Abstract
The environmental isotopes such as deuterium and oxygen-18 and the deuterium excess values have been used to assess groundwater recharge sources and their dynamics in Khan Younis City in the Gaza Strip in Palestine. Three isotopic lines for the relationship between δ2H and δ18O were used in the assessment. These lines are the global meteoric water line, the local meteoric water line and the groundwater evaporation line. The δ2H, δ18O and D-excess values indicate that deuterium and oxygen-18 isotopes originated in the groundwater from groundwater mixing with rainfall and other water sources; the groundwater in the area recharged from rainfall from a distant source that came from the Mediterranean Sea and from other sources such as wastewater, irrigation return flow and saline water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past 40 years, environmental isotopes such as deuterium and oxygen-18 have been widely used in groundwater hydrology. These isotopes are directly influenced by atmospheric processes and groundwater recharge sources. They have also been used to assess various components of the natural hydrological cycle including: determining the hydrogeological characteristics of aquifers, the sources of water in the unsaturated zone and in groundwater flow systems, groundwater flow dynamics and the interconnections between water sources (such as groundwater and surface water) and the atmosphere (Mook 2001; Blasch and Bryson 2007; Vasanthavigar et al. 2012; Chiogna et al. 2014). Additionally, these environmental isotopes have been extensively used to assess the hydroenvironmental issues associated with groundwater contamination (Krishnaraj et al. 2011; Sanchez et al. 2015; Al-Charideh and Kattaa 2016; Edirisinghea et al. 2016; Isawi et al. 2016; Gomaah et al. 2016; Mokadem et al. 2016).
Despite the complexity of water circulation within the hydrological cycle, there is a strong relationship between levels of the deuterium and oxygen-18 at a local scale to values of global scale, which is a linear relationship throughout the earth for ocean/seawater, vapour in the atmosphere and precipitation in different regions of the world and is called global meteoric water line (GMWL) (Mook 2001; Ferronsky and Polyakov 2012). The widely used mathematical relationship for the GMWL, which was developed by Craig (1961) and modified by Rozanski et al. (1993), is in accordance with the following equations:
The intercept of the above equations is called the deuterium excess (D-excess), as shown in the following relationship (Dansgaard 1964):
In addition to the GMWL, a local meteoric water line (LMWL) can be defined. The LMWL represents the δ2H and δ18O values in local rainfall for meteoric waters feeding a groundwater system, accounting to regional variations of the above relationship (Krishnaraj et al. 2011; Chiogna et al. 2014; Sanchez et al. 2015; Al-Charideh and Kattaa 2016; Edirisinghea et al. 2016; Jilali et al. 2016; Isawi et al. 2016; Gomaah et al. 2016; Mokadem et al. 2016; Peng et al. 2016). Therefore, plotting the relationship between the δ2H and δ18O values for groundwater along the LMWL and GMWL as a references is commonly used for assessing the hydrogeological factors that influence groundwater recharge and its composition (Adomako et al. 2011; Krishnaraj et al. 2011; Hamed and Dahri 2013; Ammar et al. 2016; Fynn et al. 2016; Tiwari et al. 2016). Although the D-excess values in most places around the world are about 10‰, some areas may have different slopes and intercepts due to different rainfall evaporation conditions in various air mass sources (Gat 1980; Sakai and Matsubaya 1977) and the D-excess values used to identify the meteoric water source that influences groundwater recharge (Celle-Jeanton et al. 2001; Blasch and Bryson 2007; Krishnaraj et al. 2011; Hamed and Dahri 2013; Dhaoui et al. 2016).
However, the investigation of the δ2H and δ18O values for the groundwater of the Gaza Strip as well as the study area has not been previously carried out and studied.
The first objective of this study is to establish the local meteoric water line (LMWL) for the rainfall in study area and the entire Gaza Strip from the available δ2H and δ18O values in precipitation, while the second and third objectives are to compare the isotopic composition of the groundwater evaporation line (GEL) with the LMWL to that of the global meteoric line (GMWL) and to evaluate the relationship between the δ18O values and the D-excess values in the groundwater of Khan Younis City, southern the Gaza Strip, Palestine.
Study area
General information
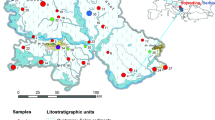
The study area for this research is Khan Younis City. The city is located in the southern part of the Gaza Strip (Fig. 1) at a latitude of 31,3439 (31°, 20′, 38.040″) north and a longitude of 34,3025 (34°, 18′, 9.000″) east. The city is generally flat and covers an area of about 6.5 km2 with topographic elevation ranging from about 54 to 76 m above the mean sea level (UNEP 2009). In 2016, the population of the city was about 236,235 inhabitants (PCBS 2012). The soil type is classified as a loessal sandy, calcaric arenosols–sandy clay loam soil type. This soil type is a transitional zone between arenosolic sandy soils and calcaric (loess) soils that have a texture of a calcareous loamy sand (Shomar et al. 2005).
Location map of the study area (http://mapsof.net/gaza-strip/gaza-strip-cities-map)
Khan Younis City is a semi-arid Mediterranean-type climate (Csa in the Köppen classification system) with an average annual rainfall of about 281.24 mm/year for the period from 1973 up to 2015. Average temperature values for the last 20 years have indicated that the average mean daily temperature ranges from 31.9 °C in summer (August) to 10.3 °C in winter (January), with an annual average solar radiation of about 18.32 MJ/cm2/day. The 20-year maximum mean monthly relative humidity is about 76% in June, and minimum mean monthly relative humidity is about 62% in December (Eshtawi 2015).
Groundwater system
Khan Younis City depends entirely on groundwater for various domestic and drinking purposes. The city does not have any other water supply source. Due to high water demand and overpumping (heavy pumping and low recharge from rainfall during the last 50 years), the groundwater aquifer beneath the city has been overexploited (CAMP 2000). Recharge for the groundwater system originates from the direct infiltration of rainfall, untreated wastewater from cesspits and the irrigation returns from nearby surrounding agricultural areas and groundwater flow from the eastern boundary of the area (Al-Agha 2005).
The aquifer beneath the city is part of the coastal aquifer basin located in the eastern coast of the Mediterranean Sea. It is classified as an unconfined shallow aquifer that receives rainfall recharge (i.e. the groundwater resource would be renewable under conditions where groundwater pumping was controlled). The volume of water stored in the aquifer varies annually and seasonally depending on rainfall intensity and water abstraction. The thickness of the aquifer is between 60 and 120 m. It is generally 10–15 km wide and consists of Pleistocene age Kurkar Group deposits including calcareous and silty sandstones, silts, clays, unconsolidated sands and conglomerates. The top of Saqiya Formation of Tertiary age forms the base of the unconfined aquifer. This formation consists of a thick sequence of marls, clay stones and shales that slopes towards the sea, with a low permeability, and is a 400–1000-m-thick wedge beneath ground surface (CAMP 2000).
A recent hydrochemical study by Abu Jabal et al. (2015) has indicated that the prevalent water type in groundwater in the study area is Na+–Cl−–SO42−, with alkaline earth metals exceeding the alkali metals and that the dominant processes controlling the groundwater hydrochemistry are evaporation, ion exchange and anthropogenic activity (mainly the uncontrolled recharge of wastewater through cesspits). Additionally, Abu Jabal et al. (2017) have indicated that the groundwater is mainly classified as being a very hard-brackish water type, with high Na+, Cl−, SO42− and NO3− concentrations and the shallow groundwater aquifer beneath about 80% of the study area is significantly contaminated.
Groundwater sampling and analytical methods
Thirty-five groundwater samples were collected from twenty groundwater wells throughout Khan Younis City (Fig. 2). The monitoring programme was carried out in two rounds at different times. In the first round, 18 samples were collected in December 2012, while in the second round 17 samples were collected in May 2013. Samples were collected in 50-millilitre clean dry glass bottles. During the sampling, care was taken to avoid introducing air bubbles into the bottles. To avoid evaporation from the samples, the bottles were completely filled to the top, tightly double-capped and stored in the dark (IAEA 2010).
Stable isotope (deuterium and oxygen-18) analyses were performed at the Central Laboratory for Environmental Isotope Hydrology, at the Egyptian Atomic Energy Authority in Cairo. The isotopic analyses for the samples were performed using CO2–H2O equilibration technique using an isotopic ratio mass spectrometer (IRMS) (Thermo–Finnigan Delta plus XL) (Horita 1988; Horita et al. 1989; Coplen et al. 1991). For the isotopic exchange between water and gas phase, 5 ml aliquots of each sample was equilibrated with either hydrogen (H2) gas or carbon dioxide (CO2) gas under constant temperature of 18 °C. Platinum rods used as a catalyst in case of deuterium only. Samples were left to equilibrate for a period of 2 h for deuterium and for a period of 10 h for oxygen-18. After the equilibration stage, dry gas was subsequently introduced into the dual inlet part of the spectrometer alternately with a reference gas of known isotopic composition [as a referenced to the international reference standard called Standard Mean Ocean Water (SMOW)].
Stable deuterium and oxygen-18 isotope values were reported in the usual δ notation in units of ‰ versus Vienna Standard Mean Ocean Water (VSMOW) for oxygen and hydrogen as shown in the following equations (Mazor, 2004). The analytical uncertainties were ± 0.1‰ for δ18O and ± 1‰ for δ2H.
Results and discussion
Isotopic composition of precipitation
For the purpose of this study, the local meteoric water lines (LMWLs) had to be determined. As there are no available data for the δ2H and δ18O values of the precipitation of the Gaza Strip as well as the study area, it was important that an appropriate rainfall collection site that would be close to Khan Younis City and that would provide useful information for the entire Gaza Strip was selected for the purpose of this study. The selected site was Rafah, Egypt, rainfall station. The station is located at an air distance of about 14 kilometres south of the study area. It is located at GPS coordinates of latitude of 31′13″59″N and longitude of 34′13″59″E.
The δ2H and δ18O values for the rainfall in the Rafah (Egypt) rainfall station are available at: http://www-naweb.iaea.org/napc/ih/IHS_resources_isohis.html, which is the database of the Global Network of Isotopes in Precipitation (GNIP) project that was established through the cooperation between the World Metrological Organization (WMO) and the International Atomic Energy Agency (IAEA). Rainfall samples for deuterium and oxygen-18 analyses were collected through the period from January 31, 2001, up to March 31, 2003, for the total of 17 samples and are shown in Table 1. The minimum, maximum, average and standard deviation values for the δ2H, δ18O and calculated (Eq. 3) D-excess values for the rainfall in Rafah (Egypt) rainfall station are also presented in Table 1.
The relationships between the δ2H and δ18O values for the GMWL (Eq. 2) and the LMWL [for rainfall of Rafah (Egypt) rainfall station] are plotted in Fig. 3. The LMWL is according to the following equation:
The slope of the LMWL of 6.217 (Eq. 6 and Fig. 3) is significantly lower than the slope for the GMWL of 8.17 (Eq. 2 and Fig. 3). This low slope value for the LMWL indicates that the study area is classified as a semi-arid climatic area with variable air humidity, low rainfall intensity and intensive evaporation process (Adomako et al. 2011; Qian et al. 2013). Shah (2013) indicated that this slope variation suggests that the isotopic composition of rainfall in the area is affected by evaporation before recharging the groundwater.
The relationship and fractionation between the environmental isotopic variation as δ2H and δ18O values in the groundwater
The δ2H, δ18O and calculated D-excess values for the groundwater samples of the study area are shown in Table 2. The minimum, maximum, average and standard deviation values for the δ2H, δ18O and calculated (Eq. 3) D-excess values are also presented in Table 3. Values for δ2H range from − 13.68 to − 11.16‰ with an average of − 12.58‰ and a standard deviation of 0.68. Additionally, values for δ18O range from − 3.89 to − 2.84‰ with an average of − 3.40‰ and a standard deviation of 0.23.
The line of best fit between δ2H and δ18O values from the groundwater represents the groundwater evaporation line (GEL) (Fig. 4) that is shown by the following equation:
The variation of the δ2H and δ18O values in the groundwater is likely to be due to the variability of local conditions that are the result of both regional and local climatic factors. Furthermore, this variability is probably due to a combination of two processes: (1) rainfall infiltration and (2) groundwater mixing mechanism with water resulted from anthropogenic activities and agricultural return flow (Adomako et al. 2011). The δ2H and δ18O values for some rainwater samples [at Rafah (Egypt) rainfall station] are relatively higher than the values in the groundwater of the study area. This isotopic enrichment in the rainfall indicates that there is a reduction in the δ2H and δ18O values in the groundwater. This reduction could be attributed probably due to the following three reasons: (1) the isotopic composition of rainfall might have affected by evaporation before water has infiltrated into the aquifer; (2) the groundwater has been recharged from rainfall from a distant source from the Mediterranean Sea to the west of the study area; and (3) isotopic exchange for deuterium and oxygen-18 has taken place between the groundwater and the aquifer rock material (Ako 2011).
Relationships between the GEL for the groundwater, the GMWL and the LMWL
Tracing the origin of deuterium and oxygen-18 isotopes in the groundwater of the study area prior to evaporation is carried out by determining the slope of the change in GEL as it deviates from the LMWL and the GMWL (Shah 2013).
Relationship between the GEL for groundwater and the GMWL
The comparison between the slope of the GMWL of 8.17 (Eq. 2) with the slope of the GEL is used to explore the origin of the groundwater (Al-Charideh et al. 2009). If the GEL slope ≅ 8.17, the groundwater is likely to have been derived from meteoric water (water derived from precipitation) without being influenced by evaporation or other post-precipitation effects. However, if the GEL indicates a slope of less than 8.17: (1) the groundwater is likely to have been derived from meteoric water with significant evaporation that has been taken place during or after rainfall and (2) there has been significant groundwater mixing with recharged water that has high δ2H and δ18O values (i.e. it is possible that surface water not associated with local rainfall has contributed to recharging the aquifer) (Gat and Carmi 1970; Gat and Dansgaard 1972; Ammar et al. 2016).
The slope of the GEL for the groundwater of the study area is of 2.492 (Eq. 7 and Fig. 4) and is less than the slope of GMWL of 8.17. This slope reduction probably indicates that: (1) the groundwater is originated from meteoric water influenced by evaporation during or after rainfall; and/or (2) there is mixing of the groundwater with recharge water from other sources that have higher δ2H and δ18O values. (Amiri et al. 2016). Leontiadis et al. (1996) emphasized that these processes typically take place in regions with a semi-arid climatic area, such as the study area.
The location of the GEL for the groundwater samples in relative to the GMWL falls to the left above the GMWL (Fig. 5). This is likely to indicate that groundwater is directly affected by recharge from local meteoric water or rainfall, and the rainfall water does not undergo evaporation during infiltration (Tiwari et al. 2016). However, Mokadem et al. (2016) stated that this behaviour of the GEL indicates that the dilution and mixing processes have taken place in the groundwater and are likely to have affected the deuterium and the oxygen-18 isotopic composition of the groundwater.
The difference in the δ2H and δ18O values between the GEL and the GMWL is about − 11.5‰ for δ2H and − 2.65‰ for δ18O (Fig. 5). The data for the δ2H and δ18O values in the groundwater indicate that about 8.57% of the groundwater samples show δ2H values from − 13.36 to − 11.16‰ and − 3.08 to − 2.84‰ for δ18O. These values can be considered similar to the GEL interaction values with the GMWL. This suggests that the predominant source of the deuterium and oxygen-18 isotopes in these samples is meteoric water, which is the predominant recharge source (Chen et al. 2016).
Relationship between the GEL for the groundwater and the LMWL for the rainfall
The GEL for the groundwater of the study area has a slope value of 2.492 (Eq. 7 and Fig. 4), which is lower than the slope value for the LMWL for the rainfall of 6.217 (Eq. 6 and Fig. 3). Li et al. (2016) suggested that this slope variation between the GEL and the LMWL indicates that evaporation process occurs for the rainfall during infiltration and tends to enrich the groundwater with deuterium and oxygen-18 isotopes causing simultaneous salinity increases.
The location of the GEL for the groundwater samples in relation to the LMWL falls to the right and below the LMWL (Fig. 6).This suggests that the aquifer behaves as a homogeneous aquifer system for the composition of deuterium and oxygen-18 isotopes. Ayadi et al. (2016) indicated that this homogeneity indicates that the aquifer has uniform flow path and recharge sources. While Li et al. (2016) suggested that this behaviour indicates that evaporation is the dominant process that enriches deuterium and oxygen-18 isotopes in the groundwater.
The interaction values for the GEL for the groundwater with the LMWL for the rainfall are about − 14.5 and − 3.75‰ for δ2H and δ18O, respectively (Fig. 7). The δ2H and δ18O values in the groundwater indicate that about 17.14% of the samples show values of − 13.68 to − 13.15‰ for δ2H and − 3.89 to − 3.6‰ for δ18O. These values can be considered to be close to the interaction values and close to the LMWL. Lu et al. (2016) indicated that under these conditions, the isotopic composition of groundwater is regulated by the composition of the precipitation source. Ayadi et al. (2016) suggested that for these samples, rapid rainfall infiltration occurs before there is significant evaporation at the soil surface.
Relationship between the GMWL, the LMWL for the rainfall and the GEL for the groundwater
The relationship between the δ2H and δ18O values in the GMWL, the LMWL for the rainfall and the GEL for the groundwater is shown in Fig. 7.
The GEL for the groundwater samples in the study area plots to the right and below the LMWL for the rainfall and to the left and above the GMWL (Fig. 7). This location of the GEL indicates that the δ2H versus δ18O values in the groundwater aquifer are significantly different from values of the rainfall of the LMWL and of the GMWL. This behaviour also indicates that the groundwater can be classified as a young groundwater with a multiple recharge sources besides precipitation (Qian et al. 2013; Ayadi et al. 2016). Dhaoui et al. (2016) and Mapoma et al. (2016) clearly stated that in this case deuterium and oxygen-18 isotopes probably originated from groundwater mixing with rainfall and old residual water recharged from other sources and anthropogenic activities. These recharge sources have different origins, and recharge has taken place over different periods of time (Al-Charideh and Kattaa 2016; Ayadi et al. 2016).
In the study area, the recent recharge is derived through infiltration through the vadose zone. These recharge sources are rainfall, untreated wastewater from the cesspits and irrigation return flow from the agricultural area located east of the study area. The historical time recharge water is derived from saline water intrusion from a deep aquifer and an adjacent aquifer comprised of Eocene sediments located to the east of the Gaza Strip. The isotopic composition of groundwater in the study area is therefore influenced by the mixing of water from these recent and historical recharge sources.
Evidence from deuterium excess (D-excess) values
The D-excess parameters have been previously used to determine the sources of water vapour in the Middle East region (Celle-Jeanton et al. 2001). Calculated D-excess values (Eq. 3) for the rainfall and the groundwater samples are presented in Tables 1 and 2, respectively. The D-excess values for the rainfall range from 7.76 to 25.64‰, with an average value of 16.89‰ and a standard deviation of 5.12.
These D-excess values differ from the D-excess value for global precipitation (GMWL) of 10‰ (Eq. 1). This variation is a consequence of the geographic location and the climatic conditions of the local study area. It is possibly due to either the evaporation of droplets below the clouds or the fact that rainfall originates from moisture of different regions and/or due to exchange processes that occur between moisture and large surfaces water in the ground (Grassa et al. 2006). A second observation that can be made about the D-excess values is that the isotopic composition of rainfall has been significantly affected by evaporation with different climatic conditions, even though there are likely different moisture sources for the rainfall in the area. Evaporation is likely to have modified the δ2H and δ18O values in the precipitation before infiltrated into the groundwater (Pang et al. 2004; Ako 2011). A third observation that can be made is that the average D-excess value of 16.89‰ falls between the western Mediterranean D-excess value of 14‰ (Gat and Carmi 1970) and the Eastern Mediterranean D-excess value of 22‰ (IAEA 2005). This location of the D-excess value is a result of specific climatic conditions in the Mediterranean region that cause an instability of air over the Mediterranean Sea leading to an intensive exchange between moisture in the atmosphere and the seawater surface (Grassa et al. 2006). The average D-excess of 16.89‰ is the same as detected in rainwater falling on the Western Mediterranean region where the study area is located and the rainfall attributed to precipitation from the Mediterranean sea (Hadi et al. 2016; Mokadem et al. 2016).
About 82.53% of the rainfall samples at the Rafah (Egypt) rainfall station have D-excess values more than 10‰ suggesting that rainfall has been affected by evaporation. This evaporation process would have taken place under conditions of low humidity especially in winter season or the rainy season (Gat and Carmi 1970). The results indicate that there is an enrichment of δ2H and δ18O values in the rain clouds from recycled water vapour with contribution of continental surface waters (Fynn et al. 2016).
The calculated D-excess values (Eq. 3) in the samples of the groundwater in the study area vary from 11.56 to 17.77‰ with an average of 14.62‰ and a standard deviation value of 1.3 (Table 3). These D-excess values are higher than the global D-excess for GMWL of 10‰ (Eq. 1). The observed result for the D-excess values in the groundwater indicates the following possibilities: (1) a rapid rainwater infiltration with limited evaporation takes place in the study area (Dhaoui et al. 2016); (2) the groundwater system is recharged from a mixture of water sources from different origins over various time periods (Hadi et al. 2016); and (3) the δ2H and δ18O values of the groundwater are highly affected by mixing with non-meteoric water (recharged water from sources other than rainwater). As mentioned previously, these non-meteoric waters are likely to be wastewater, irrigation return flow and saline water.
The average D-excess values in groundwater samples in the study area of 14.62‰ are less than the average D-excess values for the rainfall of 16.89‰. This result indicates that a mixing process occurs between the non-meteoric water with the groundwater in the aquifer (Abreha 2014).
There is a clear line of best fit with a negative slope between the δ18O and D-excess values in the groundwater (Fig. 8) according to the following equation:
This negative relationship between the δ18O values and D-excess values in the groundwater of the study area indicates that the δ18O values increase (there is an enrichment of oxygen-18) while the D-excess values decrease. This condition suggests that the groundwater has mixed with recharge water of different δ18O and δ2H values from different sources that have undergone a variable degree of evaporation before recharge into the groundwater (Shah 2013; Abreha 2014).
Conclusions
The δ2H, δ18O and deuterium excess (D-excess) values of rainfall and groundwater were used to assess groundwater recharge sources and its dynamics in the urban area of Khan Younis City in the southern the Gaza Strip in Palestine. Three isotopic lines were used for the assessment of the data: the global meteoric water line (GMWL), the local meteoric water line (LMWL) and the groundwater evaporation line (GEL). Correlations between the lines and the δ2H and δ18O values of water samples indicate that: (1) deuterium and oxygen-18 isotopes originated from groundwater mixing with rainfall and old residual water from other sources (such as from anthropogenic activities) and that these water sources are of different origins and have been recharged over a range of different times; (2) the groundwater has been derived from a mixture of local rainfall which has come from an air mass that has carried moisture from a distant source in the Mediterranean Sea to the west of the study area, and non-meteoric water (other than rainwater, such as: wastewater, irrigation return flow and saline water); (3) isotopic exchange has taken place between deuterium and oxygen-18 enriched in the groundwater and the aquifer rock material; (4) the isotopic composition of rainfall has been modified by evaporation before infiltration into the aquifer; and (5) isotopically, the aquifer behaves as a single homogeneous aquifer.
References
Abreha AG (2014) Hydrogeochemical and water quality investigation on irrigation and drinking water supplies in the Mekelle region, northern Ethiopia. Master thesis, University of Twente, Netherlands
Abu Jabal MS, Abustan I, Rozaimy MR, El Najar H (2015) Groundwater beneath the urban area of Khan Younis City, southern Gaza Strip (Palestine): hydrochemistry and water quality. Arab J Geosci 8:2203–2215. https://doi.org/10.1007/s12517-014-1346-6
Abu Jabal MS, Abustan I, Rozaimy MR, El Najar H (2017) Groundwater beneath the urban area of Khan Younis City, southern Gaza Strip (Palestine): assessment for multi-domestic purposes. Arab J Geosci 10:257. https://doi.org/10.1007/s12517-017-3036-7
Adomako D, Osae S, Akiti TT, Faye S, Maloszewski P (2011) Geochemical and isotopic studies of groundwater conditions in the Densu river basin of Ghana. Environ Earth Sci 62:1071–1084. https://doi.org/10.1007/s12665-010-0595-2
Ako AA (2011) Hydrological study on groundwater in the Banana plain and Mount Cameroon area-Cameroon volcanic line (CVL). Ph.D. thesis, Kumamoto University, Japan
Al-Agha MR (2005) Hydrogeochemistry and carbonate saturation model of groundwater, Khanyounis Governorate—Gaza Strip, Palestine’. Environ Geol 47:898–906. https://doi.org/10.1007/s00254-004-1211-0
Al-Charideh A, Kattaa B (2016) Isotope hydrology of deep groundwater in Syria: renewable and non-renewable groundwater and paleoclimate impact. Hydrogeol J 24:79–98. https://doi.org/10.1007/s10040-015-1324-4
Al-Charideh A, Abou-Zakhem B, AL-Charideh A, Abou-Zakhem B (2009) Geochemical and isotopic characterization of groundwater from the Paleogene limestone aquifer of the upper Jezireh, Syria. Environ Earth Sci 59:1065–1078. https://doi.org/10.1007/s12665-009-0098-1
Amiri V, Nakhaei M, Lak R, Kholghi M (2016) Geophysical, isotopic, and hydrogeochemical tools to identify potential impacts on coastal groundwater resources from Urmia hypersaline lake, NW Iran. Envon Sci Pollut Res 23:16738–16760
Ammar SB, Taupin JD, Zouari K, Khouatmia M (2016) Identifying recharge and salinization sources of groundwater in the Oussja Ghar el Melah plain (northeast Tunisia) using geochemical tools and environmental isotopes. Environ Earth Sci 75:606. https://doi.org/10.1007/s12665-016-5431-x
Ayadi R, Zouari K, Saibi H, Trabelsi R, Khanfi H, Itoi R (2016) Determination of the origins and recharge rates of the Sfax aquifer system (southeastern Tunisia) using isotope tracers. Environ Earth Sci 75:636. https://doi.org/10.1007/s12665-016-5445-4
Blasch KW, Bryson JR (2007) Distinguishing sources of ground water recharge by using δ2H and δ18O. Ground Water 45:294–308. https://doi.org/10.1111/j.1745-6584.2006.00289.x
CAMP (2000) Coastal aquifer management program, integrated aquifer management plan (Gaza Strip), Final Report. Metcalf & Eddy, Inc., and Camp Dresser & McKee International Inc., Report submitted to the United State Agency for International Development (USAID) mission to Gaza and West Bank, in cooperation with the Palestinian Water Authority (PWA)
Celle-Jeanton H, Travi Y, Blavoux B (2001) Isotopic typology of the precipitation in the western Mediterranean region at three different time scales. Geophys Res Lett 28:1215–1218
Chen L, Ma T, Du Y, Xiao C, Chen X, Liu C, Wang Y (2016) Hydrochemical and isotopic (2H, 18O and 37Cl) constraints on evolution of geothermal water in coastal plain of Southwestern Guangdong Province, China. J Volcanol Geotherm Res 318:45–54. https://doi.org/10.1016/j.jvolgeores.2016.03.003
Chiogna G, Santoni E, Caminb F, Tononb A, Majone B, Trenti A, Bellin A (2014) Stable isotope characterization of the Vermigliana catchment. J Hydrol 509:295–305. https://doi.org/10.1016/j.jhydrol.2013.11.052
Coplen TB, Wildman JD, Chen J (1991) Improvements in the gaseous hydrogen–water equilibration technique for hydrogen isotope ratio analysis. Anal Chem 63:910–912
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Dhaoui Z, Zouari K, Taupin JD, Farouni R (2016) Hydrochemical and isotopic investigations as indicators of recharge processes of the Continental Intercalaire aquifer (eastern piedmont of Dahar, southern Tunisia). Environ Earth Sci 75:1186. https://doi.org/10.1007/s12665-016-5990-x
Edirisinghea EANV, Karunarathneb GRR, Samarakoonb ASMNB, Pitawalac HMTGA, Dharmagunawardhanec HA, Tilakarathna IANDP (2016) Assessing causes of quality deterioration of groundwater in Puttalam, Sri Lanka, using isotope and hydrochemical tools. Isot Environ Health Stud 52:513–528. https://doi.org/10.1080/10256016.2015.1127918
Eshtawi T (2015) Integrated hydrologic modeling as a key for sustainable development planning of urban water resources in the semi arid watersheds of the Gaza Strip. Ph.D. thesis, Universität Bonn, Germany
Ferronsky VI, Polyakov VA (2012) Isotopes of the earth’s hydrosphere. Springer, Berlin. https://doi.org/10.1007/978-94-007-2856-1
Fynn OF, Yidana SM, Chegbeleh LP, Yiran GB (2016) Evaluating groundwater recharge processes using stable isotope signatures-the Nabogo catchment of the White Volta, Ghana. Arab J Geosci 9:279. https://doi.org/10.1007/s12517-015-2299-0
Gat JR (1980) The isotopes of hydrogen and oxygen in precipitation. In: Fritz P, Fontes J (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, Netherland, pp 21–47
Gat JR, Carmi H (1970) Evolution of the isotopic composition of atmospheric waters in the Mediterranean sea area. J Geophys Res 75:3039–3040
Gat JR, Dansgaard W (1972) Stable isotope survey of the freshwater occurrence in Palestine and northern Jordan rift valley. J Hydrol 16:177–212
Gomaah M, Meixner T, Korany EA, Garamoon H, Gomaa MA (2016) Identifying the sources and geochemical evolution of groundwater using stable isotopes and hydrogeochemistry in the Quaternary aquifer in the area between Ismailia and el Kassara canals, Northeastern Egypt. Arab J Geosci 9:437. https://doi.org/10.1007/s12517-016-2444-4
Grassa F, Favara R, Valenza M (2006) Moisture source in the Hyblean mountains region (south-eastern Sicily, Italy): evidence from stable isotopes signature. Appl Geochem 21:2082–2095. https://doi.org/10.1016/j.apgeochem.2006.07.014
Hadi K, Kumar US, Al-Senafy M, Bhandary H (2016) Environmental isotope systematics of the groundwater system of southern Kuwait. Environ Earth Sci 75:1096. https://doi.org/10.1007/s12665-016-5886-9
Hamed Y, Dahri F (2013) Hydro-geochemical and isotopic composition of groundwater, with emphasis on sources of salinity, in the aquifer system in northwestern Tunisia. J Afr Earth Sci 83:10–24. https://doi.org/10.1016/j.jafrearsci.2013.02.004
Horita J (1988) Hydrogen isotope analysis of natural waters using an H2–water equilibration method: a special implication to brines. Chem Geol 72:89–94
Horita J, Ueda A, Mizukami K, Takatori I (1989) Automatic δD and δ18O analyses of multi-water samples using H2–and CO2–water equilibration methods with a common equilibration set-up. Appl Radiat Isot 40:801–805
IAEA (2005) Isotopic composition of precipitation in the Mediterranean basin in relation to air circulation patterns and climate. Final report of coordinated research project 2000–2004. International Atomic Energy Agency (IAEA), Vienna, Austria
IAEA (2010) Sampling procedures for isotope hydrology. Booklet by the Water Resources Program. International Atomic Energy Agency (IAEA), Vienna
Isawi H, El-Sayed MH, Eissa M, Shouakar-Stash O, Shawky H, Abdel Mottaleb MS (2016) Integrated geochemistry, isotopes, and geostatistical techniques to investigate groundwater sources and salinization origin in the Sharm El-Shiekh area, south Sinai, Egypt. Water Air Soil Pollut 227:151. https://doi.org/10.1007/s11270-016-2848-5
Jilali A, Fagel N, Amar M, Abbas M, Zarhloule Y (2016) Hydrogeochemical processes constrained by multivariate statistical methods and isotopic evidence of groundwater recharge in the aquifer of Figuig, eastern high atlas of Morocco. Arab J Geosci 9:42. https://doi.org/10.1007/s12517-015-2089-8
Krishnaraj S, Murugesan V, Vijayaraghavan K, Sabarathinam C, Paluchamy A, Ramachandran M (2011) Use of hydrochemistry and stable isotopes as tools for groundwater evolution and contamination investigations. Geosciences 1:16–25. https://doi.org/10.5923/j.geo.20110101.02
Leontiadis L, Vergis S, Christodoulou T (1996) Isotope hydrology study of areas in eastern Macedonia and Thrace, northern Greece. J Hydrol 182:1–17
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger desert, northwest China. Expo Health 8:331–348. https://doi.org/10.1007/s12403-016-0193-y
Lu Y, Tang C, Chen J, Yao H (2016) Assessment of major ions and heavy metals in groundwater: a case study from Guangzhou and Zhuhai of the Pearl river delta, China. Front Earth Sci 10:340–351. https://doi.org/10.1007/s11707-015-0513-8
Mapoma HWT, Xie X, Zhu Y, Liu Y, Sitolo-Banda GC (2016) Trace element geochemical evolution and groundwater origin in North Rukuru–Songwe alluvial aquifer of northern Malawi. Environ Earth Sci 75:877. https://doi.org/10.1007/s12665-016-5682-6
Mazor E (2004) Chemical and isotopic groundwater hydrology, 3rd edn. Marcel Dekker, Inc., New York
Mokadem N, Demdoum A, Hamed Y, Bouri S, Hadji R, Boyce A, Laouar R, Saad A (2016) Hydrogeochemical and stable isotope data of groundwater of a multi-aquifer system: northern Gafsa basin-central Tunisia. J Afr Earth Sci 114:174–191. https://doi.org/10.1016/j.jafrearsci.2015.11.010
Mook WG (2001) Environmental isotopes in the hydrological cycle, principles and applications, vol 1. International Atomic Energy Agency (IAEA) and United Nations Educational, Scientific and Cultural Organization (UNESCO), Vienna
Pang H, He Y, Zhang Z, Lu A, Gu J (2004) The origin of summer monsoon rainfall at New Delhi by deuterium excess. Hydrol Earth Syst Sci 8:115–118
PCBS (2012) Estimated population in the Palestinian territory mid-year in Khan Younis governorate. Palestinian Central Bureau of Statistics (PCBS), Ramallah, Palestine. http://www.pcbs.gov.ps/Portals/_Rainbow/Documents/khana.htm. Accessed 23 April 2017
Peng TR, Huang CC, Zhan WJ, Wang CH (2016) Assessing groundwater sources and their association with reservoir water using stable hydrogen and oxygen isotopes: a case study of the Taipei basin, northern Taiwan. Environ Earth Sci 75:753. https://doi.org/10.1007/s12665-016-5544-2
Qian H, Li P, Wu J, Zhou Y (2013) Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan plain, northwest China. Environ Earth Sci 70:57–70. https://doi.org/10.1007/s12665-012-2103-3
Rozanski K, Araguas-Araguas L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. Geophys Monogr 78:1–36
Sakai H, Matsubaya O (1977) Stable isotopic studies of Japanese geothermal system. Geothermics 5:97–124
Sanchez D, Barbera JA, Mudarra M, Andreo B (2015) Hydrogeochemical tools applied to the study of carbonate aquifers: examples from some karst systems of Southern Spain. Environ Earth Sci 74:199–215. https://doi.org/10.1007/s12665-015-4307-9
Shah ZA (2013) A qualitative and quantitative study of chemical and isotopic parameters of the groundwater system in parts of Unnao district, Uttar Pradesh, India. Ph.D. thesis, Aligarh Muslim University, India
Shomar BH, Muller G, Yahya A (2005) Geochemical features of topsoils in the Gaza Strip: natural occurrence and anthropogenic inputs. Environ Res 98:372–382. https://doi.org/10.1016/j.envres.2004.10.008
Tiwari SK, Bartarya SK, Rai SK, Gupta AK, Asthana AKL (2016) Isotopic and geochemical studies of groundwater from the Ramganga basin and the middle Ganga Plains: implication for pollution and metal contamination. Environ Earth Sci 75:1170. https://doi.org/10.1007/s12665-016-5971-0
UNEP (2009) Environmental assessment of the Gaza Strip following the escalation of hostilities in December 2008–January 2009. Report by the United Nations Environmental Program (UNEP), Nairobi, Kenya
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV, Ganesh BP (2012) To Understand the characteristics of stable isotopes and trace element in groundwater of Thirumanimuttar Sub-Basin, Tamil Nadu, India. Carp J Earth Environ Sci 7:89–100
Acknowledgements
The authors would like to thank His Excellency Ambassador Salman Al Harfi, former Ambassador for State of Palestine to the Republic of Tunisia, for his recommendation and support to the Arab Atomic Energy Agency in Tunisia to finance the isotopic analysis and also thank to the Arab Atomic Energy Agency in Tunisia, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu Jabal, M.S., Abustan, I., Rozaimy, M.R. et al. The deuterium and oxygen-18 isotopic composition of the groundwater in Khan Younis City, southern Gaza Strip (Palestine). Environ Earth Sci 77, 155 (2018). https://doi.org/10.1007/s12665-018-7335-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7335-4