbonate ion REE [CO3]+, but the proportions of different complexes vary from place to place. As whole, the most important factor determining the difference in the composition of mineral waters of the Gornovodnoe CO2-rich water is residence time, i.e., the degree of openness of the water–rock–gas system.
The cold CO2-rich groundwater of the Eastern Sikhote-Alin ridge is the result of interactions between fresh groundwater of meteoric origin, mantle gases and the host volcanic rocks. It thus highlights connectivity between deep and shallow fluids along deep fractures related to ancient terrane boundaries.
References
Bragin IV, Chelnokov GA, Chudaev OV, Kharitonova NA, Vysotsky SV (2016) Geochemistry of thermal waters of continental margin of Far East of Russia. Acta Geologica Sinica (English edition) 90(1):276–284. https://doi.org/10.1111/1755-6724.12657
Chelnokov AN, Chelnokov GA (2003) Deposits of carbonic mineral waters of Primorye. Hydrogeology and geochemistry of the waters of the folded regions of Siberia and the Far East. Vladivostok. Dal’nauka, pp 60–69 (in Russian)
Chelnokov GA, Kharitonova NA (2008) Carbonic mineral waters of the south of the Far East of Russia. Vladivostok. Dal’nauka. 165 (in Russian)
Chelnokov G, Kharitionova N, Bragin I, Vasil’eva M (2013) Deuteruim, oxygen-18 and tritium in precipitation, surface and groundwater in the Far East of Russia. Proc Earth Planet Sci 7:151–154. https://doi.org/10.1016/j.proeps.2013.03.209
Chelnokov GA, Kalitina EG, Bragin IV, Kharitonova NA (2014) Hydrochemistry and genesis of thermal waters of the Goryachii Klyuch spring in Primorskii Krai (Far East of Russia). Russ J Pac Geol 8(6):475–488. https://doi.org/10.1134/S1819714014060037
Chelnokov G, Kharitonova N, Bragin I, Chudaev O (2015) Geochemistry of mineral water and gases of the Razdolnoe Spa (Primorye, Far East of Russia). Appl Geochem 59:147–154. https://doi.org/10.1016/j.apgeochem.2015.05.001
Chelnokov George A, Bragin Ivan V, Kharitonova Natalia A (2018) Geochemistry of mineral waters and associated gases of the Sakhalin Island (Far East of Russia). J Hydrol 559:942–953. https://doi.org/10.1016/j.jhydrol.2018.02.049
Chudaev OV (2003) Composition and conditions of formation of modern hydrothermal systems of the Russian Far East. Dal’nauka. Vladivostok. p 216 (in Russian)
Chudaev OV, Chudaeva VA, Sugimori K (2001) New geochemical data of the high PCO2 waters of Primorye (Far East Russia). In: Proceedings of the 10th international symposium on WRI-10, pp 473–477
Chudaev OV, Chelnokov GA, Bragin IV, Kharitonova NA, Blokhin MG, Aleksandrov IA (2015) REE fractionation in the rivers of Eastern and Soutern Sikhote-Alin with natural and anthropogenic anomalies. Russ J Pac Geol 9(6):428–438. https://doi.org/10.1134/S1819714015060020
Chudaeva VA, Chudaev OV, Chelnokov AN, Edmunds UM, Shand P (1999) Mineral waters of Primorye. Dal’nauka. Vladivostok. p 163 (in Russian)
Deines P (1970) The carbon and oxygen isotopic composition of carbonates from the Oka Carbonatite Complex, Quebec, Canada. Geochim Cosmochim Acta 34(11):1199–1225
Dubinina EO, Kovalenker VA, Avdeenko AS, Lavrushin VYu, Stepanets MI (2005) Origin of mineral springs of the Elbrus region, northern Caucasus: isotopic-geochemical evidence. Geochem Int 43(10):988–998
Edmunds WM, Shand P, Chudaeva VA, Lutsenko TN, Chudaev OV, Chelnokov AN (1996) The mineral waters of Primorye, Russia. Final Report. British Geological Survey. Technical Report WD/96/42R
Karaka YK, James JT, Evans WC, Kennedy BM (1999). Geochemistry and hydromechanical interaction of fluid associated with the San Andrease Fault system, California. In: Heneberg WC (ed) Faults and subsurface fluid flow in the shallow crust. Geophysical Monograph 113:129–148
Kharitonova NA, Chelnokov GA, Karabtsov AA, Kiselev VI (2007) Geochemistry of Na-HCO3 groundwater and sedimentary bedrocks from the central part of the Sikhote-Alin mountain region (Far East of Russia). Appl Geochem 22(8):1764–1776. https://doi.org/10.1016/j.apgeochem.2007.03.033
Kharitonova NA, Vakh EA, Chelnokov GA, Chudaev OV, Aleksandrov IA, Bragin IV (2016) REE geochemistry in groundwater of the Sikhote-Alin fold region (Russian Far East). Russ J Pac Geol 10(2):141–154. https://doi.org/10.1134/S1819714016020032
Kharitonova NA, Shvartsev SL, Lepokurova OE, Chelnokov GA (2017) Unique CO2–saturated mineral waters of the Mukhen Deposit (Khabarovsk Krai): composition and Genesis. Dokl Earth Sci 475(2):953–957. https://doi.org/10.1134/S1028334X17080220
Kharitonova N, Chelnokov G, Bragin I, Nakamura H, Iwamori H, Morikawa N, Korzun A (2019) The geochemistry of water and gas phases from high pCO2 sparkling springs within the northern Sikhote-Alin ridge region (Russian Far East). E3S Web Conf. 98:01025. https://doi.org/10.1051/e3sconf/20199801025
Kopylova YuG, Lepokurova OE, Tokarenko OG (2009) Formation conditions of the chemistry of Tersinskie carbonic-acid mineral water. Water Resour 36(5):577–585
Kopylova YG, Lepokurova OE, Tokarenko OG, Shvartsev SL (2011) Chemical composition and genesis of the carbonic-acid mineral waters of the Tersinskoe deposit (Kuzbass). Dokl Earth Sci 436(2):284–289
Krainov SR, Ryzhenko BN, Shvets VM (2004) Geochemistry of groundwater. Theoretical, applied and environmental aspects. Nauka, Moscow. 677 (in Russian)
Lavrushin VYu (2012) Subsurface fluids of the Greater Caucasus and its surrounding. Geos, Moscow, p 384
Markov VS (1988) Underground waters of the Far East Russia. Vladivostok, p 40 (in Russian)
Marty B, Jambon A (1987) C/3He in volatile fluxes from solid earth: implications for carbon geodynamics. Earth Planet Sci Lett 83:16–26
Mityusheva TP, Simakova YS, Lavrushin VY (2007) Mineral formation in the springs of the mineral waters of the European north-east. In: Bullen TN, Wang YS (eds) Water-Rock Interaction 12 (WRI-12). Taylor & Francis Croup, London, pp 393–396
Perel’man AI (1982) Geochemistry of nature water. Moscow, Science, p 154 (in Russian)
Pinneker EV, Pissarskiy BI, Pavlova SE (1995) Helium isotope data for ground water in the Baikal rift zones, Isotopes Environ. Health Stud 31:97–106
Sano Y, Marty B (1995) Origin of carbon in Fumarolic Gas from Island Arc. Chem Geol 119(1–4):265–274
Sano Y, Wakita H (1985) Geographical distribution of 3He/4He ratios in Japan: implications for arc tectonics and incipient magmatism. J. Geophys. Res. Solid Earth 90(B10):8729–8742. https://doi.org/10.1029/JB090iB10p08729
Shand P, Edmunds WM, Chudaeva VA, Lutsenko TN, Chudaev OV, Chelnokov AN (1995) High PCO2 springs of the Primorye region, eastern Russia. In: Kharaka YK, Chudaev OV (eds) Water-Rock Interaction 8. Balkema, Rotterdam, pp. 393–396
Shand P, Johannesson KJ, Chudaev OV, Chudaeva VA, Edmunds WM (2004) Rare earth element contents of high pCO2 groundwaters of Primorye, Russia: Mineral stability and complexation controls. In: Johannesson K (ed) Rare earth elements in groundwater systems. Springer, Berlin, pp 161–187
Shvartsev SL, Kharitonova NA, Lepokurova OE, Chelnokov GA (2017) Genesis and evolution of high-pCO2 groundwaters of the Mukhen spa (Russian Far East). Russ Geol Geophys 58(1):37–46. https://doi.org/10.1016/j.rgg.2016.12.002
Tchepkaia NA, Chelnokov GA, Karabtsov AA, Tarasenko IA (2006) Hydrochemical characteristics of Lastochka Spa (Primorye, Far East of Russia). J Geochem Explor 88(1–3):148–152. https://doi.org/10.1016/j.gexplo.2005.08.028
Tolstikhin IN, Lehmann BE, Loosli HH, Gautschi A (1996) Helium and argon isotopes in rocks, minerals, and related groundwaters: a case study in northern Switzerland. Geochim Cosmochim Acta 60:1497–1514
Umeda K, Ogawa Y, Asamori K, Oikawa T (2006) Aqueous fluids derived from a subducting slab: observed high 3He Emanation and conductive anomaly in a non-volcanic region, Kii Peninsula, Southwest Japan. J Volcanol Geothermal Res 149(1–2):47–61. https://doi.org/10.1016/j.jvolgeores.2005.06.005
User’s guide AQUACHEM (2005) A computer program for speciation, reaction-path, advective transport, and inverse geochemical calculation. Waterloo
Zamana IV (2015) Hydromineral resources of Zabaikalskiy krai. Resort base and natural health properties of Tuva and country region. 2(1–1):135–137
Acknowledgements
Research presented in this article was supported jointly by projects: INTAS No. A2-075 led by Dr. W. Mike Edmunds (British Geological Survey), Russian Foundation for Basic Research (RFBR) and the Japan Society for the Promotion of Science (JSPS) according to the research Project No. 19-55-50002, and the Russian Science Foundation (No. 18-17-00245). Also, the authors gratefully thank the editor, James W. LaMoreaux, and the anonymous reviewers for reviewing and making critical comments on this manuscript. We would like to thank Dr.Tatiana Velivetskaya, Dr.Vladimir Goriachev and Mrs. Natalia Zarubina for their help on chemical and isotopic analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
The paper is devoted to Professor W.M. Edmunds (Oxford University, UK) who made significant contributions to the study of the geochemistry of ground waters of the Far East of Russia.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a part of the Topical Collection in Environmental Earth Sciences on “Mineral and Thermal Waters” guest edited by Drs. Adam Porowski, Nina Rman and Istvan Forizs, with James LaMoreaux as the Editor-in-Chief.
Rights and permissions
About this article
Cite this article
Kharitonova, N.A., Chelnokov, G.A., Bragin, I.V. et al. Major and trace element geochemistry of CO2-rich groundwater in the volcanic aquifer system of the Eastern Sikhote-Alin (Russia). Environ Earth Sci 79, 55 (2020). https://doi.org/10.1007/s12665-019-8697-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8697-y