Abstract
Thirty-six sediment samples from twelve soil profiles have been collected from Karewa deposits of south Kashmir with an objective for evaluating weathering of the source area, palaeoclimate and environmental contamination. Chemical Index of Alteration (CIA), Plagioclase Index of Alteration (PIA) and Chemical Index of Weathering (CIW) values of the sediments averaged at 53.99, 68.6 and 72.53 indicating low to moderate degree of weathering. Higher values are observed in the samples collected at top surface. The samples taken at 15 cm depth and 60 cm depth showed almost similar but lesser values than top soil samples. A–CN–K and A–CNK–FM diagrams showed an almost similar contents for Al2O3, CaO, Na2O, K2O and MgO as those of upper crust, reflecting very poor to moderate weathering history. The ratio plots of SiO2 vs Al2O3 + K2O + Na2O and CIA (molar) vs Al2O3 indicate semi-arid climatic conditions with a mean annual precipitation of 846 mm per year and mean annual paleo-temperature of 10.91 °C. Metal concentration in sediment samples ranged from 331.1 to 571 ppm (Ba), 146 to 292 ppm (Zr), 82 to 587 ppm (Sr), 70 to 130 ppm (Cr), 58.9 to 120 ppm (Rb), 37.6 to 112 ppm (V) and 43 to 112 ppm (Zn). The enrichment ratio of 6 for As, 3 for Cr and 2 for Ni, Sb and Tb indicate the enrichment of these metals in the sediment samples. The QoC plot of As, Ni, Co and Cr illustrated an anthropogenic source and Ba, Rb, Sr, Nb, and Ta were mainly derived from geogenic sources with no evidence of anthropogenic contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geochemical investigations based on the chemical analysis of sediments are used as an effective tool with multiple applications (Grunsky et al. 2009). Such investigations were initially used for mineral exploration, but their application is now used for understanding the sedimentary processes including weathering of the source rock and palaeoclimate along with environmental issues (Bhat 2017; Ranasinghe et al. 2008; Grunsky et al. 2009).
Weathering is an important mechanism for elemental fractionation away from the parental bedrock (Nesbitt and Young 1982; McLennan 1989; McLennan et al. 1993). The extent of fractionation depends upon a number of factors including the nature of the bedrock (Gromet et al. 1984; Viers et al. 2000). Local weathering conditions are generally associated with warm and humid climate. However, a more arid climate is generally associated with relatively weak chemical weathering (Nesbitt and Young 1982; Fedo et al. 1995, 1997). Thus weathering processes transform bedrocks into soils and ultimately into sediments. However, the original geochemical signatures of the source rock may be retained (Bickle et al. 2005; Blum et al. 1998). The relationship between climate and degree of weathering has shown that low to moderate rainfall results in low loss of labile elements and corresponds to lower CIA values in the resulting sediments (Suttner and Dutta 1986; Goldberg and Humayun 2010). Contrary to this, consistent rainfall will continuously flush the weathering profile with unsaturated fluids for hydrolysis, remove the products of ion exchange and thus more parental material will be subjected to decomposition over a period of time (Pham Tich Xuan et al. 2013; Feng and Kerrich 1990; Ahmad et al. 2014; Verma et al. 2012; Bhat 2017). The trace elements such as Sc, Th, Zr, Cr, Ni and Co are generally immobile during surficial processes (Taylor and Mclennan 1985; Garrels and Mackenzie 1971), and their abundances in sediments are useful indicators of source composition (Suttner and Dutta 1986; Goldberg and Humayun 2010; Sheikh et al. 2014; Saxena et al. 2015). Sediment geochemistry gives a more comprehensive overview of the processes that have operated during the production of sediments (Jeelani and Shah 2006; Jin et al. 2006; Maynard et al. 1982; Rashid et al. 2015).
In the natural environment, all known elements are present at some level of concentration in humans, animals, vegetables and minerals, and their beneficial and harmful effects appears to be present since evolution began (Niskavaara et al. 1997). Well-known examples are Se toxicity and deficiencies in China (Tan et al. 2002), As problem in Bangladesh (Nordstrom 2002) and West Bengal, India (Mukherjee et al. 2010), enrichment of U and other elements in the ‘sickness country’ in Kakadu, Australia (Selinus and Frank 2000). Recent geochemical baseline studies show that the determination and explanation of natural background levels (baseline data) of elements provide a useful tool for environmental policy (Salminen et al. 2008; Selinus and Frank 2000; Adriano 2001).
Over the recent past, geochemical mapping programme have been carried out in Kashmir Valley but with a focus on mineral exploration (Bhat and Bhat 2014; Mir and Mir 2015). The main goal of the present study is to examine the effects of paleoweathering and climate in Karewa basin of south Kashmir using selected major and trace elements. In addition, the ER (enrichment ratio) and quantification of contamination (QOC) for few metal contents have been evaluated to identify any potential environmental contamination in the study area. A thorough knowledge on the bioavailability of the trace elements, the environmental factors and the geographical distribution are required in better understanding the role of trace elements in the environment, necessitated this study, first of its kind in a non-industrial and less urbanized area of Western Himalaya.
Study area
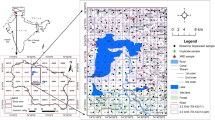
The study area covers the parts of Shopian, Pulwama and Budgam districts of south Kashmir, Jammu and Kashmir, in Survey of India toposheet No. 43K/13 and 43K/14, bound by latitudes 33˚41′10″–34˚00′00″ and longitudes 74˚45′00″–75˚00′00″ (Fig. 1). Geologically, the area is covered by the rocks of Karewa group, which are underlain by Permo–Triassic sequence and overlain by recent alluvium (Bhatt 1975). The Plio-Pleistocene Karewas are commonly divided into two lithologic units, the lower Karewas (Hirpur Formation) and upper Karewas (Nagum and Dilpur formations) (Bhatt 1989; Singh 1982). Lower Karewas are gently inclined and better exposed on the PirPanjal side. Upper Karewas are better exposed on the Great Himalayan side (Bhatt 1975, 1976; Singh 1982). The undifferentiated quaternary sediments include recent alluvium, alluvial tracts, flood plains, river terraces, and talus and scree fans (Bhatt 1976).
Methodology
Twelve soil profiles of Karewa deposits from parts of Shopian, Pulwama and Budgam districts of south Kashmir were selected and studied for major oxides and trace elements. A total of 36 samples have been collected from top surface, 15 cm and 60 cm depth, respectively. The samples collected from stream sediment/slope wash at top surface represent the A horizon samples of the soil profile. The samples collected at 15 cm depth represent B horizon samples of the profile. Similarly, the samples collected at 60 cm depth represent the C horizon samples of the profile. After drying, each sample was powdered and sieved through 120 micron mesh, coned and quartered and submitted for chemical analysis. Major oxides, Cr, Rb, Sr, Y, Zr, Nb, Ba, Ga, Sc, V, U, Th, Pb, Ni, Co, Cu and Zn were analysed by XRF (Make and Model: M/S Panalytical; MAGIX, 2.4 KW Sequential XRF Spectrometer). REE’s, Sn, In, Hf, Ta, Mo, W, Ge, Be were analysed by ICPMS (Make and Model: ICPMS—Perkin Elmer Sciex ELAN-6100). As, Sb, Bi and Se were analysed by VG-AAS, Li and Cs by F-AAS, Cd and Ag by GF–AAS (Make and Model: VARIAN: AA Series and Perkin Elmer: PE Series). Standard reference material GBW-07410 with known element concentrations was analysed after each batch of 20 samples for accuracy and duplicate samples after each batch of 10 samples was analysed for repeatability. In addition, repeated analysis of 5% of stream sediment samples were carried out to validate unambiguously the consistency in sampling and the accuracy in the analysis.
Chemical weathering of sediments and soils has been quantitatively estimated by calculating chemical index of alteration (CIA), plagioclase index of alteration (PIA) and chemical index of weathering (CIW) (Nesbitt and Young 1982; Harnois 1988; Fedo et al. 1995). The chemical index of alteration (CIA) was calculated with the formula:
where CaO* is the amount of CaO incorporated in the silicate fraction of the studied samples (Fedo et al. 1995). Quantitative measure of plagioclase weathering is estimated by calculating PIA as follows:
Similarly, the chemical index of weathering was calculated with the formula:
The PIA values of the studied sediments are comparable to the calculated values of CIW (Harnois 1988) which does not include K2O. Optimum fresh value for CIA and CIW is ≤ 50 and optimum weathered value for CIA and CIW is 100.
Correlation of soil elemental composition and ratios provides a robust estimation of palaeo-rainfall and palaeo-temperature (Marbut 1935; Sheldon et al. 2002). Maynard (1992) proposed formulae for calculation of precipitation based on the premise that alkalis and alkaline earth (Ca, Mg, Na, K) are discriminated against Al. The palaeo-precipitation has been calculated using the following formula:
P (mean annual precipitation) = 14.265(CIA-K) − 37.632 (Maynard 1992; Sheldon et al. 2002).
Similarly, the mean annual temperature has been estimated using the formula:
T (mean annual temperature) = − 18.51 (S) + 17.2989 (Sheldon et al. 2002),
where S is the molecular ration of Na2O and K2O to Al2O3.
The environmental impact of metals and the pollution level in the sediments have been estimated by parameters including enrichment ratio (ER) and quantification of contamination (QoC) (Simex and Helz 1981; Asaah et al. 2006; George et al. 2016).
The enrichment ratio (ER) is calculated as:
where Cn is the concentration of an element measured in a sample and Bn is the background or baseline concentration. An enrichment ratio, ER of 1 indicates that the soil or sediment is neither enriched nor depleted in a particular element relative to the average crustal concentration. On the other hand an ER of > 1 or < 1 is an indication of enrichment or depletion.
Similarly, the quantification of contamination is calculated using the equation:
where X is the average concentration of the metal in the sample under investigation, and Xc is the average concentration of the metal in background (Asaah et al. 2006).
Results
The statistical analysis of major, trace and REE’s of the sediment samples along with comparison to UCC is shown in Table 1. Major elemental analysis depicts that sediment samples are rich in SiO2 followed by Al2O3, Fe2O3, CaO, MgO, K2O, Na2O, P2O5, TiO2 and MnO. SiO2 being the dominant oxide makes up nearly 60% of the oxide composition of the samples, ranging from 37.1 to 66.1 Wt% with an average of 58 Wt %. SiO2 is followed by Al2O3 with concentration ranging from 9.4 to 14.4 wt% at an average of 12.86 wt%. The concentration of Fe2O3 ranges from 4.8 to 6.5 wt% at an average of 5.6 wt%. CaO content ranges from 1.2 to 17.6 wt% at an average of 5.2 wt%. The concentration of MgO, K2O and Na2O is low and decreases from former to latter. The rest of the oxides are very less in concentration making less than 1% of the total oxide composition. Average major oxides of the samples are plotted against the profile depth (Fig. 2). SiO2, Al2O3 and K2O decreases from bottom to top of the profile, whereas CaO and MgO increase from bottom to top of the profile. From bottom to top, Fe2O3, Na2O and TiO2 first increase and then decrease, whereas MnO and P2O5 concentrations initially decrease and then increase up the profile.
The dominance of heavy metals in the sediment samples is in the following order: Ba > Zr > Sr > Cr > Rb > V > Zn > Ni > Y > Cu > Pb > Ga. In particular, the average Ba concentration is 460 ppm, Zr and Sr about 200 ppm, Cr and Rb near 100 ppm. The concentration of the other trace elements is less than 50 ppm. The lower detection limit (LDL) of Ba is 50 ppm, Zr, Sr, V, Y and Ga (5 ppm), Cr (15 ppm), Rb (3 ppm), Zn (10 ppm), Ni and Pb (2 ppm) and Cu (1 ppm), respectively (Bhat 2017). The concentration of Ba, Cr, Cu, Ni, Rb, Th, V, Zn, Sb and Be decreases from bottom to top of the profile whereas, Sc, Sr, Ta and U increases from bottom to top of the profile. Co, Y, Zr, Li, Hf and W clearly deviate from their variation trends from bottom to top of the profile. The concentration of the trace elements first shows a decrease and then increases towards the top of the profile whereas Ga, Nb, Cd and As showed an opposite trend from bottom to top of the depth profile (Table 2).
The dominance of rare earth elements in the sediment samples are in the following order: Ce > La > Nd > Pr > Sm > Gd > Dy > Er > Yb > Tb > Eu. The concentration of Ce ranges from 55.8 to 117.8 ppm with an average of 80.91 ppm. The concentration of La ranges from 29.8 to 55.4 ppm with an average of 41.27 ppm. The concentration of Nd ranges from 24.7 to 48.9 ppm with an average of 35.9 ppm. The concentration of other REE’s is less than 10 ppm. The concentration of Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu exhibits an increasing trend from bottom to top of the profile, however, La, Ce, Pr, Nd and Sm first decreases and then sharply increases from bottom to top of the profile (Fig. 3).
Discussion
Weathering of the source area and palaeoclimate
The CIA has been developed by Nesbitt and Young, 1982 for palaeoclimatic reconstruction at early proterozoic sediments of Huronian Supergroup, and is the most widely used chemical index to determine the degree of weathering of source area. The CIA values of fresh rocks and minerals are consistently near 50. The samples with CIA values below 60 display low chemical weathering, between 60 and 80 indicate moderate chemical weathering and more than 80 exhibit extreme chemical weathering (Fedo et al. 1995). CIA values of the studied sediment samples vary from 31.6 to 74.6 with an average value of 53.99 indicating low to moderate degree of weathering. Higher CIA values are found in the samples collected at top surface and the samples taken at 15 cm depth and 60 cm depth showed almost similar values of CIA but lower than the samples collected at the top of the profile (Fig. 2). Thus, the chemical weathering rates are higher at the surface and decreases with increasing depth (Bhat 2017).
The degree of the chemical weathering is also estimated using the plagioclase index of alteration (Fedo et al. 1995; Nesbitt et al. 1980; Nath et al. 1997) in molecular proportions. Very high PIA values indicate that most of the plagioclase has been converted to clay minerals. This, in turn, accords with data obtained using the CIW index. The PIA values of sediment samples ranged from 29.76 to 83.49 with an average of 68.6 indicating low to moderate weathering. Higher PIA values are found in the samples collected at top surface and the samples taken at 15 and 60 cm depth, respectively but lower than top soil (Fig. 2).
Similarly the chemical index of weathering (CIW) index is not sensitive to post-depositional K-enrichments and is similar to CIA. Chemical Index of Weathering is a molecular immobile/mobile ratio based on the assumption that Al remains in the system and accumulates in the residue, while Ca and Na are leached away (Gas’kova et al. 2017; Harnois 1988; Puchelt 1972). The CIW values of the sediments vary from 33.20 to 85.99 with an average value of 72.35 indicating low to moderate degree of weathering. Similar to CIA and PIA, CIW values are found in the samples collected at top surface and the samples taken at 15 and 60 cm depth showed lower and almost similar values of CIW (Fig. 2). Low intensity weathering may be because of the nearness of provenance of sediments. Though hydro-climatic conditions are able to carry the mechanical disintegration of source rock material but due to short distance of sediment transportation from source rock, the maturity of sediments is not achieved (Nesbitt and Young 1982; Fedo et al. 1995).
Nesbitt and Young (1982), and Nesbitt et al. (1996) used the ternary diagrams (A–CN–K) using Al2O3 − (CaO + Na2O) − K2O and (A–CNK–FM) using Fe2O3 + MgO − (CaO + Na2O + K2O) − Al2O3 as three axis to deduce weathering trends. On both the A–CN–K and A–CNK–FM diagrams, samples of the study area show almost similar contents in Al2O3, CaO, Na2O, K2O and MgO as those of upper crust, reflecting very poor to moderate weathering history (Fig. 4a, b). Weathering has not reached to a stage at which significant amounts of the alkali and alkali earth elements will be removed from the sediments.
Triangular diagrams showing weathering trend of studied samples (after Nesbitt and Young 1982). a A–CN–K triangular diagram. b A–CNK–FM triangular diagram
The ratios of SiO2/(Al2O3 + K2O + Na2O) of the sediments show that all the samples are plotted in the semi-arid climatic zone (Fig. 5a). The prevalent dry condition will reduce weathering process; resulting less chemical maturity (Goldberg and Humayun 2010; Prasad et al. 2014; Raj et al. 2015). The CIA (molar) index of fresh feldspars (and for un-weathered, non-peraluminous igneous rocks) is one, but rises towards infinity as chemical weathering progresses. With increasing chemical alteration, CIA (molar) values increase due to the alteration of feldspars to clay minerals. CIA (molar) values smaller than one for clastic sediments may indicate the presence of carbonates, which must be removed by leaching the sediments with acetic acid prior to chemical analysis. The plot between CIA (molar) and Al2O3values (Fig. 5b) for sediments indicate semi-arid conditions during the deposition of the sediments. The results are in agreement with the previous studies carried out in the study area. Rashid et al. (2015) inferred low to moderate chemical weathering in the source region for clay-silt dominant sediments of Wular Lake, North Kashmir. Similarly, Mir and Jeelani (2015) indicated moderate weathering and immaturity of the sediments in river Jhelum and its tributaries in Kashmir valley basin. Ahmad and Chandra (2013) and Chandra et al. (2016) studied the loess paleosol sediments of the Karewa group of Kashmir valley. They found a mixed source rocks for the sediments with a large provenance, which apparently has undergone weak to moderate recycling processes and low to moderate chemical weathering. Bhat et al. (2019) have indicated low to moderate values of CIA, PIA and CIW in Karewa deposits of south Kashmir and hence low to moderate degree of weathering. Further, the studies have indicated a mean annual palaeo-precipitation of 846 mm per year and mean annual paleo-temperature of 10.91 °C. An important caveat to bear in mind that none of the studied samples represents a palaeosol. However, the alkali and alkaline earth elements present in the samples are in excess to that of typical soil. Thus, the present estimation can be seen as rather conservative and possibly represents the minimal values. Overall, an apparently semi-arid climate prevailed during the deposition of Karewa sediments of Kashmir Valley with a poor to moderate weathering history.
Environmental risk assessment
Due to the universal formulas of enrichment ratio (ER) and quantification of contamination (QOC), these parameters are relatively simple and easy tools for assessing the enrichment degree and comparing the contamination of different environmental media (Benhaddya and Hadjel 2013; George et al. 2016; Boyle and Jonasson 1973). Geochemical normalization has been used to calculate enrichment ratio (ER) (Simex and Helz 1981) to identify anomalous metal contributions and to assess anthropogenic intrusions of the metals in the samples. The highest enrichment ratios of around six for As at all depths indicate that this metal shows moderately to severe enrichment. The enrichment ratio for Cr is around three and two indicating that these metals show moderate enrichment. The elements showing ER above one include Zr, Co, Y, Hf, Th and Sc.
The quantification of contamination (QoC) mainly describes the quantification of anthropogenic concentration of metal employing the concentration in the background metals to represent the lithogenic metal (Asaah et al. 2006; George et al. 2016). The values of the index are mainly expressed as percentage, demonstrating the magnitude of lithogenic and anthropogenic impacts (George et al. 2016). The QoC plot showed that the average concentration of Ba, Rb, Sr, Nb, and Ta was mainly derived from geogenic sources with no evidence of anthropogenic impacts (blue area) (Fig. 6). While the values of As, Ni, Co and Cr illustrated to have an anthropogenic source of contamination in all the sites (yellow area). The other elements showing a low anthropogenic impact include Zr, Hf, Th and Sc (yellow area) (Fig. 6). The contamination factor for all the metals is almost similar from top to bottom of the profile. The high concentration of these heavy metals particularly As and Cr is due to combustion of coal in brick kilns (Bhat et al. 2017).
Conclusions
Thirty-six sediment samples from twelve soil profiles were collected from Karewa deposits of south Kashmir to evaluate weathering characteristic, palaeoclimate and environmental contamination. On both the A–CN–K and A–CNK–FM diagrams, samples of the study area show almost similar contents in Al2O3, CaO, Na2O, K2O and MgO as those of upper crust, reflecting very poor to moderate weathering history. Further, the sediments were deposited in semi-arid type of climate with mean annual precipitation of 846 mm per year and mean annual temperature of 10.91 °C. The potential trace metal enrichment in sediments related to human activities was evaluated using an enrichment factor and quantification of contamination factor. The highest enrichment ratios of six for As indicate that this metal is highly enriched in the samples. The enrichment ratio for Cr is above three and for Ni, Sb and Tb, the enrichment ratio is above two. The enrichment ratio for Cr is around three and two indicating that these metals show moderate enrichment. The elements showing ER above one include Zr, Co, Y, Hf, Th and Sc. The QoC plot of As, Ni, Co and Cr illustrated to have an anthropogenic source of contamination.
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer Verlag, New York
Ahmad I, Chandra R (2013) Geochemistry of loess-paleosol sediments of Kashmir Valley, India: provenance and weathering. J Asian Earth Sci 66:73–89
Ahmad AHM, Noufal KN, Masroor AH, Khan T (2014) Petrography and geochemistry of jumara dome sediments, Kachchh Basin: implications for provenance, tectonic setting and weathering intensity. Chi J Geochem 33(1):9–23
Asaah VA, Abimboal AF, Suh CE (2006) Heavy metal concentration and distribution in surface soils of the bassa industrial zone 1, Douala, Cameroon. Arab Jour Sci Eng 31(2):147
Benhaddya ML, Hadjel M (2013) Spatial distribution and contamination assessment of heavy metals in surface soils of hassiMessaoud. Algeria Environ Ear Sci 71(3):1473–1486
Bhat NA (2017) Geochemistry of surface water and stream sediments in Karewa basin of South Kashmir, India: implications on human health, agriculture and environmental studies. PhD Thesis; Banarus Hindu University
Bhat AA, Bhat NA (2014) Geochemical mapping of Kashmir Nappe and Karewa group of rocks covering parts of Anantnag, Baramulla and Srinagar districts, J&K in toposheet nos. 43K/13 and 43K/14. Geological Survey of India, Unpublished report FS 2013-14
Bhat NA, Nath S, Guha DB, Singh BP, Bhat AA (2018) Geochemistry of stream sediments of Karewa Basin of South Kashmir, India: their environmental and agricultural implications. Ind J Geosci (Under review)
Bhat NA, Singh BP, Bhat AA, Nath S, Guha DB (2019) Application of geochemical mapping in unraveling paleoweathering and provenance of Karewa sediments of South Kashmir, NW Himalayas, India. J Geol Soc Ind 93(1):68–74. https://doi.org/10.1007/s12594-019-1124-x
Bhatt DK (1975) On the Quaternary geology of the Kashmir Valley with special reference to stratigraphy and sedimentation. Geol Sur Ind Misc Pub 24:188–204
Bhatt DK (1976) Straigraphical status of Karewa Group of Kashmir, India. Him Geol 6:197–208
Bhatt DK (1989) Lithostratigraphy of Karewa group, Kashmir Valley, India and a critical review of its fossil record. In: Memoir of the Geological Survey of India, vol 122. Geological Survey of India, Calcutta
Bickle MJ, Chapman HJ, Bunbury J, Harris NBW, Fairchild IJ, Ahmad T, Pomiès C (2005) Relative contributions of silicate and carbonate rocks to riverine Sr fluxes in the headwaters of the Ganges. Geochim Cosmochim Acta 69:2221–2240
Blum JD, Gazis CA, Jacobson AD, Chamberlain CP (1998) Carbonate versus silicate weathering in the Raikhot catchment within the high Himalayan crystalline series. Geol 26:411–414
Boyle RW, Jonasson IR (1973) The geochemistry of As and its use as an indicator element in geochemical prospecting. J Geochem Explor 2:251–296
Chandra R, Ahmad I, Quarshi A (2016) Pedological and geochemical characterization of loess-paleosol sediments of Karewa basin: implications for paleoclimatic reconstruction of Kashmir valley. J Geol Soc India 4:38–54
Fedo CM, Nesbitt HW, Young GM (1995) Unravelling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 23(10):921–924
Fedo CM, Young GM, Nesbitt HW, Hanchar JM (1997) Potassic and sodic metasomatism in the Southern province of the Canadian shield: evidence from the paleoproterozoic serpent formation, huroniansupergroup. Canad Precam Res 84:17–36
Feng R, Kerrich R (1990) Geochemistry of fine grained clastic sediments in the Archean Abitibi greenstones belt, Canada: implications for provenance and tectonic setting. Geochim Cosmochim Acta 54:1061–1081
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. Norton, New York
Gas’kova OL, Strakhovenko VD, Ovdina EA (2017) Composition of brines and mineral zoning of the bottom sediments of soda lakes in the Kulunda steppe (West Siberia). Russ Geo Geophy 58(10):1199–1210
George RM, Martin. GD, Nair. SM, Thomas SP Jacob S (2016) Geochemical assessment of trace metal pollution in sediments of the Cochin backwaters, Envir Foren, 17:2, 156–171, https://doi.org/10.1080/15275922.2016.1163623
Goldberg K, Humayun M (2010) The applicability of the chemical index of alteration as a paleoclimatic indicator: an example from the Permian of the Paraná Basin, Brazil. Palaeo Palaeo Palaeo 293:175–183
Gromet LP, Dymek RF, Haskin LA, Korotev RL (1984) The ‘North American shale composite’: its compilation, major and trace element characteristics. Geochim Cosmochim Acta 48:2469–2482
Grunsky EC, Drew LJ, Sutphin DM (2009) Process recognition in multi-element soil and stream-sediment geochemical data. Appl Geochem 24:1602–1616
Harnois L (1988) The C.I.W. index: a new chemical index of weathering. Sed Geol 55:319–322
Jeelani G, Shah AQ (2006) Geochemical characteristics of water and sediment from the Dal Lake, Kashmir Himalaya: constraints on weathering and anthropogenic activity. Environ Geol 50(1):12–23
Jin ZD, Li FC, Cao JJ, Wang SM, Yu JM (2006) Geochemistry of Daihai Lake sediments, inner Mongolia, North China: implications for provenance, sedimentary sorting, and catchment weathering. Geomor 80:147–163
Marbut CF (1935) Atlas of American agriculture. III. Soils of the United States. Government Printing Office, Washington
Maynard JB (1992) Chemistry of modern soils as a guide to interpreting Precambrian paleosols. J Geol 100:279–289
Maynard JB, Valloni R, Yu HS (1982) Composition of modern deep-sea sands from arc-related basin. In: Leggett JK (ed) Trench forearc geology: sedimentation and tectonics on modern and ancient active plate margins, vol 10. Geological Society, London, Special Publications, pp 551–561
McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. Rev Miner 21:170–199
McLennan SM, Hemming S, McDaniel DK, Hanson GN (1993) Geochemical approaches to sedimentation, provenance and tectonics. Geol Soc Amer Spec Pap 284:21–40
Mir RA, Jeelani G (2015) Textural characteristics of sediments and weathering in the Jhelum River basin located in Kashmir Valley, western Himalaya. J Geol Soc India 86:445–458
Mir IA, Mir RA, 2015, Interim report on geochemical mapping of Srinagar, Pampur and Ganderbal areas of Srinagar and Ganderbal districts, Jammu and Kashmir in toposheet no. 43 J/11 and part of 43 J/12. Geological Survey of India, Unpublished report FS 2014-15
Mukherjee PK, Pal T, Chattopadhyay S (2010) Role of geomorphic elements on distribution of arsenic in groundwater—a case study in parts of Murshidabad and Nadia districts, West Bengal. Ind Jour Geosci 64(1–4):77–86
Nath BN, Bau M, Ramalingeswara RB, Rao CM (1997) Trace and rare earth elemental variation in Arabian Sea sediments through a transect across the oxygen minimum zone. Geochim Cosmochim Act 61(12):2375–2388
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nat 299:715–717
Nesbitt HW, Markovics G, Price RC (1980) Chemical processes affecting alkalis and alkali earths during continental weathering. Geochim Cosmochim Acta 44:1659–1666
Nesbitt HW, Young GM, McLennan SM, Keays RR (1996) Effect of chemical weathering and sorting on the petrogenesis of siliciclastic sediments, with implications for provenance studies. J Geol 104:525–542
Niskavaara H, Reimann C, Chekushin V, Kashulina G (1997) Seasonal variability of total and easily leachable element contents in top soils (0–5 cm) from eight catchments in the European Arctic (Finland, Norway and Russia). Environ Poll 96:261–274
Nordstrom DK (2002) Worldwide occurrences of arsenic in groundwater. Science 296:2143–2145
Prasad V, Farooqui A, Sharma A, Phartiyal B, Chakraborty S, Bhandari S, Singh A (2014) Mid–late Holocene monsoonal variations from mainland Gujarat, India: a multi-proxy study for evaluating climate culture relationship. Palaeo Palaeo Palaeo 397:38–51
Puchelt H (1972) Barium. Handbook of geochemistry (Wedepohl KH et al (eds)), 56B1–56O2, Springer, Berlin
Raj R, Chamyal LS, Prasad V, Sharma A, Tripathi JK, Verma P (2015) Holocene climatic fluctuations in the Gujarat Alluvial plains based on a multiproxy study of the Pariyaj Lake archive, western India. Palaeo Palaeo Palaeo 421:60–74
Ranasinghe PN, Fernando GWAR, Dissanayake CB, Rupasinghe MS (2008) Stream sediment geochemistry of the Upper Mahaweli River Basin of Sri Lanka—geological and environmental significance. J Geochem Explor 99:1–28
Rashid SA, Ganai JA, Masoodi A, Khan FA (2015) Major and trace element geochemistry of lake sediments, India: implications for weathering and climate control Arab. J Geosci 8:5677–5684
Salminen R, Gregorauskiene V, Tarvainen T (2008) The normative mineralogy of 10 soil profiles in Fennoscandia and north-western Russia. App Geochem 23:3651–3665
Saxena A, Trivedi A, Chauhan MS, Sharma A (2015) Holocene vegetation and climate change in central Ganga plain: a study based on multiproxy records from Chaudhary-KaTal, Raebareli District, Uttar Pradesh, India. Quat Inter 371:164–174
Selinus O, Frank A (2000) Medical geology. In: Moller L (ed) Environmental medicine. Joint Industrial Safety Council, Stockholm, pp 64–82
Sheikh JA, Jeelani G, Gavali RS, Shah RA (2014) Weathering and anthropogenic influences on the water and sediment chemistry of Wular Lake, Kashmir Himalaya. Environ Ear Sci 71:2837–2846
Sheldon ND, Retallack GJ, Tanaka S (2002) Geochemical climofunctions from North American soils and application to Paleosols across the eocene-oligocene boundary in oregon. Jour Geol 110:687–696
Simex SA, Helz GR (1981) Regional geochemistry of trace elements in Checapeake Bay. Environ Geol 3(6):315–323
Singh IB (1982) Sedimentation pattern in the Karewa Basin, Kashmir Valley, India, and its geological significance. J Palaeon Soc Ind 27:71–110
Suttner LJ, Dutta PK (1986) Alluvial sandstones composition and paleoclimate, I, framework mineralogy. J Sed Pet 56:329–345
Tan J, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L (2002) Selenium in soil and endemic diseases in China. Sci Tot Environ 284:227–235
Taylor SR, Mclennan SM (1985) The continental crust: its composition and evolution. Blackwell, Oxford
Verma M, Singh BP, Srivastava A, Mishra M (2012) Chemical behavior of suspended sediments in a small river draining out of the Himalaya, Tawi River, northern India: implications on provenance and weathering. Him Geol 33(1):1–14
Viers J, Dupré B, Braun JJ, Deberdt S, Angeletti B, Ngoupayou JN, Michard A (2000) Major and trace element abundances, and strontium isotopes in the Nyong basin rivers (Cameroon): constraints on chemical weathering processes and elements transport mechanisms in humid tropical environments. Chem Geol 169:211–241
Wedepohl HK (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Xuan PT, Pho NV, Gas’kova OL, Bortnikova SB (2013) Geochemistry of surface waters in the vicinity of open pit mines at the cay cham deposit, Thai Nguyen Province, Northern Vietnam. Geochem Inter 51(11):931–938
Acknowledgements
The work has been carried out under Geological Survey of India, Field Season Programme 2013–14. The authors gratefully acknowledge Director General, Geological Survey of India, Dy. Director General and HOD, Geological Survey of India, Northern Region and Dy. Director General, State Unit Jammu and Kashmir for providing necessary facilities for completion of the work. The authors are also thankful to the officers of Geochemical Lab. GSI, Lucknow for analysis of the samples. The authors are highly indebted to Miss Samreen Shaikh, Geologist, GSI for improving the language of the manuscript and also to the anonymous reviewers for their valuable scientific comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhat, N.A., Singh, B.P., Nath, S. et al. Geochemical characterisation of stream sediments and soil samples from Karewa deposits of south Kashmir, NW Himalaya, India. Environ Earth Sci 78, 278 (2019). https://doi.org/10.1007/s12665-019-8212-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8212-5