Abstract
It is important to have qualitative as well as quantitative understanding of the hydraulic exchange between lake and groundwater for effective water resource management. Dal, a famous urban fresh water lake, plays a fundamental role in social, cultural and economic dynamics of the Kashmir Valley. In this paper geochemical, isotopic and hydrological mass balance approaches are used to constrain the lake water–groundwater interaction of Dal Lake and to identify the sources of lake water. Water samples of precipitation (n = 27), lake water (n = 18) and groundwater (n = 32) were collected across the lake and its catchment for the analysis of δ18O and δ2H. A total of 444 lake water samples and 440 groundwater samples (springs, tube wells and dug wells) were collected for the analysis of Ca2+, Mg2+, HCO3 −, SO4 2−, Cl−, NO3 −, Na+ and K+. Water table and lake water level were monitored at 40 observation locations in the catchment. Water table map including pH and EC values corroborate and verify the gaining nature of the Dal Lake. Stable isotopes of lake water in Boddal and Gagribal basins showed more deviation from the global meteoric water line than Hazratbal and Nigeen basins, indicating the evaporation of lake water. The isotopic and geochemical mass balance suggested that groundwater contributes a significant proportion (23–40%) to Dal Lake. The estimated average groundwater contribution to Dal Lake ranged from 31.2 × 103 to 674 × 103 m3 day−1 with an average of 276 × 103 m3 day−1. The study will be useful to delineate the possible sources of nutrients and pollutants entering the lake and for the management of lake water resources for sustainable development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lakes are major surface fresh water resources (Shiklomanov 1993; Dessie et al. 2015) and are the main sources of meeting the demand for drinking, irrigation, water transport recreation, fishery and industrial purposes (Meybeck 1995; Leonard and Crouzet 1999; Kalff 2001; Shiklomanov and Rodda 2003; Wu et al. 2017). Globally, there are more than 110,000 natural lakes (>1 km2) covering a total area of 2.3 × 106 km2 (Kebede et al. 2006; Chebud and Melesse 2009) and millions of smaller lakes (Meybeck 1995; Finlayson et al. 1999; Kalff 2001). Despite the importance of the lakes, they are facing major problems, which include lowering of water level, siltation, acidification, toxic contamination and eutrophication (Kira 1997; Ballatore and Muhandiki 2002), posing risks to the environment and human health (Li et al. 2016). To cope with such problems and for the management purposes, accurate determination of the water balance of lakes is required.
Groundwater is a life-sustaining resource (Rosenberry et al. 2015) that supplies water to billions of people (Giordano 2009; Sun et al. 2009; Siebert et al. 2010; Gleeson et al. 2012; Li et al. 2013a), plays a central part in recharging the lake basins of the world (Nakayama and Watanabe 2008). It is hydraulically connected to lakes and an understanding of this interaction is fundamental for effective water resource management (Winter et al. 1998; Hayashi and Rosenberry 2002; Brodie et al. 2007; Cook et al. 2001; Owor et al. 2011). Interaction between them influences the characteristics of lake water, including the stability of water level and water quality (Winter et al. 1998; Barr et al. 2000; Schneider et al. 2005). These interactions are complex both in time and space, and are not only influenced by climate, landform, geology, and biotic factors but also by fabricated human activities (Kenoyer and Anderson 1989; Shaw and Prepas 1990; Sebestyen and Schneider 2001).
Environmental isotopes are naturally occurring tracers (Behrens et al. 1971; Martinec et al. 1974; Rodhe 1981; Obradovic and Sklash 1986; Maule and Stein 1990; Maurya et al. 2011; Cable et al. 2011; Wassenaar et al. 2011; Jeelani et al. 2013; Qian et al. 2013) that provide quantitative information about lake inflow components (groundwater, surface water, and precipitation) (Maloszewski and Zuber 1982, 1983; Mattle et al. 2001; Qian et al. 2014). Since each component of a lake’s water budget acquires a different isotopic signature (Bennett et al. 2008; Gibson et al. 2010a, b), well-mixed lakes act as “mixing bowls” (Gibson and Edwards 2002; Turner et al. 2010; Brooks et al. 2014; Gibson et al. 2015) and reflect the net influence of all the components. The isotopic signatures of lake water which comprises the mixture of recharge of these accordingly attribute the particular isotopic signature (Dawidek and Ferencz 2014; Kummu et al. 2014). Due to the enrichment of δ18O and δ2H in lake water that undergoes evaporation (Gonfiantini 1986; Gibson et al. 2002; Gibson and Edwards 2002), the resulting isotopic signature is different from that of ground water and provides an ideal conservative tracer for evaluating the extent of mixing of surface water and groundwater (Gonfiantini 1986; Shivanna et al. 2004; Jeelani et al. 2010). The light isotopic species of water (δ16O) has a higher vapor pressure and diffusivity than the isotopically heavy species (δ18O and δD) and is preferentially removed from a lake during evaporation (Gibson and Edwards 2002; Dawidek and Ferencz 2014; Kummu et al. 2014). The lake thereby becomes enriched in the heavy isotopic species of water. The degree of enrichment depends on climatic conditions, including average annual temperature, relative humidity, and precipitation and evaporation rates. Using these isotopes (δ18O or δ2H) isotope-balance approaches (Coplen, 2011; Sacks et al. 2014; Jeelani et al. 2015) have been used in numerous hydrological studies to quantify groundwater inflow to lakes (Turner et al. 1984; Krabbenhoft et al. 1990; LaBaugh et al. 1997).
Hydrogeochemical interaction in various settings is a well-recognized method used into trace the source, for assessing the sensitivity of lakes, seepage estimation and destination of surface water, groundwater and lake water. Physical parameters (pH, EC, and TDS) and major elements (Ca2+, Mg2+, Cl− and HCO3 −) (Zimmerman and Ehhalt 1970; Bentley et al. 1986; LaBaugh et al. 1995, 1997; Bocanegra et al. 2012; Goldhaber et al. 2014; Li et al. 2013b) can be used to trace the flow of groundwater (Allison and Hughes 1978). Groundwater discharge zones can be located by searching for anomalies in pH, electrical conductivity (EC) and total dissolved solids (TDS) values. Most conservative element chloride has been used to check water budgets by the mass balance method (e.g., Balleau 1973; Allison and Hughes 1978; Allison 1988; Allison et al. 1994; Bajjali 2006; Jeelani 2008). Eriksson and Khunakasem 1969 first proposed that chloride concentration in ground water to estimate a recharge flux in aquifers. Understanding the geochemical mass balance of lake (pH, EC, TDS, Ca2+, Mg2+, Cl− and HCO3 −) and their interactions with groundwater is important (1) for water resource management (Showstack 2004) (2) for understanding nutrient balances (Ala-aho et al. 2013; Kidmose et al. 2013) and (3) for quantifying the vulnerability of lakes to pollution (Klove et al. 2011). Lake–groundwater interactions are nevertheless complex, and transport of these solutes is highly variable, both spatially and temporally (Winter et al. 1998). It depends on contribution of subsurface water (groundwater), aquifer geology, topography, and meteorological conditions (Rosenberry and LaBaugh 2008).
Dal Lake has tremendous societal and economic significance in Kashmir Valley. However, it is continuously degraded with the increasing population and anthropogenic pressure. Tourism has also developed rapidly leading to the increasing number of hotels, houseboats in and around the lake. It is important to have both a qualitative and quantitative understanding of lake–groundwater interaction to support the development of effective management plans for sustainable use of water resources. Despite the limited work on water quality of the lake, no study has yet identified the sources of water and the contribution of these sources into the lake. The objectives of the present work are (1) to characterize the geochemical and isotopic signatures in lake water, streams and groundwater, (2) to constrain the different sources of the lake water (3) to assess the connection between groundwater and lake water and (4) to gain a comprehensive understanding of hydrological processes in the Dal Lake.
Study area
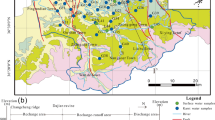
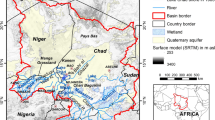
Dal, the second largest lake in the Jammu and Kashmir state (Fig. 1), lies between latitudes 34°04′ and 34°11′N and longitudes 74°48′ and 74°53′E in the northeast township of Srinagar city. The lake has a total open water spread area (water surface area) of 12 km2 (excluding areas covered by floating gardens). Some of the morphometric characteristics/hydrological parameters of the Dal Lake are summarized in Table 1. Total area of catchment is about 314 km2, and Teilbal–Dachigam sub-catchment contributes 70% areal distribution of drainage catchment (Jeelani and Shah 2006; Saleem et al. 2015). Panjal Traps (basalts, andesites), Triassic Limestone (limestone, sandstone, dolomite, shale), Agglomeratic Slates (pyroclastic slates, conglomerates, quartzite), Karewas (sand, clay, silt and loess) and Recent Alluvium are the dominant geological formations (Fig. 1) in the lake catchment, as shown in geological map (Thakur and Rawat 1992 and Jeelani and Shah 2006). The lake basin is surrounded mostly by alluvium and Karewas, which form good aquifers with moderate yields (CGWB 2013). Water table depth in the alluvial aquifers in summer ranges from 1.5 to 4 m bgl (below ground level), while in winter it increases and varies from 1.7 to 6 m bgl (CGWB 2013). Toward the eastern side of the lake, the alluvium is in contact with the Zabarwan Range, which consists mostly of Panjal Traps and Triassic Limestone. It is a multi-basin lake, which is divided into four basins; Hazratbal, Boddal, Gagribal and Nigeen basins. The average volume of water estimated in Hazratbal was 35 × 106 m3, Boddal was 18.5 × 106 m3, Gagribal was 13.7 × 106 m3, and Nigeen was 3.3 × 106 m3. Perennial streams are found only in the north and eastern side of the lake including Teilbal main stream, Habbak, Shalimar, Nishat, and Brain streams (Saleem 2015). There are two outlets from the lake, namely the Dalgate and Amir Khan nallah and former is controlled by a weir and lock system. Total annual average outflow from these two outlets has been estimated as 346 × 106 m3 a−1. The drainage pattern in the study area is sub-dendritic patterns with the general flow direction being from east to south west (Fig. 1). The total annual precipitation and average temperature recorded by meteorological station at Shalimar meteorological station (34°8′58″N and 74°52′54″E) in 2015 was 1753 mm and 12.7 °C, respectively. The climate of the area is Sub-Mediterranean type (Bagnolus and Meher-Homji 1959) dominated by the Western disturbances in winter and ISM (Indian summer monsoon) in summer (Jeelani et al. 2017).
Materials and methods
Water sampling
For major ion chemistry, lake water (n = 444), groundwater (n = 480) and stream samples (n = 36) were collected in high density polyethylene (HDPE) bottles during 2015 from representative sites (Fig. 2) on monthly basis. Groundwater samples were collected from open dug wells (n = 36), closed tube wells (n = 372) and springs (n = 108) located nearby the lake, and its catchment. Stream water samples were also collected from inflow streams (Teilbal, Habbak, Shalimar, Nishat, and Brain) before the confluence with lake water. Water samples were collected in 125 ml HDPE bottles from precipitation (n = 27), lake water (n = 18) and groundwater (n = 32) for δ18O and δ2H analysis. Precipitation samples were collected in homemade precipitation collectors, fitted at many selected sites across the study area. Each collector consisted of a 10-L plastic container fitted with a funnel and a long narrow tube to avoid evaporation.
Analytical techniques
Physical parameters like, pH, total dissolved solids (TDS) and electrical conductivity (EC) were measured in field (in situ) using portable HACH+ (HQD Sension+). Standard methods were adopted for determination of major ions in water samples (APHA 2005). Ca2+, Mg2+, Na+ and K+ were measured by atomic absorption spectrophotometer. HCO3 − and Cl− were analyzed by acid–base and AgNO3 titration, respectively. SO4 2− and NO3 − were determined by colorimetric method (HACH DR/890). Stable water isotope ratios hydrogen (δ2H) and oxygen (δ18O) were analyzed at Physical Research Laboratory (PRL), Ahmedabad, by Dual Inlet Isotope Ratio Mass Spectrometer through gas equilibration method (Revesz and Coplen 2006). Isotope results are reported in the conventional delta notation (δ) (Coplen 1996), as given by the following equation:
where R sample represents the isotope ratio of the sample (2H/1H or 18O/16O) and R standard represents the corresponding ratio of the standard, VSMOW). The δ values are expressed in parts per thousand (permil, ‰). The precision of measurement for δ2H is ±1‰ and that of δ18O is ±0.1‰.
Water level measurement
Water table (WT) is hidden beneath the land surface; it can be directly observed through monitoring wells. It was monitored/observed on seasonal basis (2015) at forty (40) observation sites including; dug wells, tube wells, and springs (Fig. 2). It was recorded with a hand-held sensor capable of accuracy to about 0.1 inch, whereas elevation of ground surface was measured with the help of GPS (Trimble Juno). Besides, the water level of the lake was also measured simultaneously nearer to these observation locations along the periphery of the lake. The recorded data was later interpolated in Arc-GIS 10.2 software using geostatistical analyst tool for contour maps.
Isotopic mass balance
Isotopic mass balance (IMB) equation provided by Gonfiantini (1965), Dincer (1968), Krabbenhoft et al. (1990), and subsequently by Gibson et al. (2002), Gibson and Reid (2010) and Sacks et al. (2014) were simplified and used. The Dal Lake, complex dynamic system is connected to different components of hydrological cycle, through evaporation, precipitation, groundwater, surface inflows and outflow. The generalized mass balance equation for unsteady lakes is:
where G i and S i are the groundwater and surface water inflow rates, respectively, G o and S o are the groundwater and surface water outflow rates, respectively, P and E are the precipitation and evaporation rates, respectively, v is the volume of water in the lake, and t is time. The annual isotope mass balance equation for a well-mixed lake may be written as:
where each water budget elements (term) in (Eq. 2) has been multiplied by its respective isotopic composition expressed in delta notation (δ) to obtain the isotopic mass balance equation in order to separate and quantify groundwater inflow.
By assuming that the Dal Lake is isotopically well mixed (i.e., δS o = δL = δG o) can be simplified to the following equation:
where δ values in ‰ represent the composition of δ18O and δ2H for all components of the lake system; ∆ V is change in lake volume with the isotopic composition of the lake δ L; S i and G i are the inflow of surface water and groundwater, with their isotopic compositions δS i and δG i, respectively; P is precipitation, with its isotopic signature δ P; S o and G o are the outflow of surface water and groundwater with their isotopic compositions δS o and δG o, respectively; and E is evaporation, with the isotopic signature of evaporative flux δ E. In the present study, seasonal weighted values of δP and δE are used. Seasonal δP and δE values are multiplied by seasonal percentage of total precipitation and evaporation, respectively.
The linear resistance model given by Craig and Gordon (1965) was used to determine the isotopic composition of evaporated water from lake. The equation from Horita and Wesolowski (1994) and Clark and Fritz (1997) was used to solve for the isotopic value for evaporation.
where δ L is the isotopic value for the lake, δ E Isotopic value for evaporated water vapor, ε l–v is fractionation factor for equilibrium, liquid–water vapor exchange, Δε bl–v is the kinetic fractionation factor for evaporation. Following equation (Eq. 6) was used to solve the Δε bl–v for oxygen and hydrogen isotopes (Skrzypek et al. 2015)
where h is the relative humidity.
Meteorological data
Daily data of air temperature, precipitation, vapor pressure, humidity and pan evaporation were procured from the meteorological station in Shalimar (latitude: 34°8′58″N, longitude: 74°52′54″ and elevation: 1608 m amsl) for the year 2015, which is just about 2 km in east side of the lake. Monthly values for atmospheric precipitation were multiplied by the lake surface area on the monthly precipitation occurred to determine the monthly volume of precipitation input to the lake (Lancaster 2013).
Evaporation from the lake surface was estimated from pan evaporation using conversion coefficient (Dunne and Leopold 1978; Qu 1994; Skrzypek et al. 2015):
where L E is evaporation from Dal lake, E pan is evaporation from pan (data from Shalimar meteorological station), k is the conversion coefficient (0.59).
Chloride mass balance
Chloride mass balance (CMB) is well-recognized and widely used method to trace recharge sources and to estimate their contributing fractions to a given system. In this study, the groundwater mass balance technique given by Eriksson and Khunakasem (1969) was revisited and applied.
where Q GW = groundwater recharge flux (mm season−1); ClGW = chloride concentration in ground water (mg L−1); P = precipitation (mm season−1); and Clwp = weighted average chloride concentration in precipitation (mg L−1). The weighted average chloride in precipitation was calculated using the following equation:
where ClPi is chloride in precipitation (mg L−1) of the ith sample; P is precipitation (mm) of the ith sample; and n is the number of samples.
Results
Water table (WT) and lake water level (LwL) followed a natural cyclic pattern of seasonal fluctuations. The WT elevation (hydraulic head) in alluvium (on-shore) varied from 1587 to 1590 m amsl, while the LwL varied from 1583 to 1585 m amsl. Higher LwL was observed in summer, followed by declining LwL in autumn and low LwL in winter. Lowest hydraulic head was found at Dalgate, Sadribal, Saidakadal, Rainawari, Khanyar and Nigeen sites and highest at Harwan, Shalimar and Nishat sites. WT varied from 1 to 2 m amsl during the summer season and 0.5–1 m amsl during the winter season.
Water samples from lake, groundwater and streams in the Dal catchment showed distinct chemical variations as summarized in Table 2. pH of groundwater was found to be lower than that of the lake water. It ranged from 7.2 to 7.7 with an average of 7.4 in groundwater and 7.2–8.7 with an average of 8.3 in lake water. Electrical conductivity (EC) of the groundwater was 1–3 times higher than the lake water. It ranged from 177 to 1344 µs cm−1 with an average of 601 µs cm−1 in groundwater and 175–287 µs cm−1 with an average of 218 µs cm−1 in the lake water. The overall trend of concentration of major ions was found to be similar in lake water, groundwater and streams with dominant cation Ca2+ followed by Mg2+ and Na+, and dominant anion HCO3 − followed by SO4 2− and Cl−. The concentration of Ca2+ and Mg2+ varied from 24.4 to 43 and 1.6 to 3.5 mg L−1 in lake water, 9.6 to 120.2 and 6.7 to 81.6 mg L−1 in stream water, 9.6–192.3 and 8.6–86.3 mg L−1 in groundwater, respectively. The concentration of Cl− was lower in streams (2.5–4.9 mg L−1) than the lake water (2.8–17 mg L−1) and groundwater (2.8–38.3 mg L−1). The precipitation weighted average chloride (Clwp) concentration of precipitation samples ranged from 2 to 6 mg L−1.
The summary of stable isotopic composition of streams, precipitation, groundwater and lake water is presented in Table 3. The isotopic composition of lake water ranged from −8.9 to −4‰ with an average of −6.7 for δ18O and from −52 to −30‰ with an average of −41 for δ2H (Table 3). Groundwater δ18O ranged from −8.6 to −4.7‰, with an average of −7.9‰ and δ2H ranged from −29.9 to −53.7‰ with an average of −45.5‰. The δ18O and δ2H values in streams ranged from −8.9 to −6 with an average of −7.8 and −52.4 to −25.4 with an average of −44.7, respectively. The isotopic composition of precipitation ranged from −11.9 to −1.6 with an average of −6.8 for δ18O and δ2H ranged from −81.7 to 6 with an average of −37.8. The groundwater was found to be highly depleted in heavier isotopes, while the lake water was enriched in heavier isotopes.
Discussion
Variation in groundwater and lake water level
Throughout the year, WT was found to be higher than the adjacent LwL across the shoreline, irrespective of the season. When the hydraulic head of the WT is higher than elevation (head) of LwL, the groundwater flows into the lake (higher to lower head). From the hydrological data (Fig. 3), it clearly excluded that the groundwater is fed by the lake water. Water level contour map also corroborates and supports that the lake basin is effluent in nature (Fig. 3). The higher hydraulic gradient of WT was found toward northeast (NE) and northwest (NW) side, which indicates maximum flux of groundwater into the lake. Lowest flux in east (E) and southeast (SE) sides is attributed to the occurrence of volcanic rocks (Panjal Traps). It is pertinent to mention here that the Panjal Traps are massive volcanic rocks with lesser fracture density. The lesser hydraulic gradient in western side of the lake is found due to the clay-rich alluvium with lower hydraulic conductivity and permeability.
The difference between the WT and adjacent LwL of water level is higher (1.5 m) toward NE, NW and E than SE, SW and W also indicated the higher flux of groundwater toward these sides. Seasonally the difference is higher (2 m) in summer and lower (1 m) in winter reflecting the higher groundwater flux into the lake during summer. The observed WT fluctuations have been mainly caused by seasonal variations in weather with differential recharge from rainfall and snow/ice melt.
However, many factors influence LwL, including rainfall, snow/ice melt, evaporation and water consumption. Drawdown of LwL was also influenced by the amount and timing of groundwater and surface water inputs to the streams feeding the lake, which are in turn controlled by different hydrological processes in the catchment.
Hydrogeochemical approach
pH values of groundwater varies in a narrow range, while the pH of lake water varies widely. The wide range in pH of lake water is due to the diel and seasonal changes in photosynthetic rate in aquatic plants (Williams 1998; Saleem et al. 2015). Lowest EC in groundwater was recoded at high altitude springs and tube wells (Harwan, Dara, Brain, and Chesmashahi), and higher was found in Habbak, Batpora, Shalimar, Nehru Park and Nishat areas. From Fig. 4, it is clear that the EC showed a general decreasing trend from all sides of the lake toward the lake interior. From large discrepancies in EC between lake and groundwater and decreasing trend with distance from the shoreline, it can be excluded that the groundwater is fed by lake water. pH and EC values also corroborate and support the results from the LwL–WT contour maps that the Dal Lake is effluent in nature.
Major ions of lake water were compared with groundwater samples, to see the contribution of groundwater to lake using geochemical mass balance (GMB) approach. pH, EC, TDS, Ca2+, Mg2+, Cl−, and HCO3 − were used for GMB respect to different zones (sides) of the lake [i.e., northeast (NE), northwest (NW), east (E), west (W), southeast (SE) and southwest (SW) sides] (Fig. 4). NO3 − and SO4 2− did not exhibit substantial differences, and their sources in the lake are from anthropogenic activities so that they were not used for the GMB method. Groundwater showed higher values of Ca2+ (>185%), HCO3 − (>158%), Mg2+ (>1300%), Cl− (>181%), Na+ (>400%) and K+ (>400%) than lake and stream water. Higher concentrations of chemical constituents in groundwater suggest the carbonate and silicate weathering (Jeelani and Shah 2006; Saleem et al. 2015). These results show that there appears to be good relationship in groundwater with the water quality of the lake. However, the groundwater samples showed no evidence of elevated SO4 2− and NO3 − concentrations than lake water. This suggests that groundwater is not the primary source of SO4 2− and NO3 − to the lake; rather, they may primarily come from anthropogenic sources. From Piper trilinear (Fig. 5) and Stiff diagrams (Fig. 6), two hydrochemical water types were identified in lake water: Ca2+–Mg2+–HCO3 − type and mixed type, whereas only one water type Ca2+–Mg2+–HCO3 − was found in the groundwater and streams. This clearly indicates that the contribution of lake water to groundwater is negligible and there is a strong hydraulic connection between the streams and groundwater.
Based on GMB, the zone (side) wise relative proportion of major ions Fig.7 revealed that groundwater contributes 19–58% to the lake (Figs. 7, 8 and 9). The higher contribution of groundwater was found from NW (58%) and E (56%) sides. Lowest contribution was found from SE (20%) and W (37%) sides of the lake (Fig. 8). EC and WT contour maps also corroborate and support the higher fraction of groundwater from NW and E sides of the lake.
In other words, the overall annual contribution of groundwater in eastern and western side of the lake was 18 and 21%, respectively (Fig. 10). The contribution of streams in eastern and western side of the lake was estimated to be 19 and 17%, respectively. Higher contribution of groundwater in western side is because of lesser streams along that side. Rest of the contribution in eastern (13%) and western side (11%) of the lake was found from other sources including: (1) outflows from sewage treatment plants, (2) small drains, (3) sullage from hotels/houses, and (4) waste water from the houseboats present within the lake basin. Teilbal stream was found to have a distinct effect on the chemical quality of Hazratbal basin on northern side and contributes 100% of water in northern side. However, it does not contribute much to the water quality in other sides (southern) of the lake basin. The contribution of other streams (Shalimar, Nishat, Brain) is very less compared to the main stream.
Chloride mass balance
The results indicate a range of recharge values to groundwater that varies between 18.5 and 70 mm/season. Maximum recharge (70 mm) was found in spring season, when the total rainfall was recorded as 230 mm (Fig. 11), while minimum recharge (41 mm) was estimated in winter, when the precipitation was higher. The groundwater recharge is influenced by the melting of accumulated winter snow which is higher during winter. In summer, the catchment of the lake the extent of snow packs decreases significantly. The same is reflected in the recharge estimates. The groundwater recharge in summer (35 mm) was higher than in autumn season (18 mm). Based on the mean annual values recharge to the groundwater is probably 165 mm (10% of the annual rainfall), excluding recharge from additional water.
Isotopic approach
δ18O versus δ2H plot (Fig. 12a) of surface water, lake water and groundwater from the Dal catchment clearly shows that the samples from groundwater and streams have similar isotopic values than the lake water. Groundwater and stream water samples were characterized by lighter isotopes, with higher slope (6.8 and 8.5) and intercept (8 and 21) than the local meteoric water line (LMWL). On the other hand, the isotopic values of lake water were relatively enriched in the heavy isotopes, with lower slope (3.9) and intercept (15) than LMWL. This suggested that the isotopic composition of lake water is modified by evaporation, while the isotopic composition of the stream and groundwater has retained their original signature. The degree of evaporation is also reflected in the isotopic composition of different basins (Fig.12b) of the lake: Hazratbal basin (δ18O = −8.2), Boddal basin (δ18O = −6.8), Nigeen basin (δ18O = −6.9) and Gagribal basin (δ18O = −5.3). The lower isotopic values in Hazratbal basin reflects the dominant influence of with the main inflow stream (Telbal nala), whereas the higher isotopic value in Gagribal basin is attributed to the evaporation (the last basin with outflow). Samples collected to the west and south east side of the lake (Boddal and Gagribal basins) plot further away from the GMWL suggesting higher evaporation rate due to the stagnation of water. Different evaporation rates/trends in four basins reflect inputs contribution from different sources with different isotopic values. It also suggests that these basins have different residence time of water, which is then reflected in the isotopic composition (Gonfiantini 1986; Gibson et al. 2002). Increase in the residence time of water in the lake makes the water more enriched in δ18O. The most depleted values were during spring in Nigeen basin, and the most isotopically enriched values were observed during the autumn months in Gagribal basin. During spring season, the lake water is heavily influenced by snow melt, so lake water samples plot very closely to the meteoric water line. When lake water is exposed to sunlight and heat during the summer and autumn, lighter isotopes (δ16O and δ1H) are evaporated preferentially (Gibson and Edwards 2002). With fewer light isotopes, the remaining lake water is enriched in δ18O and δ2H, causing isotopic composition of that water to show more positive δ18O and δ2H values.
The percentage of groundwater component that mixes with lake has been computed using IMB equation (Eq. 4) on seasonal basis. Throughout the study, IMB results showed direct relationship between groundwater and lake water. It was found that groundwater is entering into the lake at all locations and there is no contribution of lake water into the groundwater recharge. It was found that groundwater contributes 23–40% to the lake. Maximum contribution was found in winter season (40%) and lower contribution in autumn season (23.5%). Spring and summer contribute 24.7 and 34.8% respectively (Table 4).
Water budget of lake/hydrological budget
The monthly estimated discharge measurements (total inflow and total outflow from streams) were combined with the results from the chemical and isotope mass balance approaches. The results were used to determine daily/monthly contribution of groundwater to the water budget of the lake (Table 4, Fig. 13). All the components were taken into consideration including; stream inflows, precipitation, evaporation, groundwater and outflow except runoff. It was observed that total volume of water in Dal Lake varied substantially in time and space (basin wise). The total volume of water stored in the lake varied from 79.11 × 106 m3 to 56.78 × 106 m3, highest was recorded in the summer months and lowest in winter months. Spatially, Hazratbal basin exhibits more volume than Boddal, Gagribal and Nigeen basins. In Hazratbal basin, it ranged from (28.13 × 106 to 39 × 106 m3), whereas in Boddal, Gagribal and Nigeen basins, the total volume of water varied from (15 × 106 to 20.82 × 106 m3), (10.81 × 106 to 15.45 × 106 m3) and (2.7 × 106 to 3.78 × 106 m3), respectively. Maximum lake volume and water was found in summer months, particularly in June and lower in winter months, especially in January. Precipitation over the catchment ranged from 14.8 to 440 mm month−1 with an annual total of 1753 mm. Spring season contributes ~40% of the total annual precipitation. The results revealed that annual contribution of precipitation to the lake is 6.43 × 105 m3 (5.6%). Evaporation was also found to be one of the main components of the water budget, and it ranged from 0 to 0.44 m3 day−1 with an average of 0.24 m3 day−1. Total 88 m3 of water is lost as evaporation from the lake annually. Similarly, the seasonal groundwater contribution to the lake varied from 31.2 × 103 to 674 × 103 m3 day−1 with an average of 276 × 103 m3 day−1. Groundwater contributes maximum during summer and minimum in winter. It was observed that 112 × 103 to 2389 × 103 m3 of water is discharged daily from the lake through two main outflows (Dalgate and Amirkhan), highest being recorded in May and lowest in December.
Uncertainties
Lake–groundwater interactions have their own uncertainties and, until now, they have very rarely been combined (geochemical, isotopic and hydrological approaches) in a single study as we have done in this work. There are uncertainties at the time of measurement and computation due to human, instruments and shortage of data. Water balance estimates of Dal Lake involved different parameters and variables recorded in meteorological station (Shalimar) and sometimes obtained from equations/manually. Human or instrumental errors are found associated with such estimates. Similarly, estimation of precipitation can have a wide range of error, depending on the gage placement in the catchment. Errors in measurement of individual rainfall events directly on lake can be as high as 7%. Errors in estimates of evaporation from lake can also vary widely depending on instrumentation and methodology. If pans are used that are located a distance from the lake of interest, errors can be considerable. Evaporation estimates for this study may also have more uncertainty because of the lack of direct estimates taken from Dal Lake. Isotopic mass balance is based on several factors, and some of these were neglected in this approach, such as runoff component, leading to considerable uncertainties. The cross section of a stream channels where a discharge measurement is made is an important control on the quality of the measurement. Yet, because selection of the measurement sites was good but there can be considerable variation in measured discharge related to the section of the channel chosen for a discharge measurement. Errors in estimates of stream discharge (inflow and outflow) which was measured using area-velocity method are often considered to be within 10 percent. The measurement errors are in 7% range. Sensitivity analysis has been made to all variables considered in the lake water balance. The isotopic mass balance equation is more sensitive to evaporation and precipitation. 10% change in the evaporation can cause up to 14% increase or decrease in groundwater contribution. Similarly by changing rainfall over the lake by 10% from what has been measured a change up to 17% increase or decrease in groundwater contribution. The more uncertainty and sensitivity errors arise either due to random and coarse observations. It can be reduced by additional (sampling count) and systematic observations, ensuring proper instrument configuration (advanced instrumentation) and postprocessing technique.
Conclusion
This study presents a lake–groundwater investigation to quantify or classify the relative role of groundwater inflow to Dal Lake. Stable isotopes coupled with physical flux measurements were crucial for accomplishing the major objectives. The study concludes that LwL is lower than the adjacent WT, suggesting the effluent nature of the lake. The concentration of major ions in lake remained relatively constant throughout the year and was influenced by groundwater inflows, reflecting its quality and the local geology. WT, EC and GMB values suggested that NE and NW sides of the lake have higher contribution from groundwater. Isotopic composition of δ18O and δ2H indicates that water in the different basins of the lake is significantly influenced by evaporation and shows evaporative enrichment. The groundwater generally shows an average stable isotopic signal of the local precipitation indicating active recharge with annual average of 10%. The total volume of water in lake varied from 79.11 × 106 m3 to 56.78 × 106 m3. Both geochemical and isotopic approaches suggest that groundwater (flux) contributes major component (23–40%) to lake water budget. It was found that the average groundwater inflow to Dal Lake was 32 × 103 m3 day−1 in winter months and 508 × 103 m3 day−1 in summer month with an annual average of 276 × 103 m3 day−1. Uncertainty in precipitation (10%) and evaporation (10%) measurements suggests that groundwater inflow rates may increase or decrease 17 and 14%, respectively.
References
Ala-aho P, Rossi PM, Klove B (2013) Interaction of esker groundwater with headwater lakes and streams. J Hydrol 500:144–156
Allison GB (1988) A review of some of the physical, chemical and isotopic techniques available for estimating groundwater recharge. Estimation of natural groundwater recharge. Springer, Dordrecht, pp 49–72
Allison GB, Hughes MW (1978) The use of environmental chloride and tritium to estimate total recharge to an unconfined aquifer. Soil Res 16(2):181–195
Allison GB, Gee GW, Tyler SW (1994) Vadose-zone techniques for estimating groundwater recharge in arid and semiarid regions. Soil Sci Soc Am J 58(1):6–14
APHA (2005) (American Public Health Association) Standard method for examination of water and wastewater, 21st edn. APHA, AWWA, Washington
Bagnolus F, Meher-Homji VM (1959) Bio-climatic types of south East Asia. Travaux de la Section Scientific at Technique Institut Franscis de Pondicherry. p 227
Bajjali W (2006) Recharge mechanism and hydrochemistry evaluation of groundwater in the Nuaimeh area, Jordan, using environmental isotope techniques. Hydrogeol J 14(1–2):180–191
Ballatore TJ, Muhandiki VS (2002) The case for a world lake vision. Hydrol Process 16:2079–2089. doi:10.1002/hyp.1142
Balleau P (1973) Flow-through of water and chloride ion in a water table aquifer of the Bassendean sands, Perth basin. In: Proceedings of the Institute of Engineers, Australia, Hydrology symposium, vol 73, no 3. National Conference Publication, Perth
Barr AD, Turner JV, Townley LR (2000) WSiBal: a coupled water, conservative solute and environmental isotope mass balance model for lakes and other surface water bodies. IHAS Publ Int Assoc Hydrol Sci 262:539–544
Behrens H, Bergmann H, Moser H, Rauert W, Stichler W, Ambach W, Pessl K (1971) Study of the discharge of Alpine glaciers by means of environmental isotopes and dye tracers. Zeitscherkd Glazialgeo 7:79–102
Bennett KE, Gibson JJ, McEachern P (2008) Water yield estimates for critical loadings assessment: comparisons of gauging methods vs. an isotopic approach. Can J Fish Aquat Sci 65:83–99
Bentley HW, Phillips FM, Fritz SDP, Fontes JC (1986) Handbook of environmental isotope geochemistry, vol 2, 427p
Bocanegra E, Quiroz Londono OM, Martınez DE, Romanelli A (2012) Quantification of the water balance and hydrogeological processes of groundwater–lake interactions in the Pampa Plain, Argentina. Environ Earth Sci 68(8):2347–2357
Brodie R, Sundaram B, Tottenham R, Hostetler S, Ransley T (2007) An overview of tools for assessing groundwater–surface water connectivity. Bureau of Rural Sciences, Canberra
Brooks JR, Gibson JJ, Birks SJ, Weber M, Rodecap K, Stoddard JL (2014) Stable isotope estimates of evaporation: inflow and water residence time for lakes across the United States as a tool for national lake water quality assessments. Limnol Oceanogr 59:2150–2165
Cable J, Ogle K, Williams D (2011) Contribution of glacier meltwater to streamflow in the Wind River Range, Wyoming, inferred via a Bayesian mixing model applied to isotopic measurements. Hydrol Process 25(14):2228–2236
CGWB (2013) Groundwater information booklet of Srinagar district, Jammu & Kashmir, (Central Ground Water Board), Government of India, Ministry of Water Resources
Chebud YA, Melesse AM (2009) Modeling lake stage and water balance of Lake Tana, Ethiopia. Hydrol Process 23:3534–3544. doi:10.1002/hyp.7416
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton
Cook PG, Herczeg A, McEvan KL (2001) Groundwater recharge and stream baseflow, Atherton Tableleands, Queensland. CSIRO Land Water, CSIRO, Clayton South, Australia. Technical Report 08/10
Coplen TB (1996) New Guidelines for reporting stable hydrogen, carbon and oxygen isotope-ratio data. Geochem Cosmochem Acta 60:3359
Coplen TB (2011) Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom 25:2538–2560. doi:10.1002/rcm.5129
Craig H, Gordon LI (1965) Deuterium and oxygen 18 variations in the ocean and the marine atmosphere 277–374
Dawidek J, Ferencz B (2014) Water balance of selected floodplain lake basins in the Middle Bug River valley. Hydrol Earth Syst Sci 18:1457–1465. doi:10.5194/hess-18-1457-2014
Dessie M, Verhoest NE, Pauwels VR, Adgo E, Deckers J, Poesen J, Nyssen J (2015) Water balance of a lake with floodplain buffering: Lake Tana, Blue Nile Basin, Ethiopia. J Hydrol 522:174–186
Dincer T (1968) The use of oxygen 18 and deuterium concentrations in the water balance of lakes. Water Resour Res 4(6):1289–1306
Dunne T, Leopold LB (1978) Water in environmental planning. Macmillan, London
Eriksson E, Khunakasem V (1969) Chloride concentration in groundwater, recharge rate and rate of deposition of chloride in the Israel Coastal Plain. J Hydrol 7(2):178–197
Finlayson CM, Davidson NC, Spiers AG, Stevenson NJ (1999) Global wetland inventory–current status and future priorities. Mar Freshw Res 50(8):717–727
Gibson JJ, Edwards TWD (2002) Regional surface water balance and evaporation transpiration partitioning from a stable isotope survey of lakes in northern Canada. Global Biogeochem Cycles. doi:10.1029/2001GB001839
Gibson JJ, Reid R (2010) Stable isotope fingerprint of open-water evaporation losses and effective drainage area fluctuations in a subarctic shield watershed. J Hydrol 381(1):142–150
Gibson JJ, Prepas EE, McEachern P (2002) Quantitative comparison of lake throughflow, residency, and catchment runoff using stable isotopes: modelling and results from a survey of Boreal lakes. J Hydrol 262:128–144
Gibson JJ, Birks SJ, Jeffries DS, Kumar S, Scott KA, Aherne J, Shaw P (2010a) Site-specific estimates of water yield applied in regional acid sensitivity surveys in western Canada. J. Limnol 69:67–76
Gibson JJ, Birks SJ, McEachern P, Hazewinkel R, Kumar S (2010b) Interannual variations in water yield to lakes in northeastern Alberta: implications for estimating critical loads of acidity. J Limnol 69(Suppl. 1):126–134. doi:10.3274/JL10-69-S1-13
Gibson JJ, Birks SJ, Yi Y, Vitt D (2015) Runoff to boreal lakes linked to land cover, watershed morphology and permafrost melt: a 9-year isotope mass balance assessment. Hydrol Process 29:3848–3861. doi:10.1002/hyp.10502
Giordano M (2009) Global groundwater issues and solutions. Annu Rev Environ Resour 34:153–178
Gleeson T, Wada Y, Bierkens MF, van Beek LP (2012) Water balance of global aquifers revealed by groundwater footprint. Nature 488(7410):197–200
Goldhaber MB, Mills CT, Morrison JM, Stricker CA, Mushet DM, LaBaugh JW (2014) Hydrogeochemistry of prairie pothole region wetlands: role of long-term critical zone processes. Chem Geol 387:170–183
Gonfiantini R (1965) Some results on oxygen isotope stratigraphy in the deep drilling at King Baudouin Station, Antarctica. J Geophys Res 70(8):1815–1819
Gonfiantini R (1986) Environmental isotopes in lake studies. In: Fontes JC, Fritz P (eds) Handbook of environmental isotope geochemistry, vol 2. Elsevier, Amsterdam, pp 113–168
Hayashi M, Rosenberry DO (2002) Effects of ground water exchange on the hydrology and ecology of surface water. Ground Water 40(3):309–316. doi:10.1111/j.1745-6584.2002.tb02659.x
Horita J, Wesolowski DJ (1994) Liquid-vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochim Cosmochim Acta 58:3425–3437
Jeelani G (2008) Aquifer response to regional climate variability in a part of Kashmir Himalaya in India. Hydrogeol J 16(8):1625–1633
Jeelani G, Shah AQ (2006) Geochemical characteristics of water and sediment from the Dal Lake, Kashmir Himalaya: constraints on weathering and anthropogenic activity. Environ Geol 50:12–30
Jeelani G, Bhat NA, Shivanna K (2010) Use of δ18O tracer to identify stream and spring origins of a mountainous catchment: a case study from Liddar watershed, western Himalaya, India. J Hydrol 393(3):257–264
Jeelani G, Kumar US, Kumar B (2013) Variation of δ18O and δD in precipitation and stream waters across the Kashmir Himalaya (India) to distinguish and estimate the seasonal sources of stream flow. J Hydrol 481:157–165
Jeelani G, Kumar US, Bhat NA, Sharma S, Kumar B (2015) Variation of δ18O, δD and 3H in karst springs of south Kashmir, western Himalayas (India). Hydrol Process 29(4):522–530
Jeelani G, Deshpande RD, Shah RA, Hassan W (2017) Influence of southwest monsoons in the Kashmir Valley, western Himalayas. Isot Environ Health Stud. doi:10.1080/10256016.2016.1273224
Kalff J (2001) Limnology inland water ecosystems. Prentice Hall, Upper Saddle River
Kebede S, Travi Y, Alemayehu T, Marc V (2006) Water balance of Lake Tana and its sensitivity to fluctuations in rainfall, Blue Nile basin, Ethiopia. J Hydrol 316(1–4):233–247
Kenoyer GJ, Anderson MP (1989) Groundwater dynamic role in regulating acidity and chemistry in a precipitation-dominated lake. J Hydrol 109:287–306
Kidmose J, Nilsson B, Engesgaard P, Frandsen M, Karan S, Landkildehus F, Jeppesen E (2013) Focused groundwater discharge of phosphorus to a eutrophic seepage lake (Lake Væng, Denmark): implications for lake ecological state and restoration. Hydrogeol J 21(8):1787–1802
Kira T (1997) Survey of the state of world lakes. In: Jorgensen, SE, Matsui S (eds) Guidelines of lake management: the World’s Lakes in Crisis, no. 8. International Lake Environment Committee and United Nations Environment Programme, Kusatsu, Japan
Klove B, Ala-Aho P, Bertrand G, Boukalova Z, Erturk A, Goldscheider N, Lundberg A (2011) Groundwater dependent ecosystems. Part I: hydroecological status and trends. Environ Sci Policy 14(7):770–781
Krabbenhoft DP, Anderson MP, Bowser CJ (1990) Estimating groundwater exchange with lakes: 2. Calibration of a three-dimensional, solute transport model to a stable isotope plume. Water Resour Res 26(10):2455–2462
Kummu M, Tes S, Yin S, Adamson P, Jozsa J, Koponen J, Richey J, Sarkkula J (2014) Water balance analysis for the Tonle Sap Lake-floodplain system. Hydrol Process 28:1722–1733. doi:10.1002/hyp.9718
LaBaugh JW, Rosenberry DO, Winter TC (1995) Groundwater contribution to the water and chemical budgets of Williams Lake, Minnesota, 1980–1991. Can J Fish Aquat Sci 52:754–767
LaBaugh JW, Winter TC, Rosenberry DO, Schuster PF, Reddy MM, Aiken GR (1997) Hydrological and chemical estimates of the water balance of a closed-basin lake in north central Minnesota. Water Resour Res 33(12):2799–2812
Lancaster B (2013) Rainwater harvesting for drylands and beyond: guiding principles to welcome rain into your life and landscape. Rainsource Press, Tucson
Leonard J, Crouzet P (1999) Lakes and reservoirs in the EEA area. Topic report No 1. European Environment Agency, ISBN 92-9167-119-3
Li P, Wu J, Qian H (2013a) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225. doi:10.1007/s12665-012-2049-5
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013b) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32(3):195–206. doi:10.1007/s10230-013-0234-8
Li P, Feng W, Xue C, Tian R, Wang S (2016) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, northwest China. Expo Health. doi:10.1007/s12403-016-0237-3
Maloszewski PW, Zuber A (1982) Determining the turnover time of groundwater systems with the aid of environmental tracers. 1. Models and their applicability. J Hydrol 57:207–231
Maloszewski P, Zuber A (1983) Interpretation of artificial and environmental tracers in fissured rocks with a porous matrix. In: Isotope Hydrology. International atomic energy agency (IAEA), Vienna, pp 635–651
Martinec J, Siegenthaler U, Oeschger H, Tongiorgi E (1974) New insights into the run-off mechanism by environmental isotopes. In: Isotope techniques in groundwater hydrology, I. Proceedings of a symposium
Mattle N, Kinzelbach W, Beyerle U, Huggenberger P, Loosli HH (2001) Exploring an aquifer system by integrating hydraulic, hydrogeologic and environmental tracer data in a three-dimensional hydrodynamic transport model. J Hydrol 242(3):183–196
Maule CP, Stein J (1990) Hydrologic flow path definition and partitioning of spring meltwater. Water Resour Res 26(12):2959–2970
Maurya AS, Shah M, Deshpande RD, Bhardwaj RM, Prasad A, Gupta SK (2011) Hydrograph separation and precipitation source identification using stable water isotopes and conductivity: River Ganga at Himalayan foothills. Hydrol Process 25(10):1521–1530
Meybeck M (1995) Global distribution of lakes. In: Physics and chemistry of lakes. Springer, Berlin, Heidelberg, pp 1–35
Nakayama T, Watanabe M (2008) Missing role of groundwater in water and nutrient cycles in the shallow eutrophic Lake Kasumigaura, Japan. Hydrol Process 22(8):1150–1172
Obradovic MM, Sklash MG (1986) An isotopic and geochemical study of snowmelt runoff in a small arctic watershed. Hydrol Process 1(1):15–30
Owor M, Taylor R, Mukwaya C, Tindimugaya C (2011) Groundwater/surface water interactions on deeply weathered surfaces of low relief: evidence from Lake Victoria and Kyoga, Uganda. Hydrogeol J 19:1403–1420
Qian H, Li P, Wu J, Zhou Y (2013) Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan Plain, Northwest China. Environ Earth Sci 70(1):57–70. doi:10.1007/s12665-012-2103-3
Qian H, Wu J, Zhou Y, Li P (2014) Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan Plain. Hydrol Process 28:3554–3562. doi:10.1002/hyp.9915
Qu YG (1994) Water balance and forecasting of water level change in Qinghai Lake. J Lake Sci 6(4):298–307 (in Chinese with English abstract)
Revesz K, Coplen TB (eds) (2006) Methods of the Reston Stable Isotope Laboratory: U.S. Geological Survey Techniques and Methods Report 10, variously paged
Rodhe A (1981) Spring flood meltwater or groundwater? Hydrol Res 12(1):21–30
Rosenberry DO, LaBaugh JW (2008) Field techniques for estimating water fluxes between surface water and ground water, techniques and methods 4-D2. USGS, Reston
Rosenberry DO, Lewandowski J, Meinikmann K, Nutzmann G (2015) Groundwater-the disregarded component in lake water and nutrient budgets part 1: effects of groundwater on hydrology. Hydrol Process 29(13):2895–2921
Sacks LA, Lee TM, Swancar A (2014) The suitability of a simplified isotope-balance approach to quantify transient groundwater–lake interactions over a decade with climatic extremes. J Hydrol 519:3042–3053
Saleem M (2015) Assessment of chemical quality of Dal Lake, Srinagar, India. M.Phil. Thesis submitted to Department of Earth sciences, University of Kashmir, Hazratbal Srinagar
Saleem M, Jeelani G, Shah RA (2015) Hydrogeochemistry of Dal Lake and the potential for present, future management by using facies, ionic ratios, and statistical analysis. Environ Earth Sci 74(4):3301–3313
Schneider RL, Negley TL, Wafer C (2005) Factors influencing groundwater seepage in a large, mesotrophic lake in New York. J Hydrol 310(1–4):1–16. doi:10.1016/j.jhydrol.2004.09.020
Sebestyen SD, Schneider RL (2001) Dynamic temporal patterns of near shore seepage flux in a headwater Adirondack lake. J Hydrol 247:137–150
Shaw RD, Prepas EE (1990) Groundwater–lake interactions. Accuracy of seepage meter estimates of lake seepage. J Hydrol 199:105–120
Shiklomanov IA (1993) World freshwater resources. In: Gleick PH (ed) Water in crisis: a guide to the world’s fresh water resources. Oxford University Press, New York
Shiklomanov IA, Rodda JC (2003) World water resources at the beginning of the twenty-first century. Cambridge University Press, Cambridge
Shivanna K, Kulkarni UP, Joseph TB, Navada SV (2004) Contribution of storms to groundwater recharge in the semi-arid region of Karnataka, India. Hydrol Process 18:473–485
Showstack R (2004) Stable water usage, but some groundwater concerns, USGS report indicates. Eos Trans Am Geophys Union 85(12):114
Siebert S, Burke J, Faures JM, Frenken K, Hoogeveen J, Doll P, Portmann FT (2010) Groundwater use for irrigation—a global inventory. Hydrol Earth Syst Sci 14(10):1863–1880
Skrzypek G, Mydłowski A, Dogramaci S, Hedley P, Gibson JJ, Grierson PF (2015) Estimation of evaporative loss based on the stable isotope composition of water using hydrocalculator. J Hydrol 523:781–789
Sun R, Jin M, Giordano M, Villholth KG (2009) Urban and rural groundwater use in Zhengzhou, China: challenges in joint management. Hydrogeol J 17(6):1495–1506
Thakur VC, Rawat BS (1992) Geologic map of Western Himalaya: Dehra Dun. India, Wadia Institute of Himalayan Geology, scale, 1(1,000,000)
Turner JV, Allison GB, Holmes JW (1984) The water balance of a small lake using stable isotopes and tritium. J Hydrol 70:199–220. doi:10.1016/0022-1694(84)90122-7
Turner KW, Wolfe BB, Edwards TWD (2010) Characterizing the role of hydrological processes on lake water balances in the Old Crow Flats, Yukon Territory, Canada, using water isotope tracers. J Hydrol 386:103–117
Wassenaar LI, Athanasopoulos P, Hendry MJ (2011) Isotope hydrology of precipitation, surface and ground waters in the Okanagan Valley, British Columbia, Canada. J Hydrol 411(1):37–48
Williams WD (1998) Management of inland saline waters. In: Guidelines of lake management, vol 6, pp 1–108
Winter TC, Harvey JW, Franke OL, Alley WM (1998) Ground water and surface water-a single resource. US Geol Surv Circular 1139
Wu J, Xue C, Tian R, Wang S (2017) Lake water quality assessment: a case study of Shahu Lake in the semi-arid loess area of northwest China. Environ Earth Sci 76:232. doi:10.1007/s12665-015-4208-y
Zimmerman U, Ehhalt D (1970) Stable isotopes in the study of the water balance of Lake Neusiedl, Austria: investigation of the reliability of the stable isotope method. In: Isotope Hydrology, pp 129–138
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saleem, M., Jeelani, G. Geochemical, isotopic and hydrological mass balance approaches to constrain the lake water–groundwater interaction in Dal Lake, Kashmir Valley. Environ Earth Sci 76, 533 (2017). https://doi.org/10.1007/s12665-017-6865-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6865-5