Abstract
Humans are daily exposed to the natural radioactivity present in rocks, soils, and water. The distribution of these elements in the surface is not uniform, being influenced by the variation of the physical, geological, and meteorological parameters. The concentration activities of natural radionuclides 238U, 226Ra, 228Ra, and 222Rn were determined in the groundwater supplying the Salutaris Mineral Waters Park, in Paraíba do Sul, in the state of Rio de Janeiro. The concentrations of 238U varied from 0.95 to 2.70 μg L−1 with a mean concentration of 1.96 μg L−1, 226Ra ranged from 1.50 to 12.6 mBq L−1 with an average of 5.03 mBq L−1, 228Ra presented levels between 1.80 and 2.80 mBq L−1, with an average of 2.40 mBq L−1, and 222Rn, with levels of 5.90–1.94 × 104 mBq L−1 with an average concentration of 7.50 Bq L−1. The contribution of the consumption of these radionuclides dissolved in the water distributed in the Park to the effective annual dose ranged from 0.03 to 0.10 mSv year−1, with an average of 0.08 mSv year−1. The results showed that all effective annual dose values per ingestion of these mineral waters were below the individual dose limit of 0.10 mSv year−1 recommended by the World Health Organization (WHO).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drinking water may contain natural radionuclides, such as uranium and thorium decay series, their decay products, as radium and radon, potassium (40K) and artificial radionuclides (Altikulaç et al. 2015) in different concentrations. Radon, especially the 222Rn isotope, is the most abundant in mineral waters and is the main responsible for its radioactivity, with 226Ra being the second largest contributor (WHO - World Health Organization 2004; Feitosa et al. 2008). This work, however, will be restricted in the analysis of the radionuclides belonging to the 238U and 232Th series.
Studies of groundwater radioactivity levels have shown that the presence of these elements in Brazilian waters is quite variable. Radium isotopes, 226Ra and 228Ra, can be found in concentration ranges from 0.001 to 3.79 Bq L−1 and 0.002–3.80 Bq L−1, respectively, uranium was detected in concentrations between 0.002 and 930.0 μg L−1 and the 222Rn isotope between 0.40 and 3542 Bq L−1. These differences are related to the local geology, the physicochemical characteristics of the waters and the individual properties of the radionuclides (Lauria and Godoy 2000; De Oliveira et al. 2001; Godoy and Godoy 2006; Corrêa et al. 2015; Yuce et al. 2009; Bonotto 2017; Wakasugi and Damato 2017; Yuce et al. 2017; Godoy et al. 2019; Pfanz et al. 2019).
Uranium and thorium are considered lithophile elements and mainly make up the acidic igneous rocks, such as granite (Bonotto 2004; Santos 2010). Sedimentary rocks generally contain low levels of radioactivity; however, sandstones, phosphate rocks, and carbonates are important exceptions since they may have high concentrations of natural radionuclides. The sandstones, for example, have about 1.4 ppm of uranium content (Galbeman 1977; Bonotto and Da Silveira 2006; Santos 2010).
The 226Ra, half-life (T1/2) of 1600 years, and the 228Ra, T1/2 = 5.7 years, can easily dissolve in water and be transported into the aquifer, its most common sources in drinking water are from the radioactive decay of 238U and 232Th found naturally in the earth’s crust (Landstetter and Katzlberger 2009).
Radium isotopes present high radiotoxicity and human health hazard in case of ingestion, due to its similarity to calcium, an element commonly fixed in bones (Sánchez et al. 1999). Radium ingested through food and water is incorporated into the human body, leading to a significant increase in effective dose due to internal radiation. The presence of this element in the human skeleton can reach concentrations 25 times higher than the daily intake (Camargo and Mazzilli 1998).
The three major radon isotopes are 219Rn, 220Rn, and 222Rn, all of them are an alpha particle emitting noble gas, with a half-life of 3.9 s, 54.5 s and 3.8 days, respectively, the last one being the most abundant on the environment. They are originated from 223Ra, 224Ra, and 226Ra decay, respectively. Due to its low reactivity and non-participation in chemical processes such as precipitation and complexation in a liquid medium, radon can be freely transported from the rocks to the water and migrate at great distances (Lauria et al. 2014).
Intake of radon dissolved in water can also represent a direct health risk due to the irradiation of sensitive cells in the gastrointestinal tract and other organs by the alpha radiation emitted in its decay (Crawford-Brown 1990; da Silva 2000; Yuce and Gasparon 2013). Thus, radon can potentially lead to the development of neoplasms or other diseases, being the 222Rn isotope of greatest significance due to its higher half-life and abundance (ICRP 1993; Hopke et al. 2000; Groves-Kirkby et al. 2016).
Recently, studies on the potability of Paraíba do Sul mineral waters were performed by Cruz (2016), Alves et al. (2017), De Oliveira (2017) and Pereira et al. (2018). However, the concentration levels of 238U, 228Ra, 226Ra, and 222Rn and the radiological impacts of water consumption were not reported in the literature previously. Therefore, the present study aims to determine the natural radioactivity levels from those radionuclides in the mineral waters of Salutaris Park and to evaluate the radiation doses to which the population is exposed.
Study area
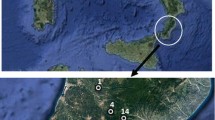
This study was developed in the Salutaris Mineral Waters Park, one of the main attractions in Paraíba do Sul, a municipality located in the Center-South region of the state of Rio de Janeiro, and the first state hydromineral resort, as shown in Fig. 1, with a population estimated at 41.084 people (IBGE - Instituto Brasileiro de Geografia e Estatítica 2018). In 1887, it was verified the presence of ferruginous, magnesian and alkaline waters in the locality, and in 1898, the bottling and commercialization of these waters had begun. Currently, however, such activity is discontinued, and the park has stimulated the city’s tourist activity (De Souza 2014; SECEC 2019).
The park has three mineral water sources in operation: Nilo Peçanha, Maria Rita and Alexandre Abraão fonts that distribute water collected from their respective tubular wells (identified in this work as P1, P2, and P3, respectively), illustrated in Fig. 2. These waters are available to the population free of charge through a fountain located inside the park. Table 1 presents the description and geographical coordinates of the sampled points.
From the geological point of view, the city is located in the Ribeira Belt, more specifically, in the Klippe Paraíba do Sul domain, characterized by the presence of gneiss, granite, and metasediments (Heilbron et al. 2000; Tupinambá 2007). The Salutaris Park is located on rocks of the Juiz de Fora Complex, interspersed with Paleoproterozoic basement units of the Quirino Complex and the supracrustal sequence of the Paraíba do Sul Group, consisting of hornblende–orthogneisses, orthogranulites, biotite–garnet gneisses, calcitic and dolomitic marbles (CPRM 2012; Gomes et al. 2013; Corval et al. 2014).
The Nilo Peçanha well (P1) captures waters that circulate through the orthogranulite, which in turn is composed of quartz, feldspar, muscovite and pyroxene. The well Maria Rita (P2) and Alexandre Abraão (P3) are close to the diabase dike, which has minerals such as pyroxene, biotite, amphibole, and apatite. All the lithotypes cited have in common an abundance of the mineral pyroxene, which according to Pertlik et al. (1974) and Bonotto and Da Silveira (2006) may contain considerable uranium content that can vary from 0.1 to 50 ppm. Other accessory minerals that usually present uranium are biotite (8.1 ppm) and apatite (10–100 ppm) identified in the diabase dike (Pertlik et al. 1974; Bonotto and Da Silveira 2006).
Methodology
Two sampling campaigns were carried out in the three wells at Salutaris Park, in Paraíba do Sul, in July/2017 and March/2018, representing dry (average monthly rainfall of 30 mm) and wet season (average monthly rainfall of 150 mm), respectively (INMET 2018).
The 238U isotope was determined through an inductively coupled plasma mass spectrometer (ICP-MS) from Thermo Scientific®, XSeries 2, and using SPS-SW1 and SLRS-6 as standard-certified reference materials. The samples were pre-filtered on 0.45-μm and 47-mm diameter cellulose ester membranes and acidified with concentrated nitric acid (HNO3). This method was based on Landstetter and Katzlberger (2009). The results obtained for the certified reference materials are within the same range, indicating the accuracy of the ICP-MS procedure without the need to use isotope dilution.
The radium long half-life isotopes, 226Ra and 228Ra, were determined by gamma spectrometry with the aid of a Canberra® high-purity germanium (HPGe) radiation detector. Samples were prepared based on the technique proposed by Dulaiova and Burnett (2004), the 228Ra was determined analyzing 338 and 911 keV photopeaks and the 226Ra through 295, 351, and 609 keV peaks.
Thirty liters of water from each sample point was drained by a fiber impregnated with potassium permanganate solution (KMnO4) at a rate of 1.0 L min−1, in which the isotopes of interest were retained (Fig. 3a). Afterward, the fibers were washed with distilled water to remove the impurities, taken in the muffle and burned at 550 °C for 1 h, to obtain a finely divided powder. This was encapsulated and analyzed after 21 days to allow radioisotopes to attain secular equilibrium with their short-lived decay products. The background was determined from the distilled water analysis using the same procedures applied to the samples.
The 222Rn isotope was determined by a portable electronic solid-state alpha detector, RAD7, from Durridge®, using a Big Bottle method adaptation, as shown in Fig. 3b. 4.0 L of a sample from each sampling point was collected in a glass bottle, avoiding water bubbling to minimize the loss of dissolved radon. A total monitoring time of 90 min was used for each point. In this type of instrument, the alpha radiation emitted at decay from 222Rn to 218Po is detected and converted into an electrical signal by the equipment.
Finally, the effective annual dose to which the population will be exposed when consuming the mineral waters pumped through the wells located in Salutaris Park can be calculated from Eqs. 1 and 2, below, uranium concentration in µg L−1 was converted to uranium activity (in Bq L−1) using the conversion factor 1.24 × 104 Bq g−1:
where DIg = effective annual dose due to ingestion of water. C = radionuclides concentration (238U, 228Ra, 226Ra and 222Rn in Bq L−1). CA = average annual water consumption (estimated at 730 L year−1, WHO - World Health Organization 2004). FC = dose conversion factor (4.5 × 10−8, 2.8 × 10−7, 6.9 × 10−7 and 10−8 Sv. Bq−1 for 238U, 226Ra, 228Ra and 222Rn, respectively (WHO, 2017).
where DIh = effective annual dose by inhalation. CRn = radon concentration in Bq L−1. RRn = air water concentration ratio (10−4). FC = dose conversion factor (9.0 nSv Bq−1 h−1 m3) (UNSCEAR 2000). F = radon and its progeny equilibrium factor (0.4). H = average occupation time per individual (7000 h year−1).
Results and discussion
The hydrogeochemical characterization of the analyzed waters was performed by Cruz (2016) and Silva (2019), which characterized the waters sampled as sodium bicarbonate (P1 and P3), and sodium bicarbonate mixed (P2) in the dry period, and magnesium bicarbonate in the rainy season. As the aforementioned authors, the chemical classification of the sampled water types was performed using the Piper and Stiff diagrams, Figs. 4 and 5, respectively, which consider the largest ionic constituents as variables, being Na+, K+, Mg2+ and Ca2+ the cationic components and HCO3− + CO32−, SO42− and Cl− the anionic components, the water classifications of the wells analyzed in the dry and wet seasons remained the same as those determined by Cruz (2016) and Silva (2019).
In addition, physicochemical parameters (conductivity, pH, Eh, temperature, and total dissolved solids) were determined, in situ, in the water samples taken from Salutaris Park, Rio de Janeiro, Brazil. These parameters were taken seasonally at the three wells, and the results are presented in Table 2.
Based on the diagrams presented, there is a change in the chemical composition of water between the dry and rainy season. It is noted that in the wet season, there was an increase in the contribution of magnesium ion in the composition of these waters, so that the waters of the three wells were classified as magnesium bicarbonate.
In addition to the mentioned characteristics, the physicochemical parameters and the presence of certain ions directly affect the presence of free elements in these waters. Radius mobility or the effectiveness of its adsorption vary with pH (Szabo et al. 2005), salinity (Wood et al. 2004), and redox potential (Szabo and Zapecza 1987).
The weathering of geological materials may have influenced the types of waters found, as they are a reflection of the lithology on which the aquifer is located. This influence can also be seen through variation of Eh values between wet and dry seasons. In the dry season, negative Eh values were observed, indicating a reduction potential and in the wet season, positive Eh values, where oxidation might be dominant.
The hydrogeochemical modeling performed using the PHREEQC 3.3.12 software indicates that fluorite, barite, and apatite are sub saturated in the entire sample set, with average saturation indexes (SI) of – 2.4, – 0.3, and – 7.9 in the dry period, and – 1.8, – 0.2, and – 7.9 in the wet period, respectively, but with the caveat that the presence of apatite mineral was not detected in hydrogeochemical modeling in P1. On the other hand, mica and feldspar are saturated in the entire sample suite. Mica and feldspar SIs average are 8.7 and 0.8 in the dry period, and 8.8 and 1.0 in the wet period, respectively, with an exception in P1 on wet season where the feldspar mineral seems to remain in solution with the SI of – 0.7. Table 3 shows the SIs of the main minerals modeled in each well.
The WHO guidelines provide recommendations for managing the risk from hazards that may compromise the safety of drinking water, including some physicochemical parameters. According to WHO (2017), the optimum pH required will vary in different supplies according to the water composition and the nature of the construction materials used in the distribution system, but it is usually in the range of 6.5–8.5, the palatability of water with a TDS level of less than about 600 mg/L is generally considered to be good, and concentrations greater than 1000 mg/L make drinking water unpalatable. Furthermore, the temperature will impact on the presence of inorganic constituents and chemical contaminants on water, and hot waters enhance the growth of microorganisms. Ideally, drinking waters should be around 25 °C (EPA 2001; WHO 2011, 2017).
The values were taken into consideration as characteristic values to see the differences during sampled seasons. Although the seasonal differences observed and taking into account, the parameters analyzed the waters from Salutaris Park are according to the limits recommended for human consumption.
Activity concentrations of natural radionuclides
Table 4 shows the isotope concentration values in the mineral waters of Salutaris Park in the two sampled periods. Radon concentrations ranged from 5.9 × 10−3 to 3.2 Bq L−1 in the dry period and from 6.50 to 19.4 Bq L−1 in the wet period. It is believed that the lowering of the aquifer level in the dry season and the consequent emergence of gaps between the mineral grains, facilitated the gas escape, and contributed to the low concentrations found. However, further seasonal behavior investigation of those waters is needed to explain better the differences in concentrations obtained.
The recommended limits of dissolved radon in water vary from country to country, ranging, for example, from 11.0 Bq L−1, recommended by the United States Environmental Protection Agency (EPA), to 100.0 Bq L−1, established by the European Union (EPA 1999; Zabadi et al. 2015). In Brazil, there is still no specific legislation for this element, however, the Ministry of Health established as a standard of water radioactivity for human consumption the limit of gross alpha radioactivity, that is, the sum of radioactivity coming from all emitters alpha media, 0.1 Bq L−1 and for gross beta radioactivity of 1.0 Bq L−1 (BRASIL 2017). In this work, the alpha particle emitters are the isotopes of 238U, 226Ra and 222Rn and the beta emitter, 228Ra.
The radon concentration results obtained in this study showed that in the dry period, all the results were below the recommended by the international organizations. However, on a wet period, only P3 well, Alexandre Abraão, was within limits established by international legislation.
The highest activity values of 226Ra, during the dry season, were observed in Alexandre Abraão and Maria Rita wells, P3 and P2, respectively, in the wet season, Maria Rita, P2 and Nilo Peçanha, P1, wells showed the highest concentration. This isotope presented a variation of 1.5–12.6 mBq L−1 and 1.5–4.7 mBq L−1, in the dry and wet periods, respectively. The concentrations of 228Ra, on the other hand, showed greater uniformity between the points sampled, varying between 1.8 and 2.5 mBq L−1 in the dry season and 2.2 to 2.8 mBq L−1 in the wet season, and the highest concentrations were found in the Nilo Peçanha well, P1, both in the dry and wet periods. These activity values are under the limits suggested by the Ministry of Health of 0.1 Bq L−1 and 1.0 Bq L−1, respectively.
In relation to the dissolved 238U concentrations, a similarity is observed in the levels of this isotope in P2 in both periods sampled, in the rainy season, the well P3 stands out with concentrations of 2.70 μg L−1, but in the dry period, the wells P1 and P2 presented the highest levels of this uranium isotope, 2.67 and 2.04 μg L−1, respectively.
Based on the chemical toxicity of uranium, WHO and EPA established the maximum permissible concentration of this element in drinking water (EPA 1999; WHO 2017). As regards national legislation, there are two regulatory limits: 15 μg L−1 adopted by the National Environment Council (CONAMA) for water management purposes (CONAMA 396/2008) and 30 μg L−1 defined by Consolidation Regulation 5, of September 28, 2017, the provisions of this ordinance do not apply, however, to natural mineral water (BRASIL 2017). Currently, Brazil follows the WHO guidelines for drinking water quality (Lauria et al. 2014); therefore, all samples analyzed were in accordance with current legislation.
Dose due to 238U, 228Ra, 226Ra, and 222Rn concentration in water
As seen above, the main human exposure routes to natural radioactivity are by inhalation, in the case of radon, as it is emitted from the water into the atmosphere, and by ingestion, whether in the consumption of water or foods containing 238U, 228Ra, 226Ra, and 222Rn. The annual effective doses were calculated using Eqs. 1 and 2, for ingestion and inhalation, respectively, using the mean concentrations of the radioisotopes sampled between the dry and wet seasons, the results obtained are found in Table 5 (UNSCEAR 2000; Duggal et al. 2017). Figure 6 shows the average contribution of each isotope to ionizing radiation exposure of the population by the consumption of the Park’s mineral water.
Concerning the annual effective dose values, it is noted that the total dose from exposure to radionuclides dissolved in the waters of the Nilo Peçanha and Maria Rita wells, P1 and P2, is at the threshold recommended by the legislation of 0.1 mSv year−1, however, the waters of the Alexandre Abraão well, P3, presented effective dose below the recommended limit (ICRP 2007). Therefore, it is recommended that the waters extracted from these wells should be periodically analyzed to verify if their consumption can cause health problems to the population.
The guidelines proposed by the International Commission on Radiological Protection (ICRP) are based on situations of prolonged radiation exposure of the public, the proposed levels may be considered, according to the WHO, conservative and should not be interpreted as mandatory limits. Exceeding a level of guidance should be considered as a stimulus for additional investigations, but not necessarily as an indication that drinking water is not safe for consumption (WHO 2017).
Conclusion
The activity concentrations of 238U, 226Ra, 228Ra, and 222Rn in the mineral water samples collected from the three wells that supply the active sources of the Salutaris Mineral Waters Park were measured to verify compliance with national and international regulations. These results ranged from 0.95 to 2.70 μg L−1, 238U, with an average concentration of 1.96 μg L−1, 1.50–12.6 mBq L−1, 226Ra, with an average of 5.03 mBq L−1, 1.80–2.80 mBq L−1, 228Ra, mean of 2.40 mBq L−1 and 5.90–1.94 × 104 mBq L−1, 222Rn, with an average of 7.50 Bq L−1.
The mean activity concentrations of radionuclide measured in the study are lower than the levels recommended by the EPA, WHO, and the Brazilian Ministry of Health. However, occasional samplings in the rainy season indicated levels of dissolved radon above the limit levels. Results showed that the mean annual effective due ingestion of these mineral water samples, 0.08 mSv year−1, is lower than the recommended value of 0.10 mSv year−1, as established by the WHO.
Ingestion of water containing high radionuclide levels contributes to the increase of human exposure to ionizing radiation that may potentially cause several deleterious effects on health. Due to those results obtained, it would be necessary to investigate thoroughly the natural radioactivity levels present in these mineral waters, including other natural radioisotopes that may be present, to better evaluate their potability.
References
Altikulaç A, Turhan Ş, Gümüş H (2015) The natural and artificial radionuclides in drinking water samples and consequent population doses. J Radiat Res Appl Sci 8:578–582
Alves IFDC, Silva-Filho EV, Marques ED, Kütter VT, De Oliveira DN, Silva CR, Gomes OVO (2017) Riscos de ingestão de flúor, estudo de caso para água mineral do interior do estado do Rio de Janeiro. Ver Bras Cien Amb 46:60–74
Bonotto DM (2004) Radioatividade nas águas, da Inglaterra ao Guarani. UNESP, São Paulo
Bonotto DM (2017) The dissolved uranium concentration and 234U/238U activity ratio in groundwater from spas of southeastern Brazil. J Environ Radioact 166:142–151
Bonotto DM, Da Silveira EG (2006) Geoquímica do urânio aplicada a águas minerais. UNESP, São Paulo
Brasil (2017) Ministry of Health. Consolidation of the norms on actions and health services and the Unified Health System. Consolidation Ordinance No. 5, of September 28th of 2017
Camargo IMC, Mazzilli B (1998) Estimativa de risco devido à ingestão de isótopos de urânio em fontes de águas minerais. Rev Saude Publica 32:317–320
Corrêa JN, Paschuk SA, Kappke J, Denyak V, Schelin HR, Claro FD, Perna AFN, Reque M, Rocha Z, Santos TO (2015) Monitoramento da radioatividade alfa relacionada ao radônio-222 em águas de poços da região metropolitana de Curitiba (PR). Eng Sanit Ambient 20:243–250
Corval A, Miranda AWA, Tapajós T (2014) Modelos geodinâmicos para o segmento central da Faixa Ribeira e de reativação da porção meridional da Plataforma Sul-Americana no Cretáceo Inferior. [S.l., s.n.]
CPRM - Serviço Geológico do Brasil (2012) Geologia e recursos minerais da folha Três Rios SF.26-Z-B-I, estado do Rio de Janeiro escala 1,100.000. Belo Horizonte, CPRM
Crawford-Brown JD (1990) Analysis of the health risk from ingested radon. In: Cothern CR, Rebers PA (eds) Radon, radium and uranium in drinking water. Lewis Publishers, Chelsea, Michigan, pp 17–26
Cruz IFD (2016) Modelagem hidrogeoquímica das águas minerais do Parque Salutaris, Paraíba do Sul, RJ. Dissertation, Federal Fluminense University
Da Silva CM (2000) Urânio, radônio-222 e polônio-210 em águas de abastecimento público da região metropolitana do Recife. Dissertation, Federal University of Pernambuco
De Oliveira, DN (2017) Hidrogeoquímica e indicadores de qualidade das águas superficiais do município de Três Rios/RJ e seu entorno. Dissertation, Federal Fluminense University
De Oliveira J, Mazzilli BP, Sampa MHO, Bambalas E (2001) Natural radionuclides in drinking water supplies of São Paulo State, Brazil and consequent population doses. J Environ Radioact 53:99–109
De Souza DA (2014) Valor de uso, Estudo de caso sobre o Parque das Águas Minerais Salutaris, Paraíba do Sul. Monograph, Federal Rural University of Rio de Janeiro, RJ
Duggal V, Sharma S, Mehra R (2017) Radon levels in drinking water of Fatehabad district of Haryana, India. Appl Radiat Isot 123:36–40
Dulaiova H, Burnett WC (2004) An efficient method for γ- spectrometric determination of radium-226, 228 via manganese fibers. Limnol Oceanogr Methods 2:256–261
EPA - United States Environmental Protection Agency (1999) Radon in drinking water, Factsheet. EPA815-F-99-007
EPA - United States Environmental Protection Agency (2001) Parameters of water quality: interpretation and standards. EPA, Ireland
Feitosa FAC, João MF, Feitosa EC, Demetrio JG (2008) Hidrogeologia, Conceitos e Aplicações. CPRM, LABHID, Rio de Janeiro
Galbeman JW (1977) Migration of uranium and thorium- exploration significance. American Association of Petroleum Geologists, Tulsa
Godoy JM, Godoy ML (2006) Natural radioactivity in Brazilian groundwater. J Environ Radioact 85:71–83
Godoy JM, Ferreira PR, De Souza EM, Da Silva LI, Bittencourt ICS, Fraifeld F (2019) High uranium concentrations in the groundwater of the Rio de Janeiro State. J Br Chem Soc 30:224–233
Gomes OV de O, Cruz IFD, Marque ED, Tapajós T, Corval A, Valente S de C, Garcia JMP, Miranda AWA, Silva-Filho EV (2013) Caracterização hidrogeoquímica preliminar das águas do Parque Salutaris, Paraíba do Sul-RJ. In: Congress proceedings of XIV Congresso Brasileiro de Geoquímica, Diamantina
Groves-Kirkby CJ, Denman AR, Campbell J, Crockett RGM, Phillips PS, Roger S (2016) Is environmental radon gas associated with the incidence of neurodegenerative conditions? A retrospective study of multiple sclerosis in radon affected areas in England and Wales. J Environ Radioact 154:1–14
Heilbron M, Mohriak W, Valeriano CM, Milani E, Almeida JCH, Tupinambá M (2000) From collision to extension: the roots of the south-eastern continental margin of Brazil. In: Talwani M, Mohriak W (eds) Atlantic rifts and continental margins. [S.l.]: American Geophysical Union (Geophysical Monograph Series, 115)
Hopke PK, Borak TB, Doull J, Cleaver JE, Eckerman KF, Gundersen LCS, Harley NH, Hess CT, Kinner NH, Kopecky KJ, Mckone TE, Sextro RG, Simon SL (2000) Health risks due to radon in drinking water. Environ Sci Technol 34:921–926
IBGE - Instituto Brasileiro de Geografia e Estatítica (2018). Available in: https://cidades.ibge.gov.br. Accessed Nov 2018
ICRP - International Commission on Radiological Protection (1993) Protection against radon-222 at home and at work. ICRP, New York
ICRP - International Commission on Radiological Protection (2007) The 2007 Recommendations of the international commission on radiological protection. ICRP, New York. Available https://doi.org/10.1177/ANIB_37_2-4. Accessed Nov 2018
INMET - Instituto Nacional de Meteorologia (2018). Available in: https://www.inmet.gov.br/portal/index.php?r=estacoes/estacoesautomaticas. Accessed in: out 2018
Landstetter C, Katzlberger C (2009) Determination of 3H, 226Ra, 222Rn and 238U in Austrian ground- and drinking water. J Radioanal Nucl Chem 282:467–471
Lauria DC, Godoy JM (2000) Origem e transporte de rádio nas águas subterrâneas de Buena/RJ. In: 1st Joint World Congress on Groundwater. Congress proceedings of 1st Joint World Congress on Groundwater, Fortaleza
Lauria DC, Veiga LHS, Franklin MR (2014) Radioatividade em água potável, Ocorrência, Regulamentação e Aspectos Proteção Radiológica. IRD/CNEN, Rio de Janeiro
Pereira RM, Salomão MS, Pedroso EC (2018) Distribuição e controle das fontes de água mineral com elementos raros (Li, V) no Estado do Rio de Janeiro. Anu Inst Geocienc 41:164–178
Pertlik F, Roger JJN, Adams JAS (1974) Uranium. In: Wedepohl KH (ed) Handbook of geochemistry. Springer, Berlin
Pfanz H, Yüce G, Gulbay AH, Gokgoz A (2019) Deadly CO2 gases in the Plutonium of Hierapolis (Denizli, Turkey). Archaeol Anthropol Sci 11:1359–1371
Sánchez AM, Montero MPR, Escobar VG, Vargas MJ (1999) Radioactivity in bottled mineral waters. Appl Radiat Isot 50:1049–1055
Santos FPC (2010) Radiouclídeos naturais em águas minerais comercializadas na cidade de Recife- PE. Dissertation, Federal University of Pernambuco
SECEC - Secretaria de Cultura e Economia Criativa do Rio de Janeiro (2019). http://mapadecultura.rj.gov.br/manchete/parque-das-aguas-minerais-salutaris. Accessed July 2019
Silva CR (2019) Avaliação dos níveis de radioatividade natural em centros urbanos e suas implicações a saúde pública. Dissertation, Federal Fluminense University
Szabo Z, Zapecza OS (1987) Relation between natural radionuclide activities and chemical constituents in ground water in the Newark Basin, New Jersey. In: Graves B (Ed) Radon, radium, and other radioactivity in ground water. Lewis Publishers, Chelsea, pp 283–308
Szabo Z, De Paul VT, Kraemer TF, Parsa B (2005) Occurrence of radium-224, radium-226, and radium-228 in water of the unconfined Kirkwood–Cohansey aquifer system, southern New Jersey. U.S. Geological Survey Scientific Investigations Report 2004–5224. USGS, Reston, Virginia
Tupinambá M (2007) Geologia da Faixa Ribeira setentrional: Estado da arte e conexões com a Faixa Araçúai. Geonomos 15:67–79
UNSCEAR- United Nations Scientific Committee on the Effects of Atomic Radiations (2000) Sources, effects and risks of ionizing radiation. United Nations, New York
Wakasugi DSM, Damato SR (2017) Avaliação da concentração de 226Ra, 228Ra e 210Pb em águas minerais dos Parques das Águas de Lambari e Águas de Contendas- MG. In: XVI Congresso Brasileiro de Geoquímica. Congress proceedings of XVI Brazilian Congress of Geochemistry, Búzios
WHO - World Health Organization (2004) Guidelines for drinking-water quality. WHO, Geneva
WHO - World Health Organization (2011) Guidelines for Drinking- Water Quality, 4th edn. WHO, Geneva
WHO - World Health Organization (2017) Guidelines for Drinking- Water Quality, 4th edn. Incorporating the First Addendum, WHO, Geneva
Wood WW, Kraemer TF, Shapiro A (2004) Radon (222Rn) in groundwater of fractured rocks: a diffusion/ion exchange model. Ground Water 42:552–567
Yuce G, Gasparon M (2013) Preliminary risk assessment of radon in groundwater: a case study from Eskisehir, Turkey. Isot Environ Healt S 49:163–179
Yuce G, Ugurluoglu D, Dilaver AT, Eser T, Sayin M, Donmez M, Ozcelik S, Aydin F (2009) The effects of lithology on water pollution: natural radioactivity and trace elements in water resources of Eskisehir Region (Turkey). Water Air Soil Poll 202:69–89
Yuce G, Fu CC, D'Alessandro W, Gulbay AH, Lai CW, Bellomo S, Yang TF, Italiano F, Walia V (2017) Geochemical characteristics of soil radon and carbon dioxide within the Dead Sea Fault and Karasu Fault in the Amik Basin (Hatay), Turkey. Chem Geol 469:129–146
Zabadi H, Mallah K, Saffarini G (2015) Indoor exposure assessment of radon in the elementar schools, Palestine. Int J Radiat Res 13:221–228
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, C.R.e., Machado, D.V. & da Silva-Filho, E.V. Determination of the natural radioactivity in the mineral water distributed in the Salutaris Park, Paraíba do Sul, Brazil. Environ Earth Sci 78, 639 (2019). https://doi.org/10.1007/s12665-019-8661-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8661-x