Abstract
Problems associated with surface and groundwater pollution by aluminium and iron is becoming a serious environmental challenge facing the limited sources of quality drinking water. Al and Fe sesquioxides predominates in most of the lateritic soils within the tropical region. Their degree of dissolution and mobility into the surface and groundwater system is determined by the chemistry of the prevailing aqueous environment. This work assessed the potential effect of environmental pollution on the chemical composition of rain and its resultant runoff, hence its contribution to the degree of dissolution of Al and Fe oxides. This was achieved by first determining the sources of pollutants which could possibly affect the physicochemical composition of runoff using remotely sensed information and field observations. Thereafter rain and runoff water samples were collected from theses pre-determined sources, and were analysed for their physicochemical compositions. Similarly, soil samples were also collected from the field and analysed for their mineral and chemical compositions. An empirical method was then employed to determine the degree of dissolution of Fe and Al oxides in aqueous solutions of varying hydrogen ion concentration which was prepared using the combinations of sulphuric acid, nitric acid, ammonium hydroxide, and deionised water. The results revealed that the degree of dissolution of Fe and Al oxides in lateritic soils increases with increasing acidity and/or alkalinity of the aqueous solutions. Increase in the acidity and/or alkalinity of the prevailing rain and runoff was attributed to their high level of ammonium, sulphate, and nitrate content which was introduced into the environment through anthropogenic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface and groundwater contamination by heavy metals such as aluminium and iron is becoming a major environmental challenge posing danger to limited source of drinking water. Though aluminium and iron are not regarded as toxic substances in lower concentration in humans, however, increase in their concentration above the Environmental Protection Agency (EPA) limit of 0.2 mg/dm3 (Environmental Protection Agency 2001) could lead to diseases such as Alzheimer (Rondeau et al. 2000); Iron toxicosis (Osweiler et al. 1985), and memory loss (Hillman 2001). High concentration of aluminium has also been found to be toxic to plants and fishes causing stunted growth and death to these organisms, respectively (Herrmann 1987). Clogging of water distributing utilities such as pipelines has been reported to occur as a result of accumulation of excessive iron precipitate, leading to low functionality of this transport system (Environmental Protection Agency 2001). Colouration of fabrics and metallic tastes has also been associated with excessive iron concentration in water, leading to the reduction in its aesthetic and economic values for both domestic and industrial purposes (World Health Organization 1996).
Sources of these heavy metal (Fe and Al) pollutants are partly attributed to anthropogenic activities which include; exploration, production and use of hydrocarbon, mining, and other industrial activities (Wang et al. 2007; Norgate et al. 2007), and partly due to geogenic factors (Jia et al. 2018). However, researches have shown that Al and Fe are found in abundance in lateritic soils which are rich in sesquioxides of Fe and Al (Fitzpatrick and Schwertmann 1981; Goldberg 1989). This soil type underlay most of the regions in tropical hot humid climates such as most part of the south-eastern Nigeria. In this region, these soils are severely eroded by water (Igwe 2005; Emeh and Igwe 2017), and the resultant sediments which comprises of metallic oxides are been transported by runoff that discharges them directly into the nearby streams; thus contaminating the surface water. In areas where there is absence of surface water, percolation prevails, which may lead to contamination of the underlying unconfined shallow aquifers. The instability of the soil aggregates on exposure to rain and runoff are attributed to their lack of soil essential minerals such as calcium, magnesium and potassium which promotes the formation of water stable aggregates (Oti 2002; Igwe 2005). Change in pH of the aqueous environment and introduction of chemical compounds such as ammonium hydroxide, ammonium acetate, and humic acid into the environment via anthropogenic activities have also been associated with increase in the degree of dispersion and deflocculation of soil aggregates which are rich in Al and Fe sesquioxide, thus promoting their instability (Frenkel et al. 1992; Emeh and Igwe 2018).
Most of the dispersed soil particles are composed of iron and aluminium oxides and hydroxides which are dissolved by the prevailing aqueous environment during their transportation phase through surface runoff or percolation. However, the degree of solubility of Al and Fe has been widely reported to be dependent on the hydrogen ion concentration of the aqueous environment (Arias et al. 1995; May et al. 1979; Arlauckas et al. 2004). Variation in hydrogen ion concentration of the aqueous environment depends on the amount of environmental pollutant such as ammonium, and oxides of sulphur, nitrogen, and carbons introduced to the environment. These pollutants are mostly from industrial emissions due to the exploration and combustion of hydrocarbons, production and usage of agricultural fertilizers, and other industrial effluents (Hill 2010; Efe and Mogborukor 2012). Large concentration of these pollutants has also been identified in dumpsites and sewages (Kjeldsen et al. 2002; Aluko et al. 2003). In most developing countries, these industrial, agricultural and domestic wastes are disposed indiscriminately to the environment due to inadequate landfills, and lack of government policies on environmental pollution control. Consequently, these pollutants are reintroduced to the aqueous environment through reaction with water vapours which produces acidic precipitation (Tiwari et al. 2007). Ingress of this precipitation to open dumpsites, agricultural farms, and other non-point sources of pollutants can further affect the chemical composition of the resultant runoff (Hall and Anderson 1988; Horner et al. 1994), thereby modifying their hydrogen ion concentration.
However, little has been known about the effect of these chemically modified runoffs on the dissolution of Fe and Al oxides in the underlying lateritic soils which may consequently lead to their excessive concentration in surface and ground water systems. Since increase in population and urbanization is associated with increase in environmental pollution, especially in developing countries such as Nigeria (Hill 2010; Ubani and Onyejekwe 2013), it is imperative to assess its contribution to the chemical composition of runoffs; hence its effect on the degree of dissolution of iron and aluminium sesquioxides in lateritic soil. Therefore, the objectives of this research are: (1) to determine the percentage composition of Al and Fe sesquioxides in the underlying lateritic soils, (2) ascertain the effect of environmental pollution on the chemical composition of the prevailing rainwater and runoff, and then (3) evaluate the effect of variation in hydrogen ion concentration (pH) of the aqueous environment on the dissolution of iron and aluminium in sesquioxides rich lateritic soils.
Materials and methods
Description of study area
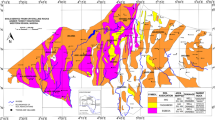
The study area lies within longitude 7°1′8.0″–7°27′44.1″E and latitude 6°52′7.7″–6°2′5.1″N in South-eastern part of Nigeria (Fig. 1). It is underlain by cretaceous–tertiary sediments within the Anambra Basin. The geologic settings, mineralogy and the sedimentological characteristics of these sediments have been described by many authors (Reyment 1965; Hoque and Ezepue 1977; Nwajide 1979; Arua and Rao 1987; Nwajide 1990; Ibe and Akaolisa 2010) and are summarized in Table 1. However, these rock Formations have so far undergone severe tropical weathering which results in the formation of dark-red coloured soils that are generally referred to as laterites. It was observed that erodibility of these lateritic soils by water erosion was high in areas with little or no concretions compared to areas with appreciable amount of concretions. This variation in water erosion resistant was explained by Okagbue and Ezechi (1988) and Emeh and Igwe (2017) to be as a result of their particle size distribution which mostly comprises of uniformly graded fine-silty sands in areas devoid of concretions. Conversely, the presence of concretions in the other areas improved their particle size gradation which subsequently increased their resistance to water erosion.
The study area is also characterized by numerous streams, especially in areas underlain by Ajali and Imo Formations. The groundwater level is shallow and unconfined with a depth of about 7 m in some areas directly underlain by Ajali Formation (Onwuka et al. 2004), while it is about 160 m in most areas underlain by Nsukka Formation (Offodile 2002). Tropical climate prevails within this area with temperature that reaches up to 31 °C during the late hot harmattan period (early March), and a minimum of about 16 °C during late December. Eze (2007) revealed that the total annual rainfall within this area is about 1581 mm which ranges from 16 mm during the peak dry period (late January–early February) to about 350 mm in the peak of the rainy season (mid July–early August). The vegetation within this area is mostly controlled by topography. The low laying areas are characterized by tropical rain forest while the areas with relatively high topography are mostly composed of shrubs and grasses. However, urbanization and industrialization has lead to the removal of most of the natural vegetation, thereby exposing the soil layers to erosive forces (Fig. 2). The population density within the study area is higher in the southern part with numerous small–medium-scale industries, open markets, and densely populated slums, while the northern part is characterized by relatively low population, few small-scale industries and agricultural activities which produces mostly arable crops. Most of the solid waste and effluents generated as the result of economic activities within this area is indiscriminately disposed to the environment (Fig. 3) due to lack of proper waste disposal systems such as landfills and incinerators.
Samples collection and analysis
To determine the water and soil sampling locations, a digital elevation model (DEM) was acquired from the United States geological survey (USGS) online archives. Topographic and drainage maps of the area were thus generated from the DEM with the aid of Surfer (version 11)—a modelling and analytical software. This helped in determining the areas with high frequency of gully erosion. Image analysis of the satellite imageries obtained from Google earth helped in determining the land use within the area. Integration of the drainage maps and land use map with the pre-existing geologic map of the study area assisted in delineating the soil and water sampling locations (Fig. 1).
Water sampling and analysis
To determine the effect of environmental pollution on the chemical composition of the prevailing rainwater and runoff within the study area, rain and runoff water samples were collected in different locations considering their economic activities. Ten rainwater samples were collected during the starting of rainy season (March–April) in different locations with varying economic activities (Fig. 1). Similarly, 20 water samples were collected from runoffs which are generated from urban dumpsites, agricultural farms, industrial areas, and low economic active area (Fig. 1). Water sample collection, handling and preservation prior to laboratory analysis was done following the methods outlined in standard methods for sampling of water and wastewater of the American Public Health Association (APHA 1998). Some physicochemical composition of the rain and runoff samples such as pH and electrical conductivity (EC) were determined in situ in the field by dipping a hand-held pH-meter and EC-meter, respectively, in about 100 ml of the water samples. The ammonium (NH4+), nitrate (NO3−), sulphate (SO42−), and carbonate (HCO3−) composition of these water samples, as well as their total dissolved solids (TDS) were determined following the methods outlined in standard methods for the examination of water and wastewater of the American Public Health Association (APHA 1998).
Soil sampling and analysis
Six soil samples were collected from Ajali Formation, while seven soil samples were collected in each of Nsukka and Ameki Formations (Fig. 1), in total 20 disturbed soil samples were collected. Soils from these geologic Formations were sampled because they are easily susceptible to water erosion (Emeh and Igwe 2017), while their sampling points were selected by considering the frequency of gully erosion occurrence and proximity to urban areas. Samples were collected 4 m away from the gully wall in a hand dug pit of about 1 m deep. This was to ensure that fresh representative samples of the eroding soil were collected. The samples were allowed to air dry for two weeks to attain complete dryness. A portion of the air dried samples were further subjected to sieve analysis to determine their particle size distribution (PSD) using the American Society for Testing and Materials (ASTM) standard sieve mesh numbers 5, 10, 18, 40, 60, 100 and 230 stacked on an electrically controlled sieve shaker which was allowed to vibrate for about 25–30 min. Particles passing sieve number 40 (particles less than 425 µm) were used to determine the soils liquid and plastic limit; hence its plasticity index following ASTM standard procedures.
The fine particle fractions (particles less than 63 µm) of six soil samples, two representative samples each from the three different geologic Formations, were further subjected to X-ray diffraction (XRD) and X-ray fluorescence (XRF) analysis to determine their mineral and chemical compositions, respectively. Prior to the XRD and XRF analysis, the fine particle fractions (< 63 µm) which were obtained through mechanical sieving of the soil samples was further pulverized using a vibrating cup miller which was set at an operating speed of 8 rpm. About 3 g of the pulverized soil samples passing through 45 µm sieve was smeared evenly on the sample holder made up of aluminium material with the aid of smooth slide. The bulk sample was analysed using Schimadzu (6000 model) x-ray diffractometer with a scanning range set between angles of 2o–60o 2θ. This was because experience has shown that most clay minerals are usually detected within this range (Brady and Boardman 1995). The running rate (scanning speed) was set at 6 degree per minute, the voltage was maintained at 40 V and at a current of 30 A, while the auto-silts (divergence, scatter and receiving) used for the various apertures control are of sizes 1.0°,1.0° and 0.3 mm. The XRD analysis was allowed to run for about 15 min.
Similarly, about 1 g of the pulverized soil sample passing through 45 µm sieve was thoroughly mixed with 4 g of lithium tetraborate (Borax) using Herzog vibrating cup miller at the speed of 8 rpm. The mixture was loaded into an aluminium cup of about 22 mm × 40 mm in size, and was pelletized using a pelletizing machine that was operated at a pressing force of 240 N and a movement stroke of 6 rpm. The pelletized sample was analysed using Thermo-scientific Advant’x (1200 model) x-ray spectrometer which was operated at a voltage of 45 V and a current of 40 A. The XRF analysis was allowed to run for about 20 min. The identification and percentage composition of the minerals and oxides were automatically done by the mineral cards of the software preinstalled in the individual machines following the methods outlined in Davis (1987).
Aluminium and iron oxides dissolution experiment
The aim of this experiment was to determine the effect of variation in hydrogen ion concentration of aqueous solutions on the dissolution of Fe and Al sesquioxides in soil samples using nitric acid (15.8 M), sulphuric acid (18.4 M), ammonium hydroxide (18.1 M), and deionised water as the reagents. The choice of the reagents was because similar compounds were found in appreciable amount in the prevailing rain and runoff water samples within the study area (Table 2).
Four acidic aqueous solutions of increasing concentration was prepared using sulphuric and nitric acid mixture, while four basic aqueous solutions of increasing concentration was prepared using ammonium hydroxide. The preparation of these solutions was achieved by diluting the acidic mixture or base with deionised water until the desired concentration is reached. The resultant acidic aqueous solutions of sulphuric and nitric acid mixtures had a concentration (M) of 0.0001, 0.001, 0.01, and 0.1 with a corresponding pH of 6, 4, 3, and 2, respectively. Subsequently, the basic aqueous solutions of ammonium hydroxide had a similar concentration with that of acid and corresponding pH of 7.5, 8, 9, and 10.5, respectively. 200 ml of these aqueous solutions were separated in different glass beakers which was labelled A1–A4 for the acidic aqueous solutions and B1–B4 for the basic aqueous solutions according to their increasing concentration.
About 30 g of fine particle portion (< 63 µm) of the soil sample was added into each of these prepared acidic and basic aqueous solutions. The mixture was thoroughly stirred with a glass rod and then was allowed to stand undisturbed for 96 h. After 96 h, about 3 cm3 of the resultant suspension was pipetted into a digestion flask, and 30 cm3 of aqua regia was added into it. The mixture was digested in a fume cupboard until a clear solution was obtained. It was further cooled, filtered, and then made up to 50 ml mark in a standard volumetric flask with deionised water. Standard solutions (mg/dm3) of 2, 4 and 6 were prepared from 1000 mg/dm3 of the above stock solution which was thereafter analysed using Shimadzu atomic adsorption spectrometer with ROM version 1.01. The calibration curve was plotted automatically for the metal of interest which was analysed using its respective wavelength, and thereafter its concentration was generated from the standard graph by the instrument. This experiment was carried out for each of the six representative soil samples.
Results and discussion
Physicochemical composition of the prevailing rainwater and runoff
The average pH value of 4.93 was recorded in the rainwater samples with a range of 3.4–6.6 (Table 2). These values indicate that the rain water samples are acidic. It was observed that the minimum value of pH (3.4) was recorded in the southern part of the study area which is more industrialized and urbanized with numerous small-scale industries and markets compared to the northern area where the maximum pH of 6.6 was recorded (Fig. 1). Unlike the rainwater samples, the runoff samples were generally basic with average pH value of 8.84 which ranges from 10.7 in runoff sample from an agricultural farm to 7.2 in runoff from residential area. Generally, it was observed from the average pH values that runoffs from agricultural area (pH 10.1) and those from urban dumpsites and drainage channels within industrial/commercial areas (pH 8.7), were more basic than runoffs from residential area (pH 7.9) which are characterized by low economic activities and relatively small quantity of indiscriminate solid waste disposal. The average value of total dissolved solutes (TDS) in runoff samples (369 mg/dm3) was very much higher than the average TDS value (69.51 mg/dm3) recorded in rainwater samples (Table 2). It was also observed that the TDS values of runoffs increases with increase in pH while that of rainwater increases with decrease in pH (Fig. 4). Thus, increase in the acidity or alkalinity of the aqueous solution resulted to increase in the total dissolved solutes. Therefore, from Fig. 4, it can be inferred that solute dissolution is dependent on the pH of the aqueous solution with R2 value of about 0.94 and 0.79 for rainwater and runoffs, respectively.
In the rainwater samples, the average concentration values (mg/dm3) of NO3−, SO42−, HCO3− and NH4+ were 18.30, 42.10, 2.68 and 2.24, respectively. The level of these ions were much higher in runoff samples with average concentration values (mg/dm3) of about 368, 312, 55 and 824 for NO3−, SO42−, HCO3− and NH4+, respectively (Table 2). It was observed that runoff generated from agricultural active areas and those from industrial/commercial areas, especially those sourced from urban dumpsites appears to have higher concentration of these chemicals compared with those from residential and low economic active areas (Table 2). The concentration of NH4+ was particularly higher in runoff samples from agricultural active areas with average value of about 1366 mg/dm3 compared with runoffs from other sources (Table 2). This relatively high concentration of NH4+ in runoff samples from agricultural active areas also resulted in high pH with average value of 10.1 and a corresponding average TDS value of about 563 mg/dm3. High concentration of NH4+ in the agricultural farm runoffs could be as a result of the organic and inorganic fertilizers used in arable farming which is the major type of crop production within the study area.
The acidic nature of the rainwater could be attributed to the relatively high concentration of NO3− and SO42− in the rainwater samples, while the basic nature of runoffs could be attributed to the high concentration of NH4+ in the runoff water samples (Table 2). This is because these acidic and basic radicals could react with water to form acidic and basic compounds such as sulphuric acid, nitric acid, and ammonium hydroxide, respectively (Burns et al. 2016; Buechel et al. 2000). High concentration of these compounds have been found in rain and runoff samples by several authors which they attributed to industrial emissions, and urban wastes and effluents into the environment as a result of anthropogenic activities (Hall and Anderson 1988; Sumner and McLaughlin 1996; Aluko et al. 2003; Tiwari et al. 2007). Aqueous solution of these compounds has also been found to cause dispersion and dissolution of soil aggregate and its constituent oxides, respectively (Park et al. 2001; Arlauckas et al. 2004; Abdalla et al. 2011, Emeh and Igwe 2018). Similarly, acidic precipitation has been related with excessive mobilization of Al from the underlying soils (Cronan and Schofield 1979).
Geochemical composition and geotechnical properties of the soil aggregates
From The XRD results, the diffraction pattern of most of the soil samples (Fig. 5a, b) revealed that the soil minerals were majorly composed of quartz, kaolinite, haematite, and montmorillonite contributing an average of 65, 20, 11, and 0.3%, respectively (Table 3). The chemical compositions of the fine particle fraction of the soils are summarized in Table 3. Apart from SiO2 which contributes about 51% of the total oxide in the soil’s fine fraction, Al2O3 was the next most abundant oxide, contributing an average of 36.30%, followed by Fe2O3 with an average of 7.15%. The percentages of all other oxides are insignificant with respect to their contribution in the fine particle fraction of the soil. Similar geochemical composition has been reported by Ibe and Akaolisa (2010) and Igwe et al. (2009) on the same study area, while Gidigasu (1972); Buhmann (1994); Bühmann et al. (2004); and Schellmann 2018 has reported the chemical composition of similar soils in other tropical and sub tropical region. These authors attributed the formation of sesquioxide rich soils to the intensive tropical weathering which causes rapid decomposition of the clay minerals thereby leaving behind oxides of aluminium, iron, and silicon. This soil type which is mainly composed of Al, Fe and Si oxides with relatively very little of the clay minerals are generally called oxisols or laterites (Schellmann 1986). The relative abundance of aluminium and iron oxides in the soils reflected the underlying geology. Percentage composition of aluminium oxides were higher in soils derived from Nsukka and Ameki Formations, compared with those derived from Ajali Formation. This variation could be as a result of the difference in the mineralogical composition and sedimentological attributes of the different parent rocks within the Anambra basin as revealed in the works of (Nwajide 1979; Arua and Rao 1987; Ibe and Akaolisa 2010).
The chemical variations of the lateritic soils also reflected on some of their physical and geotechnical properties such as colour, plasticity, and particle size distribution of the soil samples (Table 4). Soils derived from Nsukka Formation are relatively more reddish with moderate-high plasticity (PI = 24.3). Their PSD varies from well graded in the samples collected from the area with lateritic concretions with average coefficient of uniformity (cu) of 11.1, to uniformly graded soils in samples collected from the area that is devoid of concretions with average cu of 5.5 (Fig. 6). Soils derived from Ameki Formation have similar properties with those of Nsukka formation. However, their plasticity was slightly lower (PI = 18.6) and they lack concretions; hence are completely composed of uniformly graded oxisols with average cu of 3.4. Conversely, soils derived from Ajali Formation have a lighter red colour, which are composed of uniformly graded medium-fine grained sands (cu = 3.0) with small percentage of low plastic fines (PI = 3.6). Grading and plasticity of soil aggregates has a significant contribution on their degree of susceptibility to erosion. Soil aggregates which are well graded with fines of high plasticity are more resistant to water erosion compared to those that are uniformly graded with fines of low plasticity (Emeh and Igwe 2017). Slaking of lateritic soil aggregates has also been observed by Emeh and Igwe (2018) to be very much dependent on their plasticity, thus determines their degree of susceptibility to water erosion. Therefore, this could explain the reason why areas underlain by Ajali Formation were relatively more susceptible to gully erosion than those underlain by Nsukka Formation.
Another implication of the chemical composition of the fine particle fraction of the soil aggregates which are chiefly composed of Al and Fe sesquioxides is that they are easily slakable and readily dispersive when exposed to water (Oti 2002). This is because the prevailing soil aggregates are bonded by Fe and Al sesquioxides which Townsend and Reeds (1971); Cornell and Schwertmann (2003); and Igwe et al. (2009) have attributed to be the cement, binding the individual lateritic soil particles into bulk aggregates. High degree of susceptibility of these soil aggregates to slaking and dispersion were also attributed to their low exchangeable sodium percentage (ESP), reduced calcium–magnesium ratio due to their relatively high content of aluminium and iron, and relatively low content of soil Ca and Mg which helps in the formation of water stable soil aggregates (Mbagwu and Auerswald 1999; Oti 2002). Soil aggregate stability in water has been described as one of the major factors controlling soils susceptibility to water erosion (Egashlra et al. 1983; Igwe 2005); hence the high mobility of dispersed lateritic aggregates by runoff after a precipitation event into the nearby surface waters and shallow aquifers within the study area.
Effect of pH of the aqueous environment on dissolution of Fe and Al oxides in lateritic soils
The dissolution experiment revealed that the average concentration of dissolved iron from lateritic soils (Fe–Al sesquioxide rich soils) in acidic aqueous solution of 0.0001 M at pH of 6.0 was 0.07 mg/dm3. This concentration value of dissolved iron increased to 1.5 mg/dm3 when the molar concentration of acids in the aqueous solution was increased to 0.001 M at pH of 4. Further increment in the molar concentration of the acidic aqueous solution to 0.01 M (pH 3) and 0.1 M (pH 2) resulted to subsequent increase in the average concentration of dissolved iron with values of 6.1 mg/dm3 and 23.9 mg/dm3, respectively (Table 5). Similar trend was found in the average concentration value of dissolved Al (Fig. 7a). However, unlike Fe which was detected in acidic aqueous solution of 0.0001 M and 0.001 M, Al was not detected at these molar concentrations of the acidic aqueous solution. Only about 0.04 mg/dm3 of dissolved aluminium was detected in 0.01 M acidic aqueous solution, while 0.39 mg/dm3 was found in 0.1 M acidic aqueous solution (Table 5).
Comparing the degree of dissolution of Fe and Al from lateritic soils in the acidic aqueous solution, it was observed that the amount of dissolved iron was about 185 times more than that of aluminium in 0.0001 M acidic aqueous solution and about 69 times in 0.1 M of the same solution. Generally, the total dissolved Fe and Al in the acidic aqueous solution was approximately 32 mg/dm3, which Fe contributed about 99%. Thus, it could be inferred that aluminium oxide is sparingly soluble in aqueous solution of sulphuric and nitric acid mixture of about 0.0001 M. Nevertheless, its solubility gradually increases with increase in the molar concentration of the acidic mixture. Similar observation in the solubility of Al in acids was reported by Stumm and Morgan (1970) and May et al. (1979) where they posited that aluminium is a strongly hydrolyzing metal, and therefore, is relatively insoluble in the neutral pH range of 6.0–8.0.
It was further observed that the solubility of these oxides were higher in basic aqueous solution of ammonium hydroxide than in acidic aqueous solution. The amount of total dissolved iron increases from 0.34 mg/dm3 in 0.0001 M (pH 7.5) solution of ammonium hydroxide to 1525 mg/dm3 in 0.1 M (pH 10.5) of the same solution, while the total number of dissolved aluminium increases from 0.00 mg/dm3 in 0.0001 M of the basic solution to 3.04 mg/dm3 in 0.1 M of the same solution (Table 5). Like in acidic aqueous solution, the average amount of dissolved Fe in basic aqueous solution was very high compared to that of Al (Fig. 7b), Fe contributing about 99% of the total dissolved Fe and Al.
In general, the results revealed that aluminium and iron oxide in lateritic soils are more soluble in basic aqueous solution of ammonium hydroxide than in acidic aqueous solution of sulphuric and nitric acid mixture. Their degree of dissolution was observed to increase with increase in the molar concentrations of the basic or acidic aqueous solution (Figs. 7a, b). The reason for this observed variation in the solubility of Fe and Al oxide in acidic and basic aqueous solution may be due to the presence of CaO, MgO, and K2O from calcite and clay minerals in the lateritic soil samples (Table 3). These alkali and alkaline earth metallic oxides have high affinity for hydrogen ions; hence are readily soluble in aqueous acidic solutions (Averill and Eldredge 2011). Therefore, their presence in the soil–water interaction may neutralise the pH of the acidic aqueous solution, thus reducing the solubility of Fe and Al oxides.
These findings collaborated with results of Driscoll and Schecher (1990) who reported that the solubility of Al is enhanced under acidic (pH < 6.0) or alkaline (pH > 8.0) conditions, and/or in the presence of complexing ligands, therefore, making it more available for biogeochemical transformations. Though this research does not account for the presence of the complexing ligands, however, it is a common knowledge that these organic complexes are found in most dumpsites; hence could contribute to the degree of dissolution of aluminium oxides in soils.
Health and environmental implication
From the results so far, it have been revealed that most of the chemical compound that could reduce or increase the pH of the aqueous environment are found in rainwater and its resultant runoff. The influx of these chemicals in the environment through anthropogenic activities have been found to have significant effect on the degree of dissolution of Al and Fe oxides which predominates in the underlying soils within the study area. These dissolved cations are easily mobilized by runoff to the nearby surface waters, or through leaching into the shallow unconfined aquifers; hence may lead to excessive increase in the concentration of Al and Fe in the surrounding water bodies. Presence of Al and Fe in surface water and groundwater are normally attributed to the geochemical composition of the underlying sediments (Elueze et al. 2007; Akpan-Idiok et al. 2012; Jia et al. 2018). Meanwhile, relatively high concentration of these heavy metals in surface and groundwater samples from urban areas compared with those from economic low active areas on same geological Formation (Aniebone 2015; Okoye et al. 2016), have not been sufficiently explained. However, evidence from this laboratory experiment suggests that environmental pollution which affects the chemistry of the prevailing aqueous environment could account for the observed variation in the concentration of Fe and Al in surface and groundwater samples from urban and rural area. These water bodies serve as sources of drinking water and for other domestic and agricultural purposes for most of the people living within this area. Thus, it is imperative to consider the possible impact of environmental pollution on the concentration of Fe and Al while carrying out surface and groundwater geochemical analysis.
The concentration values of Al observed in 0.0001–0.01 M solution of both acidic and basic solutions (Table 5) are within the EPA permissible limit of 0.2 mg/dm3 for Al in drinking water (Environmental Protection Agency 2001). However, at concentration of 0.1 M of both the acidic and basic aqueous solutions, the amount of dissolved Al which is approximately 0.39 mg/dm3 and 3.0 mg/dm3, respectively, is above the EPA permissible limit. Similarly, the concentration of iron in all the solutions was higher than the EPA permissible limit of 200 µg/L of dissolved Fe in drinking water (Environmental Protection Agency 2001). These concentrations of iron were very much higher in 0.01 M and 0.1 M aqueous ammonium hydroxide solutions, with average concentration values of about 300 mg/dm3 and 1500 mg/dm3, respectively. Ingress of runoff with these concentrations of Fe into the surface water could possibly contaminate the water body, which may lead to iron toxicosis when consumed (Osweiler et al. 1985). Stunted plant growths have been reported in farms within the proximity of the study area (Enete et al. 2011); hence could be as a result of high concentration of dissolved aluminium in the underlying soils, since similar conditions have been linked with excessive uptake of Al in plants (Göransson and Eldhuset 1987).
Conclusion
From the field observations, laboratory tests, and experimental results, it can be concluded that the underlying soil within the study area is predominately composed of sesquioxide of iron and aluminium. The degree of dissolution of these oxides is dependent on the pH; hence the molar concentration of acids and bases in the prevailing aqueous environment. However, their solubility is more in basic aqueous solution of ammonium hydroxide than in acidic aqueous solution of sulphuric and nitric acid mixture. Iron appears to be relatively more soluble than aluminium in both acidic and basic aqueous solutions. Sulphates, nitrates, and ammonium which are the basic and acidic precursors in the aqueous environment and which increases the degree of dissolution of Fe and Al oxides are found in appreciable quantity in the prevailing rainwater and runoffs. Their concentration in these aqueous solutions is, however, dependent on the type of anthropogenic emissions and effluents introduced into the environment. Therefore, environmental pollution has the potential of increasing the degree of dissolution of Fe and Al from Fe–Al sesquioxide rich soils; hence their increased mobility into the surface and groundwater systems.
References
Abdalla MA, Jaafar MH, Al-Othman ZA, Alfadoul SM, Ali Khan M (2011) New route for preparation and characterization of magnetite nanoparticles. Arab J Chem 4:235–237
Akpan-Idiok AU, Ibrahim A, Udo IA (2012) Water quality assessment of Okpauku river for drinking and irrigation uses in Yala, cross rivers, state, Nigeria. Res J Environ Sci 6(6):210–221
Aluko OO, Sridhar MKC, Oluwande PA (2003) Characterization of leachates from a municipal solid waste landfill site in Ibadan. Niger J Environ Health Res 2(1):32–37
Aniebone VO (2015) Hydrogeochemistry and quality assessment of some ground water samples from Enugu and environs, South–Eastern Nigeria. Glob J Geol Sci 13:15–21
APHA (1998) Standard methods for examination of water and wastewater, 19th edn. American Public Health Association, Washington
Arias MM, Barral T, Dias-Fierros F (1995) Effects of iron and aluminium oxides on the colloidal and surface properties of kaolin. Clays Clay Miner 43(4):406–416
Arlauckas SM, Hurowitz JA, Tosca NJ, McLennan SM (2004) Iron oxide weathering in sulfuric acid: implications for mars. In: 35th lunar and planetary science conference, League City, Texas, abstract no.1868
Arua I, Rao VR (1987) New stratigraphic data on the Eocene Ameki formation, south-eastern Nigeria. J Afr Earth Sc 6(4):391–397
Averill B, Eldredge P (2011) General chemistry: principles, patterns, and applications, vol 1. Saylor Foundation, Arlington, p 854
Brady JB, Boardman SJ (1995) Introducing mineralogy students to x-ray diffraction through optical diffraction experiments using lasers. J Geol Educ 43(5):471–476
Buechel KH, Moretto H, Werner D (2000) Industrial inorganic chemistry. VCH, New York, pp 29–43
Buhmann C (1994) Parent material and pedogenic processes in South Africa. Clay Mins 29:239–246
Bühmann C, Escott BJ, Hughes JC (2004) Soil mineralogy research in South Africa, 1978–2002—a review. S Afr J Plant Soil 21(5):316–329
Burns DA, Aherne J, Gay DA, Lehmann CMB (2016) Acid rain and its environmental effect: recent scientific advances. Atmos Environ 146:1–4
Cornell RM, Schwertmann U (2003) The iron oxides, 2nd edn. Wiley-VCH, Weinheim, p 664
Cronan CS, Schofield CL (1979) Aluminum leaching response to acid precipitation: effects on high elevation watersheds in the Northeast. Science 204:304–306
Driscoll CT, Schecher WD (1990) The chemistry of aluminum in the environment. Environ Geochem Health 12(1&2):28–49
Efe SI, Mogborukor JOA (2012) Acid rain in Niger delta region: implication on water resources quality and crisis. Afrrev Stech 1(1):17–46
Egashlra K, Kaetsu Y, Takuma K (1983) Aggregate stability as an index of erodibility of Ando soils. Soil Sci Plant Nutr 29(4):473–481
Elueze AA, Nton ME, Adejumo SA (2007) Hyrogeochemical assessment of surface and groundwater quality in Agbowo–Orogun area of Ibadan, Southwestern Nigeria. Glob J Geol Sci 5(1&2):13–23
Emeh C, Igwe O (2017) Variations in soils derived from an erodible sandstone formation and factors controlling their susceptibility to erosion and landslide. J Geol Soc India 90(3):362–370
Emeh C, Igwe O (2018) Effect of environmental pollution on susceptibility of sesquioxide-rich soils to water erosion. Geol Ecol Landsc 2:115–126. https://doi.org/10.1080/24749508.2018.1452484
Enete AA, Madu II, Mojekwu JC, Onyeukwu AN, Onwubuya EA, Eze F (2011) Indigenous agricultural adaptation to climate change: study of Imo and Enugu state in Southeast Nigeria. Africa Technology Policy Studies Network Working Paper. Series No. 53
Environmental Protection Agency (2001) Parameters of water quality interpretation and standards, Environmental Protection Agency, Ireland. www.epa.ie. Accessed 11 Mar 2018
Eze HI (2007) Effect of rain fall intensity and energy on gully development in North eastern Enugu state, Nigeria. Nigerian J Technol 26(1):91–96
Fitzpatrick RW, Schwertmann U (1981) The distribution and nature of secondary magnetic minerals in sesquioxidic soils along the eastern seaboard of South Africa. In: Proceedings of 10th national congress soil science society of Southern Africa, East London. Technical communication No 180, Department of Agriculture, pp 167–168
Frenkel H, Levy GJ, Fey MV (1992) Clay dispersion and hydraulic conductivity of clay-sand mixtures as affected by the addition of various anions. Clays Clay Mins 40:515–521
Gidigasu MD (1972) Mode of formation and geotechnical characteristics of laterite materials of Ghana in relation to soil factors. Eng Geol 6:79–150
Goldberg S (1989) Interaction of aluminium and iron oxides and clay mineral and their effect on soil physical properties: a review. Commun Soil Sci Plant Anal 20(11–12):1181–1207
Göransson A, Eldhuset TD (1987) Effects of aluminium on growth and nutrient uptake of Betula pendula seedlings. Physiol Plantarum 69:193–199
Hall KJ, Anderson B (1988) The toxicity and chemical composition of urban stormwater runoff. Can J Civ Eng 15:98–106
Herrmann J (1987) Aluminium impact on freshwater invertebrates at low pH: a review. In: Landner L (ed) Speciation of metals in water, sediments and soil systems. Lecture notes in earth sciences, vol 11. Springer-Verlag, Berlin, pp 157–175
Hill KM (2010) Understanding environmental pollution, 3rd edn. Cambridge University Press, New York, p 562
Hillman RS (2001) Hematopoietic agents: growth factors, minerals, and vitamins. In: Hardman JG, Limbird LE, Gilman AG (eds) Goodman and Gilman’s, the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, pp 1487–1518
Hoque M, Ezepue MC (1977) Petrology and palaeo-geography of the ajali sandstone. Nig J Min Geol 14(1):16–22
Horner RR, Skupien JJ, Livingston EH, Shaver HE (1994) Fundamentals of urban runoff management: technical and institutional issues. Terrene Inst, Washington
Ibe KK, Akaolisa CCZ (2010) Sandclass classification scheme for Ajali sandstone units in Ohafia area, Southeastern Nigeria. J Geol Min Res 2(1):016–022
Igwe CA (2005) Erodibility in relation to water-dispersible clay for some soils of Eastern Nigeria. Land Degrad Dev 16:87–96
Igwe CA, Zarei M, Stahr K (2009) Mineralogy and geochemical properties of some upland soils from different sedimentary formations in South-eastern Nigeria. Aust J Soil Res 47:423–432
Jia Y, Xi B, Jiang Y et al (2018) Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: a review. Sci Total Environ 643:967
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32(4):297–336
May HM, Helmke PA, Jackson ML (1979) Gibbsite solubility and thermodynamic properties of hydroxyaluminum ions in aqueous solutions at 25 °C. Geochim Cosmochim Acta 43:861–868
Mbagwu JSC, Auerswald K (1999) Relationship of percolation stability of soil aggregates to land use, selected structural indices and stimulated rainfall erosion. Soil Till Res 50:197–206
Norgate TE, Jahanshahi S, Rankin WJ (2007) Assessing the environmental impact of metal production processes. J Clea Prod 15(8):838–848
Nwajide SC (1979) A lithostratigraphic analysis of the Nanka sands of southeastern Nigeria. J Min Geol 16(2):103–109
Nwajide SC (1990) Cretaceous sedimentation and paleogeography of the central benue trough. In: Ofoegbu CO (ed) The benue. Tough structure and Evolution International Monograph Series, Braunschweig, pp 19–38
Offodile ME (2002) Groundwater study and development in Nigeria, vol 453. Mecon geology and engr. Services ltd., Jos, p 223
Okagbue CO, Ezechi JI (1988) Geotechnical characteristics of soils susceptible to erosion in Eastern Nigeria. Bull Int Assoc Eng Geol 38:111–118
Okoye JI, Ene GI, Ojobor CC (2016) Physico-chemical and microbiological evaluation of borehole water samples in Enugu, South-Eastern, Nigeria. J Environ Sci Toxicol Food Technol 10(11):16–19
Onwuka OS, Uma KO, Ezeigbo HI (2004) Portability of shallow groundwater in Enugu, Southeast Nigeria. Global J Environ Sci 33(1):33–39
Osweiler GD, Carson TL, Buck WB, Van Gelder GA (1985) Clinical and diagnostic veterinary toxicology. Kendall/Hunt Publishing Company, Dubuque, Iowa
Oti NN (2002) Discriminant functions for classifying erosion degraded lands at Otamiri, Southeastern Nigeria. Agro Sci 3(1):34–40
Park JY, Seong GH, Baik HH (2001) Characterization of iron(III)oxide nanoparticles prepared by using ammonium acetate as precipitating agent. Korean J Chem Eng 18(2):215–219
Reyment R (1965) Aspects of the geology of Nigeria. University of Ibadan Press, Ibadan, p 144
Rondeau V, Commenge D, Jacqmin-Gadda H, Dartigues JF (2000) Relation between aluminum concentrations in drinking water and Alzheimer’s disease: an 8-year follow-up study. Am J Epidemiol 152(1):59–66
Schellmann W (1986) A new definition of laterite. In: Banerji PK (ed) Lateritisation processes. Geological survey of India memoir 120. Pub. by order of the Governor-General of India, 1859, Calcutta, pp 11–17
Schellmann W (2018) An introduction to laterite, products and processes of intensive rock weathering. http://www.laterite.de/. Accessed 13 Mar 2018
Stumm W, Morgan JJ (1970) Aquatic chemistry. Wiley-Interscience, New York
Tiwari S, Manoj KS, Bisht DS (2007) Chemical composition of rainwater in Panipat, an industrial city in Haryana. Indian J Radio Space Phys 37:443–449
Townsend FC, Reeds LW (1971) Effects of amorphous constituents on some mineralogical and chemical properties of a Panamanian latosol. Clays Clay Miner 19:303–310
Ubani EC, Onyejekwe IM (2013) Environmental impact analyses of gas flaring in the Niger delta region of Nigeria. Am J Sci Ind Res 4(2):246–252
Wang T, Mülle DB, Graedel TE (2007) Forging the anthropogenic iron cycle. Environ Sci Technol 41(14):5120–5129
World Health Organization (1996) Guidelines for drinking-water quality, Health criteria and other supporting information, vol 2, 2nd edn. World Health Organization, Geneva
Acknowledgements
Authors are grateful to Mr. Asadu, and Mrs. Chiamaka of the Department of Geology, and Mr. Ofomata of Energy research centre of the University of Nigeria, for providing laboratory assistant. They are also grateful to Mr. Chidi Okeugo for providing assistant during the field work and to Miss Anulika Okpalanozie for proofreading and improving the English quality of this work. They also appreciate the effort of petroleum technology development fund and Mrs. Chineyenwa Azubuike for providing financial assistant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emeh, C., Igwe, O. & Onwo, E.S. Potential effect of environmental pollution on the degree of dissolution of iron and aluminium oxides in lateritic soils. Environ Earth Sci 78, 256 (2019). https://doi.org/10.1007/s12665-019-8259-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8259-3