Abstract
The São João Drainage Basin is a very important and strategic waterbody located in the Lagos Region, Rio de Janeiro State, one of the most popular tourist regions of Brazil. However, the fast economic and subsequent population growth in that region has created untreated wastes from several anthropogenic activities, which include the trace metals disposal into the surface waterbody. This study aims to relate the major ions and trace metals behavior during a 1-year sampling campaign and to provide information about the sources of chemical constituents and the factors that control their concentrations in the three main fluvial compartments of the drainage basin, including the Juturnaíba Reservoir which has a crucial role for water supply in the Lagos Region. The chemical data reveal that rainfall is the main factor responsible for the physicochemical parameters and the water dissolved constituent’s variations. The geological and anthropogenic factors which are the main sources of water constituents were assessed by the Inorganic Chemical Index. Those data corroborate the great influence of rainfall and reveal the importance of anthropogenic sources of chemical constituents in some parts of the Juturnaíba Reservoir. The geochemical modeling suggests that hydrolysis reactions are primarily responsible for the trace metals availability in the three fluvial compartments in both dry and wet seasons, followed by carbonate and sulfate complexation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The constant use of freshwater resources and the introduction of toxic materials in aquatic ecosystems require a large number of studies in order to assess and preserve the water quality. Human activities and land use often modify significantly biological, physical and chemical processes in natural waterbodies (Falkenmark 1986; Foster and Charlesworth 1996). Those system modifications can be assessed through changes in water quality that may be detected when rivers receive municipal discharges (mainly sewage and industrial waste) and drainage from agriculture areas (Chapman 1996; Ongley 1998). Pollution of freshwater bodies not only affects the water quality but also poses a threat to public health, economic development and the social prosperity of a region (Pesce and Wunderlin 2000; Rebouças et al. 2002).
Although Brazil possesses 13.7% of world’s freshwater, there are large variations in the availability of water throughout the country due to its large size and this has the potential to affect economic development. For instance, about 73% of surface freshwater is concentrated in Amazon region, which has less than 5% of the country population, whereas only 27% of surface freshwater resources are available in the other Brazilian regions where 95% of the population lives (Lima et al. 1999; Rebouças et al. 2002). Consequently, there is a risk that Brazil could suffer from water shortages in the near future due to the unequal distribution of its water resources.
In some important regions of southeastern Brazil, like São Paulo and Rio de Janeiro Metropolitan Regions, the availability of freshwater has become of significant concern because of the high population demand and because water quality is being influenced by the uncontrolled urbanization. These factors greatly increase the cost of water treatment and can lead to water use restrictions (ANA 2002, 2005).
The quality of freshwater produced in a drainage basin can be influenced by several factors, including the local climate, extent of vegetation cover, topography, geology. However, human land uses are often the most important factor that would affect directly water quality. Drainage basins are highly susceptible to the pollution due to inputs and the transport of industrial, domestic and agricultural waste (UNESCO 1998).
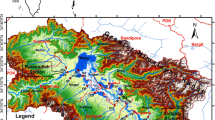
The São João River Drainage Basin has a total area of 2160 km2 and occupies part of 7 municipalities in Rio de Janeiro State: namely Cachoeiras de Macacu, Rio Bonito, Casimiro de Abreu, Araruama, São Pedro d’Aldeia, Cabo Frio, Rio das Ostras and integrally Casimiro de Abreu municipality (Fig. 1). This drainage basin is located in an important region of Rio de Janeiro State, the Lagos Region, considered one of the most important tourism regions of Brazil. It is composed by three main tributaries, namely São João, Bacaxá and Capivari Rivers. These rivers debouch into the Juturnaíba Reservoir, the main water supply source of Lagos Region, and provide freshwater for a population of about 700,000 inhabitants, which can reach 1.5 million people in summer (INEA 2012).
The main economic activity in the region until the nineteenth century was agriculture based on the cattle breeding, sugarcane and orange plantations. More recently, there has been a large expansion in tourism and in the mining of natural aggregates, particularly in sand mining (this is one of the main sand mining districts in Rio de Janeiro State). These activities have led to large population increases in the main municipalities surrounding the São João River. Despite the increased population in the region, about 40% of the area of the basin (63,400 ha) (Brasil 2008) is still covered by forest.
The relatively fast economic and subsequent population growth of the region surrounding the São João River Drainage Basin (in most cases without adequate infrastructure) have created large volumes of untreated wastes including trace metals (heavy metals, Zn, Cu, Pb, Cd, Cr, etc.). These metals could be derived both from natural sources (from rocks, soils and sediments concentrations depending on their regional/local geology background) and from anthropogenic sources (Baird 1998). In aqueous ecosystems, the metals may be in dissolved phase and associated with inorganic and organic complexes as well as in a suspended phase where they may be incorporated and bioaccumulated in aquatic organisms (Forstner and Wittman 1983; Boniforti et al. 1988; Foster and Charlesworth 1996). Dissolved trace metals may be precipitated through adsorption, which is controlled by physicochemical parameters like pH, Eh and electrical conductivity (Carballeira et al. 2000; Arias et al. 2005; Marques et al. 2010, 2012). Because trace metals naturally occur in high content in different rock types, they may become available through weathering products in soil and sediments (Rose et al. 1979; Licht 1998; Silva-Filho et al. 2014).
Thus, this study aims to characterize the dissolved major ions and trace metals behavior along the pluvial regime (dry and wet seasons) from the drainage basin which is the main waterbody and water supply of the Lagos Region. It is noted that human impacts in that region could have a great contribution for dissolved trace metals in the river water. However, the regional geology and the seasonal rains could also have a significant influence on metal concentrations in this water body.
Study area
Since the 1950s, the channel of São João River and some of its tributaries have been extensively modified in order to drain its flood plain (due to the increasing cases of malaria and yellow fever), in order to allow irrigated agriculture to take place and to allow the construction of the Federal Highway BR-101. During the 1970s and 1980s, the channel modifications were resumed in order to allow the Juturnaíba Reservoir to be constructed. As a consequence of this work, the São João River is 52% smaller in extension than its original shape (Cunha 1995). The average flow rate of São João River has been affected by the presence of the Juturnaíba Reservoir and is now 64.4 and 39.3 m3 s−1 upstream and downstream of the reservoir, respectively (INEA 2014). The upper reaches of the São João River are characterized by valleys with steep sides, which extend down the alluvial plain on which the Juturnaíba Reservoir is situated. This reservoir is located in the Silva Jardim municipality and covers an area of about 32 km2 with a maximum depth of 11 m. Before the Juturnaíba Reservoir construction, it had an area of 6 km2 (reaching 8 km2 in rainy season) and a maximum depth of 4 m (INEA 2012). One of the concerns of this newer and larger surface water reservoir is the markedly increase in the aquatic plants infestation, which is seen as a nuisance more than a threat. However, one of the relevant problems that can be occurring with the water hyacinth (Eichhornia crassipes (Mart.) Solms) infestation in the Juturnaíba dam is the increased rate of evapotranspiration (Benton et al. 1978).
The weather in the studied region is wet, with annual average temperatures ranging between 22.1 and 22.9 °C and a rainfall regime characterized by concentrated rains in the wet season (from October to March, reaching 1560 mm in average accumulated rains between 2007 and 2014) and drier weather in the dry season (from April to September, reaching 469 mm in average accumulated rains between 2007 and 2014—source: hidroweb.ana.gov.br; access on 26/06/2015).
The study area is located on the Fluminense Plain and is characterized by three main geomorphological features: (1) trays and cliffs, characterized by the Barreiras Formation, which altitudes can range from the sea level to 80 m high; (2) low rounded hills, carved in gneissic rocks (the Lagos Region Complex), with altitudes ranging from 10 to 100 m high; and (3) sandbanks and alluvial plains from the São João River and its main tributaries, ranging in altitude from sea level to 10 m high (Reis 1998; Coe et al. 2007). Those plains are low gradient depositional surfaces, formed by coastal marine sedimentation (Suguio 2003). The soils from the studied area are conditioned by the climate, presenting shallow soil profiles close to the coastline with dry weather (colluvial and alluvial soils) and deep soils profiles surrounding the transitions between the craggy mountains and the plains with wetter weather (latosols and clay soils) (Coe et al. 2007).
Concerning the regional geology (Fig. 2; Leite et al. 2004), the area displays the Quaternary alluvial deposits (fluvial-lacustrine and coastal deposits) inserted among three main geological complexes: the Lagos Region Complex (Fonseca 1993), Paraíba do Sul Complex (Campos Neto and Figueiredo 1990; Valladares 1996) and Búzios Complex (Schmitt 2001). The Lagos Region Complex (Paleoproterozoic, ~1.98 Gy) is formed by granites and granodiorites, constituting the main part of the crystalline basement in the study area. The Paraíba do Sul Complex (Neoproterozoic, ~1 Gy), which predominates on the north and west of the study region, presents supracrustal units (siliciclastic and carbonate metasediments), ranging from moderate to high metamorphic grade (paragneiss, schist and marble). The Búzios Complex (Neoproterozoic, ~1 Gy) shows the same spatial disposition of Paraíba do Sul Complex (north and west of the study region), however, displaying a narrow stretch of the area and represents supracrustal units associated with mafic/ultramafic rocks (mainly amphibolites). Those geological units are arranged in the preferential direction of NE–SW and have been subject to several phases of deformation related to the Brasiliano Cycle (Late Neoproterozoic to Early Cambrian, from 605 to 540 My—Schmitt 2001). Some other important geological units are found in the study area, including the Silva Jardim granite (Cambrian I-type granite, 505 My; Moraes 2009), and the São João Alkaline Complex (Cretaceous, ~65 My), composed of nepheline-syenite, gabbro and phonolite (Mota et al. 2009).
The Quaternary coastal sedimentation of Cabo Frio Region is represented by the São João River flood plain and its tributaries. The Quaternary deposits (Pleistocene and Holocene) are composed of continental sediments (fluvial and swamp environments); sandy sediments from paleochannels (fluvial environment); lacustrine and bottom bay sediments (clay sediments); and sandy coastal marine sediments. The latter described sediment deposit displays aligned beach ridges morphology, locally reworked by eolian action (dunes area) (Martin et al. 1997).
The Quaternary sediments also comprise the main aquifer system of the studied region, which are underlain the fractured rock aquifers in the crystalline basement. The porous-medium aquifers are formed by the heterogeneous alluvial and coastal sediments, which are constituted mainly of medium to fine sand (quartz composition) and a minor percentage of silty sand and clay sand. Furthermore, the porous-medium aquifer is unconfined and the water table varies according to rainfall season, ranging from 5 (dry season) to 10 m (wet season). The fractured aquifer behaves as a semi-confined aquifer in the most portion of the area and has a poor hydraulic connection with porous aquifer (Almeida et al. 2004; Silva Jr. 2005).
Methodology
This study was carried out by the monitoring of 7 sampling stations: namely three stations on the three rivers before debouch into Juturnaíba Reservoir (P1, P2 and P3); three in the reservoir (P4, P5 and P6); and one in the outflow of the reservoir (P7 during the period between March 2010 and May 2011 (Fig. 1). In the sampling stations located in the Juturnaíba Reservoir (P4, P5 and P6), the procedure was to obtain samples in the surface, middle and in the bottom of water column.
The samples were filtered through 0.45-µm filters and then divided into two aliquots for major ions and trace metals analysis. Samples for metal analysis were acidified with double-distilled nitric acid. Analyses for major ions (Ca++, Mg++, Na+, K+, NH4 +, F−, Cl−, NO3 −, PO4 3− and SO4 2−) were carried out by ion chromatography (850 Professional IC, Metrohm). An unacidified 1 L sample was collected for alkalinity (HCO3 −) determination, and the concentrations were obtained by using titration. Trace metal (Al, Ba, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Sb, Sr, V and Zn) determinations were carried out by ICP-MS (XSERIES 2, Thermo Fisher Scientific). Suspended particulate material (SPM) was quantified using a gravimetric method where the samples were filtered in situ with previous weighted 0.45-µm cellulose acetate filters. In the laboratory, those filters were totally dried and then weighed again, giving a result in mg l−1.
The pH, temperature and electrical conductivity (EC) determinations were measured using a multi-parameter meter (HI9828, Hanna Instruments) at the time of water sampling. The geochemical model PHREEQC (Parkhurst 1995) version 2.15 was used to carry out mineral equilibrium and dissolved species calculations.
The analytical precision for water samples was calculated by assessing the degree of variability of results for duplicate analytical samples, which were below 15% for all analyzed metals. Analytical blanks were used for each 6-sample batch. The accuracy of measurements was determined with the reference material SPS-SW1 provided by LGC (UK), and the results indicated a satisfactory recovery ranging from 80 to 90%.
Results
In order to better understand the geochemical dynamics of the river, the fluvial system will be divided in three main compartments (Fig. 3): (1) the rivers sampling stations, located, respectively, in the São João (P1), Capivari (P2) and Bacaxá (P3) Rivers; (2) the Juturnaíba Reservoir sampling stations, next to the mouth of the rivers in the Juturnaíba Reservoir (P4, P5 and P6); and (3) the Juturnaíba Reservoir outflow sampling station (P7). Table 1 presents the physicochemical and chemical data from water samples collected in the dry and wet seasons for the three fluvial compartments, and Figs. 4, 5 and 6 show the variations of the physicochemical parameters and each analyzed major ions and trace metals during the sampling period for the three fluvial compartments. It is important to know that the physicochemical parameters values and ions concentrations from Juturnaíba Reservoir compartment samples found in Table 1 are the median value from surface, middle and bottom water samples.
Among the physicochemical parameters, pH shows only small variations in the three rivers and remains almost constant in the Juturnaíba Reservoir sampling stations and also in the Juturnaíba outflow. In all three compartments, there is a suddenly decrease in the pH values in March 2011 and it could be associated with high rainfall for that month. However, in the Juturnaíba and the outflow sampling stations, there is an abruptly rise of pH value (reaching 8.9) in June 2010, which could be explained by the influence of sewage, represented by the high concentrations of PO4 and NO3 measured in that month (mainly from the São João River, P1), to the low rainfall and to the high evapotranspiration in the Lagos Region (INEA 2012), besides considering that 20% of the reservoir surface is covered with water Hyacinth (Benton et al. 1978). The EC values for all compartments are low and are generally <100 µS cm−1. The main changes in the behavior of this parameter occurred in the transition of the summer to the winter 2010 when the values stabilize. The higher EC values (>100 µS cm−1) are obviously found in the Juturnaíba sampling stations, where the dissolved water components get higher due to the less turbulent and closed environment of the reservoir.
The SPM shows the expected behavior for the rivers compartment: that is, higher suspended particle loads occurred during high rainfall period and lower loads were measured in the low rainfall period. Nevertheless, the SPM levels in the Juturnaíba compartment showed apparently random fluctuations, which suggests that the reservoir may receive suspended sediments from other sources which were not measured in this study such as some parts of the reservoir borders. The borders are constituted by exposed soil profiles, and the erosional process in the reservoir could be less dependent from the wind and rainfall regime, reflecting in the SPM behavior in the outflow sampling station (P7).
Concentrations of the major ions, Na, K, Ca and HCO3, showed little variability up to January 2011 in the rivers and Juturnaíba compartments, while Mg varied greatly during this period in those compartments, mainly in the P3. Conversely, Cl and SO4 presented significant variations on their concentrations in the rivers compartment up to January 2011, and similar in the reservoir and Juturnaíba outflow compartments. Most of those ions presented a noticeable sudden decrease in their concentrations in February 2011 followed by a rapidly rise in March 2011, particularly in the rivers compartment. This suggests that rainfall events can exert a large influence on water quality in the basin, where dissolved constituent concentrations increase during high rainfall events due to flushing of the watershed. Another relevant observation was the sudden decrease in K, Mg and HCO3 in July 2010 in Bacaxá River (P3), which suggests ion exchange was occurring by the river sediments and SPM. The ion F presents the most different behavior of the major ions. It shows a stable behavior along whole sampled period in P3 but significant variations in P1 in the rivers compartment. On the other hand, in the Juturnaíba sampling stations there is an inversion of F concentrations, the higher ones are found in P4 (São João River outflow) and the lower ones in P6 (Bacaxá River outflow). This behavior could be due to the anthropogenic source (sewage disposal) from some districts located surrounding the Juturnaíba Reservoir.
Among the analyzed macronutrients, NH4 concentrations display similar trends in the three fluvial compartments: the concentration of this constituent increased during the rainy period of 2011, showing the influence of the pluviometry regime in its behavior. The high concentrations of NH4 measured in March 2010 in the Juturnaíba Reservoir compartment may be linked to the accumulation of this ion that comes from the rivers load and from the surrounding sewage disposal. Nitrate has almost similar behavior among the river compartment sampling stations, while in the Juturnaíba compartment it displays two highest concentrations peaks in P4 (São João River outflow) in June and September 2010, which could be due to the sewage disposal allied with NH4 oxidation, and this sampling station, as in many parts of the reservoir, has a great growth of aquatic plants (water hyacinth). PO4 displays a behavior that seems to be influenced by the pluviosity in the rivers compartment, while in Juturnaíba sampling stations it presents the highest concentrations, probably due to the same factors of the N species behavior: the P accumulation by rivers discharge and the sewage disposal in the Juturnaíba Reservoir.
Concentrations of the trace metals Al and Fe also have similar behavior in the three compartments. In natural waters, Al and Fe have their availability controlled by the water pH (hydrolysis reactions) and the pH behavior corroborates partially this hypothesis. The other important controlling factor of Al and Fe availability is the pluviosity, presenting higher concentrations in dry season and lower ones in wet season, i.e., the dilution factor imposed by the rainfall. It is noticeable the highest concentrations of Al and Fe in the Juturnaíba Reservoir sampling stations, and it could be also related to the accumulation by rivers discharge in the reservoir. However, for Al, there is another source in Juturnaíba: the discarding of Al by the three water treatment plants, which is disposed directly in the reservoir, growing up the Al concentrations, mainly in the periods of low rainfall. Mn displays its behavior similar to the SPM and, consequently, also controlled by pluviosity, in the rivers compartment, while in the Juturnaíba Reservoir sampling stations the concentrations have no significant variations, showing the P6 (Bacaxá River outflow) with the highest concentrations.
The metals Cr, V and Co present similar behavior in rivers compartment as well as in the Juturnaíba compartment, and the behavior of each metal between these compartments is also very similar. The concentrations variation observed for these metals between March and November 2010 could be related to the pluviosity and ionic exchange event in the bottom and suspended sediments. Those similar metals behavior could be linked to the geological features of the study area, suggesting the influence of mafic/ultramafic rocks (high background concentrations for those metals) or some minerals which contain these metals, like magnetite and ilmenite. Conversely, Ni, which is also related to the mafic/ultramafic rocks, has opposite behavior from Cr, V and Co, showing its highest concentrations between March and November 2010 and a decrease between December 2010 and May 2011, both in rivers and Juturnaíba compartment. This fact suggests other source for Ni, like application of fertilizers (impurities from the manufacture processes) and pesticides (part of the active compounds) in different types of plantations along the drainage basin (Gimeno-Garcia et al. 1996). Cu and Zn also have similarity to Ni behavior in both rivers and Juturnaíba compartments, but Zn presents its highest concentrations in March 2011 (P1 and P2, in high rainfall period) in the rivers compartment. Although Pb also presents a similar behavior to Cu, Zn and Ni in the rivers compartment, it changes in the Juturnaíba and the Juturnaíba outflow compartment, highlighting the sudden decrease in its concentrations in March and September 2010 (P4 and P6) and coinciding to the two highest concentration peaks of NO3. This fact suggests the Pb could be scavenged by NO3, forming Pb(NO3)2, which could be a great concern for the aquatic biota, mainly fish (Mohanambal and Puvaneswari 2013). In the study region, there is a high probability of that Pb has as main source the fertilizers and pesticides (Gimeno-Garcia et al. 1996).
Ba and Sr were expected to have a similar behavior due to their geochemical affinity and, possibly, the same geological source (granitoid or carbonate rocks). However, they present very different behavior in the three compartments, suggesting diverse geochemical supergenic processes (rock weathering) and also different sources (regional rocks as well as anthropogenic source, mainly pesticides). Analyzing separately each element, their behaviors are also different between the three fluvial compartments (i.e., the highest Sr concentrations occurred in the rivers compartment during the dry season, while highest concentrations occurred in the Juturnaíba compartments during the wet season). The opposite behavior was shown by Ba in the same fluvial compartments.
The geochemical affinity between elements is well represented by the chalcophile metals Cd and Sb which behaved in a similar manner in the three fluvial compartments. This suggests that these metals were derived from the same source such as the oxidation of sulfide minerals. It is also possible that these metals were from pesticide use in the watershed (Gimeno-Garcia et al. 1996). Concentrations of these metals decreased in May, September and November 2010 which suggests that these metals are associated with some anthropogenic activities in the study region rather than with rainfall which appears to be the case for most the most of the trace metals analyzed.
In general, variations in major ion concentrations in the watershed appear to be influenced by rainfall, whereas for trace metal concentrations appear to be affected by their availability from largely anthropogenic sources. Concentrations of some major ions are probably also influenced by anthropogenic activities like sand mining and the erosion of river banks trigged by the agricultural activities (cattle breading and several kinds of plantations). This also may be the case for trace metals, which have strong relationship to pesticides applications in the several plantations along the rivers course. The behavior change of several metals and physicochemical parameters among the river and Juturnaíba compartments is obviously due to the change of limnologic environment (from river to lake), besides some anthropogenic activities that impact behavior of some chemical constituents, especially that of N species and PO4. Conversely, the Juturnaíba outflow compartment samples (P7) show almost the same behavior for most analyzed ions from Juturnaíba compartment samples. The sampling stations P3 and P6, which represent, respectively, the Bacaxá River and the Bacaxá River outflow in the Juturnaíba Reservoir, displayed for some ions behavior (Na, Cl, SO4, F, Al, Fe, Ni and Co), a significant concentration discrepancy between these two fluvial compartments, with higher concentrations in the river compartment and lower ones in the Juturnaíba compartment. It suggests that, although the contribution of the rock weathering and anthropogenic activities, the Juturnaíba Reservoir could dilute the rivers water constituents, besides to provide exchange sites (clay minerals, organic compounds, Fe and Mn oxides–hydroxides, etc.) for some constituents and/or precipitate them in the sediments, once reaching their hydrolysis pH (higher pH values in the Juturnaíba compartment).
Discussion
Evaluation of the main influences of the water chemistry from the São João River Drainage Basin
As shown in the results, the water chemical features in the study region have three main influences: (1) the pluvial regime; (2) the influence of the geological setting and (3) the anthropogenic contributions. The pluviosity of the studied region is an important control of the water constituents due to the rainfall input in the wet season and the high average temperature allied to the evapotranspiration process in the dry season. These factors could exert control on concentrations of dissolved chemical constituents through concentration and dilution. Geological factors can directly influence the stream water quality through rock weathering (soils) and subsequent deposition in surrounding streams (sediments), in addition to the influence of groundwater discharge (Hem 1985; Faure 1998). Additionally, anthropogenic activities including agricultural land use and industrial and municipal wastewater disposal are likely to be influencing water quality in the basin. Those three main influences are likely to control the concentrations of water constituents (major ions and trace metals) by pH and Eh variations. This control allows a several geochemical processes to take place in the water, including: metal adsorption by clay minerals, Fe and Mn oxide/hydroxides and organic matter; the coprecipitation of metals in the Fe and Mn oxide/hydroxides; and metals complexation with water constituents such as humic acids to form colloidal phases (Rose et al. 1979; Krauskopf and Bird 1995; Drever 1998; McSween et al. 2003).
The dominant effect that rainfall could have on water quality can be understood through the use of a Gibbs diagram (Fig. 7), which shows the great influence of the pluviosity as most of the samples plot in the dilution dominance field on the diagram and only a few samples plot in the rock weathering dominance field for both the wet and dry seasons. Although the dry season samples (mainly those from rivers and Juturnaíba compartments) mostly plot in the dilution dominance field, a significant proportion of these samples also plot in the rock weathering dominance field which might be the expected behavior. However, the wet season samples display a small trend toward the rock weathering dominance field, besides they present the high number of samples in that field. It is noticed that even in wet season (dilution process dominance), the samples show influence from the geological materials and that behavior is comparable to the major ions (Na, K, Ca and HCO3), corroborating the hypothesis of the “washing” of the drainage basin by rainfall, which concentrate water constituents from rock weathering in those fluvial compartments.
The geological and anthropogenic contributions to water quality in the basin can also be assessed by using the Inorganic Chemical Index (ICI). Originally proposed by Pacheco and Van der Weijden (1996) as “Pollution Index” and modified by Huizenga (2011), the ICI determines the percentage of the overall inorganic water chemistry that is derived from all sources, excluding chemical rock weathering (Pacheco and Van der Weijden 1996; Van der Weijden and Pacheco 2006). This index takes into account the main anions related to several anthropogenic sources, namely Cl, NO3, SO4 and PO4, mostly from agriculture and sewage disposal, and HCO3, which is considered to be the product of regional rock weathering. The equation, with the charge corrected concentrations, is given by:
For example, an ICI value of 30% means that 70% of the cations are derived from chemical weathering and 40% are derived from alternative sources (Van der Weijden and Pacheco 2006). Thus, the use of fertilizers will increase the concentrations of orthophosphate, nitrates and sulfate relative to bicarbonate, consequently increasing the inorganic chemistry index. The maximum and minimum ICI values, which characterize the weathering dominated rivers and the non-weathering dominated rivers, were calculated based on the data set from Gaillardet et al. (1999), where 62 world rivers were assessed. Of those 62 rivers, 53 were assessed as being weathering dominated, having a median ICI of 30%, and 9 were assessed to be non-weathering dominated with a median ICI value of 70%. Thus, for this study, the maximum ICI value was ≥70% and the minimum ≤40%.
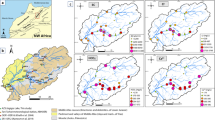
Figure 8 shows the ICI values variations during the sampled period in the three fluvial compartments. As indicated in Fig. 8, high ICI values were observed at the end of wet season 2010 which decreased in the dry season 2010 and rose again in the wet season 2011. This suggests that “washing” of the drainage basin by rainfall increases the concentrations of chemical constituents in water, showing the important influence of pluviosity. The rainfall could also be reason that the ICI of the study area does not reach small values. This fact could be allied to the regional geology of the studied region, which is predominantly comprised of gneissic and granitoid rocks and does not contain significant levels of carbonate rocks. It means that granitoid–gneiss rocks have a slower dissolution rate and lower solubility of their minerals compared to carbonate rocks and the time to reach significant HCO3 concentrations is shorter.
Another outstanding feature observed is degree to which the ICI varies between the rivers and Juturnaíba compartments in the P3 and P6 (respectively, Bacaxá River and the Bacaxá River outflow in the Juturnaíba Reservoir sampling stations). As indicated in the previous section, those stations have great differences of some major ions and metals concentrations due to some hydrogeochemical processes which may influence the ICI values in those sampling stations. On the other hand, the ICI values may reveal the anthropogenic contribution in some parts of the Juturnaíba Reservoir. Comparing the three sampling stations in the Juturnaíba compartment, it was expected the same behavior of ICI values. Nevertheless, P4 and P5 keep high ICI values, suggesting some influence of anthropogenic activities, mainly sewage disposal from the small villages next to the reservoir and also the discarding of Al by the water treatment station.
Figure 9 shows the [HCO3]/[Na] versus [Ca]/[Na] mixing diagram also reported by Gaillardet et al. (1999), which shows different fields of the main rock types, namely silicates, carbonates and evaporite rocks. It is observed that samples from the three fluvial compartments dominated by rock weathering (ICI ≤ 40%) are located close to silicate rocks field, which is the main rock type of the study region (granitoid–gneiss rocks). The majority of samples from the three fluvial compartments dominated by anthropogenic sources (ICI ≥ 70%) are concentrated close to the evaporite rocks field, and this behavior is explained by the relative high content of some water constituents, like Cl, SO4, Na and NO3, provided by the sewage disposal and agriculture activities. It is important to notice that there are no evaporite deposits next to the sampling stations, corroborating, so, the sewage influence. The samples with ICI values between 40 and 50%, which still represent major rock weathering dominance, have almost the same behavior of the ICI ≥ 70% group, with most samples get close to the silicate rocks. Conversely, the samples with ICI values between 50 and 70% have a scattered behavior, reaching almost the three rock type fields, and this great variation is pronounced in the samples from the rivers and Juturnaíba compartments. This behavior could be the result of the pluviosity influence, which could mix different sources of the water constituents in the three fluvial compartments.
Trace metals speciation, implications of its toxicity and control factors of their behavior
Among the water constituents in the Juturnaíba Drainage Basin, the trace metals are of particular concern due to their potential effects on aquatic biota and human health. Environmental contamination resulting from the extensive use of metals and semimetals in industry, agriculture, and in manufactured products has increased the threat of toxicity for plants, animals and society (Sheoran and Sheoran 2006; Silva-Filho et al. 2014). However, significant concentrations of trace metals can also be provided from geogenic sources (Rose et al. 1979; Silva-Filho et al. 2014). In aqueous ecosystems, metals may be in dissolved phase, complexed with inorganic and organic molecules as well as in a suspended phase where they may be incorporated and bioaccumulated in aquatic organisms (Forstner and Wittman 1983; Boniforti et al. 1988; Foster and Charlesworth 1996; Di Toro et al. 2001; Warren and Haack 2001). Dissolved trace metals may be precipitated through adsorption, which is controlled by physicochemical parameters like pH, Eh and electrical conductivity (Carballeira et al. 2000; Arias et al. 2005; Marques et al. 2008, 2012). In the present study, hydrogeochemical modeling was carried out in order to verify the main control factors of trace metals availability in dissolved form. The trace metals assessed in this study were Cd, Cr, Cu, Ni, Pb, Sb and Zn, which may be derived from both natural (geology) and anthropogenic sources in the watershed. Nevertheless, as shown in the previous sections, Al could also be considered a great concern, mainly in Juturnaíba Reservoir, due to the disposal of some coagulant substances (aluminum sulfate) in Juturnaíba Reservoir by Water Treatment Station. For that proposal, the PHREEQC hydrochemical modeling program was used, giving information about the main trace metals dissolved species and the possible mineral phases to be formed, given their saturation indices (SI).
Tables 2 and 3 show the main dissolved species and the probable amorphous solids of the analyzed trace metals on the three compartments and the respective trace metals concentrations of the probable effect level (PEL) for freshwater, provided by CCME (1995). This geochemical modeling was based on the median values of the physicochemical parameters and ions concentrations from each sampling stations, in the dry and wet seasons. It is noticed that Al, Cr and Sb display their main dissolved species as product of hydrolysis reactions, namely Al(OH) −4 and Al(OH) +2 for Al; Cr(OH) +2 and Cr(OH)2+ for Cr; and SbO3 − for Sb. Those metals are likely to be complexed with humic acids and others organic compounds in the water column, as well as associated, colloidal Fe/Al oxides–hydroxides (Allard et al. 2004). Still for Al, there is a little percentage related to sulfate species. Cd, Cu, Ni, Pb and Zn present as main dissolved species their non-complexing forms (monomeric forms), namely Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+. The control of their secondary dissolved species ranging between bicarbonate/carbonate complexes, hydrolysis reactions products and a little percentage is related to sulfate complexes. Nevertheless, the bicarbonate/carbonate complexes predominate due to the geochemical affinity of those metals to carbonate minerals (Krauskopf and Bird 1995; Faure 1998).
As seen in the last section, it is observed that the high rainfall (wet season) is responsible for the highest concentrations of most trace metals. By contrast, the highest concentrations of dissolved Al occurred in the dry season, probably due to the groundwater discharge influence in the baseflow of the rivers. However, the behavior presented by the trace metals in dry and wet season does not change the predominance of the main forms of trace metals dissolved species. It is also noticed that the pH values rise from the rivers compartment to Juturnaíba outflow compartment, suggesting the predominance of some species produced by hydrolysis reactions. In contrast, the bicarbonate/carbonate species is found more abundant in the rivers compartment, where the weathering processes are more intense. Sulfate species are also predictable in the studied waters; nevertheless, those species are more pronounced in acidic aquatic environments, with pH values <4.5, as environments with massive sulfide oxidation processes, like acid mine drainage and organic matter-rich aquatic environments (Eary 1998; Marques et al. 2012).
Observing the median concentrations of the analyzed trace metals, Al, Cd, Cu, Pb and Zn present concentrations above the PEL, particularly Cd, Cu and Pb, which have their median concentrations above the PEL for the dry and wet seasons in almost all fluvial compartments. This is of great concern considering that those trace metals have the potential to be incorporated in aquatic organisms, which are bioaccumulated along the food chain, potentially affecting public health through the consumption of organisms with high metal concentrations. The predominant monomeric forms presented by Cd, Cu, Pb and Zn, besides the oxides/hydroxides forms, are more reactive to metabolic processes of aquatic organisms (Alpers and Blowes 1994; Baird 1998). The hypothesis of nitrate species formation for Pb (Pb(NO3)2) in the Juturnaíba Reservoir compartment showed incongruent reactions by the hydrochemical modeling and, consequently, presenting very low concentration of this species.
Nevertheless, Al behavior could be of more concern due to the direct action on aquatic organisms, especially the fish populations. The Juturnaíba Reservoir compartment, particularly, presents the highest median Al concentration in dry and wet seasons. This important water reservoir is also a recreational area, with more than 6 fish species, which are appreciated in sport fishing. The toxicity of Al to fish is primarily due to effects on osmoregulation by action on the gill surface (McDonald et al. 1989). CCREM (1999) points out that the limit of Al to aquatic life is about 100 µg l−1, in pH values <6.5, which could affect the fish species in the reservoir. The monomeric species Al(OH) +2 , Al(OH)+2 and still Al(OH) −4 in freshwater are more reactive at cell membrane surface of aquatic organisms than polymeric forms and organically bound Al (Baird 1998; Camilleri et al. 2003; Gensemer and Playle 1999).
Trace metals can be removed from the water column by secondary mineral precipitation (e.g., as carbonates or hydroxides), coprecipitation or sorption onto organic matter (OM) or surface-reactive iron (oxyhydr) oxides that form following oxidation of ferrous iron (Balistrieri et al. 2003; Dzombak and Morel 1990; España et al. 2005; Hochella et al. 2005).
In order to verify the hydrogeochemical control of trace metals dissolved species, the saturation indexes (SI) of some secondary minerals were calculated. Those minerals have a high probability of formation according to the concentration range of their constituents. Mineral equilibrium calculations for a water sample are useful to predict the presence of precipitating minerals in aqueous systems (Deutsch 1997; Eary 1998; Marques et al. 2012). The saturation index is given by SI = log(IAP/Ksp), where IAP is the Ionic Activity Product of the ions present in a given mineral and Ksp is the solubility product, which is a constant of the given mineral. Two minerals groups were chosen for each analyzed trace metal, while one represents the product of reactions with carbonate or sulfate, the other represents the hydrolysis reactions product. These minerals groups were chosen according to the rocks predominance in the fluvial compartments, which influence the water chemistry. Thus, for Al, the chosen minerals were basaluminite (Al4(OH)10SO4) and gibbsite (Al(OH)3); for Cd, otavite (CdCO3) and Cd(OH)2; for Cr, only the amorphous chromium hydroxide (Cr(OH)3) was indicated by the modeling; for Ni, NiCO3 and Ni(OH)2; for Pb, cerussite (PbCO3) and Pb(OH)2; and for Zn, smithsonite (ZnCO3) and zincite (ZnO). For the Sb, there is no secondary mineral formation indicated for this metal in its oxidation state (valence 5) by the geochemical modeling.
As it is likely that geochemical factors such as pH and Eh exert a strong control on the availability of some chemical constituents in the water column (Hem 1985; Foster and Charlesworth 1996; Drever 1998), it is likely that these factors also influence SI values (Marques et al. 2012). For example, Jones et al. (2004) showed in a river experiment that pH (which is hugely regulated by photosynthesis) controls Zn and As availability and consequently SI values. Figures 10 and 11 display the SI values for the chosen minerals versus the pH values for each sample in dry and wet seasons. From the 8 analyzed trace metals, only Al, Cu and Cr could reach the equilibrium condition (SI = 0), and only Al and Cu reached the saturation (SI > 0) and supersaturated zone (SI > 1), i.e., the secondary minerals for those metals could be precipitated from the solution. Excepting for Al, all minerals SI values show a positive trend with pH in both the dry and wet seasons, indicating the great chemical control this parameter has on the mineral species formation in all fluvial compartments. SI values for almost all chosen minerals vary greatly during the wet season and there is a great range of pH values and there is a much less scattered distribution of SI values and lower range of pH values during the wet season. This could be due to the influence of the relative high regional evapotranspiration rate and temperatures in the dry season and the dilution factor (higher flow rate) in the wet season.
For Al, both minerals reach high SI values, being gibbsite with almost the entire samples above the equilibrium zone for dry and wet seasons. The basaluminite has the higher SI values in dry season for the rivers compartment, while in wet season the higher SI values for this mineral are found for Juturnaíba Reservoir compartment samples. The scattered behavior observed for basaluminite is due to the great variations of SO4, suggesting diffuse sources for the surface water chemistry. For Cu, Cr and Ni, the hydrolysis product minerals, respectively, cuprite, Cr(OH)2 and Ni(OH)2, have the highest SI values in both dry and wet season, while for Cd and Pb, the carbonate minerals dominate the highest SI values in both seasons. Zn displays zincite (hydrolysis product) with highest SI values in dry season and smithsonite (carbonate) in wet season. Therefore, the SI data for Cd, Pb and Zn corroborate the predominance of the carbonate/bicarbonate dissolved species along the three fluvial compartments, due to their geochemical affinity with carbonate minerals.
The predominance of the Juturnaíba Reservoir compartment samples with the highest SI values for almost all minerals in both dry and wet seasons shows that the environment of Juturnaíba Reservoir gives better conditions to mineral formation, as low turbulence, high evapotranspiration rate and relative high temperature for both seasons.
Conclusions
Anthropogenic activities in the São João River Drainage Basin impact directly on water quality, as seen by some trace metals concentrations. The main control factor of the water chemistry is the regional pluviosity, which can concentrate and dilute the water constituents, depending on the analyzed ion and the predominance of the anthropogenic activity. Although the three fluvial compartments displayed different features, like differences in mean flow rate, geology and human activities, many chemical constituents showed similar behavior in different parts of the watershed, particularly trace metal concentrations. The ICI values corroborate the great influence of pluviosity through the drainage basin by “washing” by rainfall (higher flow rate for this period). However, the use of ICI values for some proposal, like linking water chemistry to rock composition, could bring to some incorrect interpretations, as seen by the ICI > 70% samples in the mixing diagram.
Excepting Cr and Sb, all trace metals present concentrations higher than the PEL, mainly Cu and Pb, which present those concentrations in both dry and wet season. However, the Al concentrations in Juturnaíba Reservoir are of concern due to the potential effects on the aquatic biota. The main dissolved species of trace metals are the non-complexing forms, followed by oxides/hydroxides and carbonate/bicarbonate forms. The SI data show that the hydrolysis reactions regulate the most trace metals availability in the three fluvial compartments in both dry and wet season, followed by carbonate and sulfate complexation. That hydrogeochemical modeling information corroborates that the pH range is the main control factor of metal availability in the studied waters, as seen the predominance of dissolved species and the indicated minerals. Even some trace metals do not present SI values in saturation zones, like Cd, Ni, Pb and Zn; it is important to show that the saturation index, together with some physicochemical data, can give valuable information about chemical control of trace metals, which has direct influence on their availability.
References
Allard T, Menguy N, Salomon J, Calligaro T, Weber T, Calas G, Benedetti MF (2004) Revealing forms of iron in river-borne material from major tropical rivers of the Amazon Basin (Brazil). Geochim Cosmochim Acta 68:3079–3094
Almeida RMR, Lauria DC, Ferreira AC, Sracek O (2004) Groundwater radon, radium and uranium concentrations in Região dos Lagos, Rio de Janeiro State, Brazil. J Environ Radioact 73:323–334
Alpers CN, Blowes DW (1994) Environmental geochemistry of sulfide oxidation. In: American chemical society symposium series, vol 550. American Chemical Society
ANA—Agência Nacional de Águas (2002) A evolução da gestão dos recursos hídricos no Brasil. Ministério do Meio Ambiente. Brasília—DF, Brazil
ANA—Agência Nacional de Águas (2005) Caderno de recursos hídricos: Disponibilidade e demandas de recursos hídricos no Brasil. Ministério do Meio Ambiente. Brasília—DF, Brazil
Arias M, Perez-Novo C, Osorio F, Lopez E, Soto B (2005) Adsorption and desorption of copper and zinc in the surface layer of acid soils. J Colloid Interface Sci 288:21–29
Baird C (1998) Environmental chemistry, 2nd edn. Bookman, Ontario, p 622
Balistrieri LS, Box SE, Tonkin JW (2003) Modeling precipitation and sorption of elements during mixing of river water and porewater in the Coeur d’Alene River basin. Environ Sci Technol 37(20):4694–4701
Benton AR Jr, James WP, Rouse JW Jr (1978) Evapotranspiration from Hyacinth (Eichhornia crassipes (Mart.) Solms) in Texas reservoirs. Water Resour Bull 14(4):919–930
Boniforti R, Bacciola D, Niccolai I, Ruggiero R (1988) Selective extraction as estimate of bioavailability of As, Cd Co, Cr, Fe, Mn, Ni, Pb and Zn in marine sediments collected from the Central Adriatic Sea. Environ Technol Lett 9:117–126
Brasil (2008) Ministério do Meio Ambiente. Plano de manejo da área de proteção ambiental da bacia do rio São João/Mico Leão-Dourado. Brasília
Camilleri C, Markich SJ, Noller BN, Turley CJ, Parker G, Van Dan RA (2003) Silica reduces the toxicity of aluminium to a tropical freshwater fish (Mogurnda mogurnda). Chemosphere 50:355–364
Campos Neto MC, Figueiredo MCH (1990) Evolução geológica dos terrenos Costeiro, Paraíba do Sul e Juiz de Fora (RJ-MG-ES). In: Cong Bras Geol, 36, Natal. Conference paper, SBG, 6. pp 2631–2648
Carballeira A, Carral E, Puente X, Villares R (2000) Regional-scale monitoring of coastal contamination, nutrients and heavy metals in estuarine sediments and organisms on the coast of Galicia (Northwest Spain). Int J Environ Pollut 13:1–6
CCME (Canadian Council of Ministers of the Environment) (1995) Protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. CCME EPC-98E. Prepared by Environment Canada, Guidelines Division, Technical Secretariat of the CCME Task Group on Water Quality Guidelines, Ottawa. (Reprinted in Canadian environmental quality guidelines, Chapter 6, Canadian Council of Ministers of the Environment, 1999, Winnipeg)
CCME (Canadian Council of Ministers of the Environment) (2002) Canadian sediment quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg. http://ceqg-rcqe.ccme.ca/en/index.html#void
CCREM (Canadian Council of Ministers of the Environment) (1999) Canadian water quality guidelines for the protection of agricultural water uses: introduction. In: Canadian Environmental Quality Guidelines, 1999. Canadian Council of Ministers of the Environment, Winnipeg
Chapman D (1996) Water quality assessments—a guide to use of biota, sediments and water in environmental monitoring, 2nd edn. Chapman and Hall, New York, p 305
Coe HHG, Carvalho CN, Souza LOF, Soares A (2007) Peculiaridades Ecológicas da Região de Cabo Frio, RJ. Revista de Tamoios, julho/dezembro, Ano IV, no 2
Cunha SB (1995) Impactos das Obras de Engenharia sobre o Ambiente Biofísico da Bacia do Rio São João (Rio de Janeiro—Brasil). Rio de Janeiro. Technical Report
Deutsch WJ (1997) Groundwater geochemistry e fundamentals and application to contamination. CRC Press LLC, Boca Raton, p 221
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC (2001) Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem 20(10):2383–2396
Drever JI (1998) The geochemistry of natural waters. Prentice Hall, Inc, Englewood Cliffs
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York
Eary LE (1998) Predicting the effects of evapoconcentration on water quality in mine pit lakes. J Geochem Explor 64:223–236
España JS, Pamo EL, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel riverwatershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20:1320–1356
Falkenmark M (1986) Macro-scale water supply/demand comparison on the global scene. Beitr Zur Hydrol 6:15–40
Faure G (1998) Principles and applications of geochemistry. Prentice Hall, Englewood Cliffs, p 330
Fonseca AC (1993) Esboço Geocronológico da Região de Cabo Frio, Estado do Rio de Janeiro. Ph.D. Thesis, IG/USP
Forstner U, Wittman GTW (1983) Metal pollution in the aquatic environment. Springer, Berlin, p 486
Foster RB, Charlesworth SM (1996) Heavy metals in the hydrological cycle: trends and explanation. Hydrol Process 10(2):227–261
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gensemer RW, Playle RC (1999) The bioavailability and toxicity of aluminum in aquatic environments. Crit Rev Environ Sci Technol 29:315–450
Gimeno-Garcia E, Andreu V, Boluda R (1996) Heavy metals incident in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92:19–25
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254, 3rd edn. US Geological Survey. Water Supply Paper
Hochella MF, Moore JN, Putnis CV, Putnis A, Kasama T, Eberl DD (2005) Direct observation of heavy metal–mineral association from the Clark Fork River Superfund Complex: implications for metal transport and bioavailability. Geochim Cosmochim Acta 69:1651–1663
Huizenga JM (2011) Characterisation of the inorganic chemistry of surface waters in South Africa. Water SA 37(3):401–410
INEA (2014) Elaboração do plano estadual de recursos hídricos do Estado do Rio de Janeiro. Estudos Hidrológicos e Vazões Extremas. Technical Report
INEA—Instituto Estadual do Ambiente (2012) Relatório de Situação, Ano II (2011–2012) Região hidrográfica VI (Lagos/São João). Planejamento e Gestão. Technical Report
Jones CA, Nimick DA, Mccleskey RB (2004) Relative effect of temperature and pH on diel cycling of dissolved trace elements in Prickly Pear Creek, Montana. Water Air Soil Pollut 153:95–113
Krauskopf KB, Bird DK (1995) Introduction to geochemistry. WCB McGraw-Hill, Boston, p 326
Leite CAS, et al (2004) Carta Geológica do Brasil ao Milionésimo: sistema de informações geográficas-SIG. Folha SF.23 Rio de Janeiro e SG.23 Iguapé, escala 1:1.000.000. CPRM, Brasília
Licht OAB (1998) Prospecção Geoquímica: Princípios,Técnicas e Métodos. CPRM, Rio de Janeiro, p 216
Lima JEFW, Ferreira RSA, Christofidis D (1999) O uso da irrigação no Brasil. In: Estado das águas no Brasil—1999: Perspectivas de gestão e informação de recursos hídricos. SIH/ANEEL/MME; SRH/MMA, pp 73–82
Marques ED, Sella SM, de Mello WZ, Lacerda LD, Silva-Filho EV (2008) Hydrogeochemistry of sand pit lakes at Sepetiba Basin, Rio de Janeiro, Southeastern Brazil. Water Air Soil Pollut 189:21–36
Marques ED, Sella SM, Bidone ED, Silva-Filho EV (2010) Geochemical behavior and dissolved species control in acid sand pit lakes, Sepetiba sedimentary basin, Rio de Janeiro, SE Brazil. J South Am Earth Sci 30:176–188
Marques ED, Tubbs D, Gomes OVO, Silva-Filho EV (2012) Influence of acid sand pit lakes in surrounding groundwater chemistry, Sepetiba sedimentary basin, Rio de Janeiro, Brazil. J Geochem Explor 112:306–321
Martin L, Suguio K, Dominguez JML, Flexor JM (1997) Geologia do Quaternário Costeiro do litoral norte do Rio de Janeiro e do Espírito Santo. FAPESP/CPRM, São Paulo, p 112
McDonald DG, Reader JP, Dalziel TKR (1989) The combined effects of pH and trace metals on fish ionoregulation. In: Morris R, Taylor EW, Brown DJA, Brown JA (eds) Acid toxicity and aquatic animals. Cambridge University Press, Cambridge, pp 221–242
McSween HY Jr, Richardson SM, Uhle ME (2003) Geochemistry: pathways and processes. Columbia University Press, New York
Mohanambal R, Puvaneswari S (2013) A study on the acute toxicity of lead nitrate (Pb(NO3)2) on the freshwater fish Catla catla. Int J Curr Sci 8:2151–2155
Moraes JM (2009) Geologia do Granito Silva Jardim (RJ): implicações na evolução tectônica dos Terrenos Oriental e Cabo Frio. M.Sc. Dissertation. Faculdade de Geologia/UERJ
Mota CEM, Geraldes MC, Almeida JCH, Vargas T, Souza DM, Loureiro RO, Silva AP (2009) Características Isotópicas (Nd e Sr), Geoquímicas e Petrográficas da Intrusão Alcalina do Morro do São João: Implicações Geodinâmicas e Sobre a Composição do Manto Sublitosférico. Revista do Instituto de Geociências—USP. v 9, n.1, pp 89–100
Ongley E (1998) Modernization of water quality programs in developing countries: issues of relevancy and cost efficiency. Water Qual Int 3:37–42
Pacheco FAL, Van der Weijden CA (1996) Contributions from water-rock interactions to the composition of groundwater in areas with sizeable anthropogenic input. A case study of the Fundão area, central Portugal. Water Resour Res 32:3553–3570
Parkhurst DL (1995) User’s guide to PHREEQC—a computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations. U.S. Geological Survey, Water-Resources Investigations Report 95–4227
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Córdoba City (Argentina) on Suquía River. Water Res 34(11):2915–2926
Rebouças AC, Braga B, Tundisi JG (2002) Águas doces no Brasil: capital ecológico, uso e conservação. 2.ed. São Paulo: Escrituras Editora
Reis AP (1998) Projeto Carta Geológica do Estado do Rio de Janeiro. Folhas: Morro De São João e Barra De São João. Escala: 1:50.000. DRM, Niterói
Rose AW, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration. Academic Press, London, p 657
Schmitt RS (2001) Orogenia Búzios—Um evento tectono-metamórfico cambroordoviciano caracterizado no Domínio Tectônico de Cabo Frio, Faixa Ribeira—sudeste do Brasil. Ph.D. Thesis, IG/UFRJ
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19(2):105–116
Silva Jr GC (2005) ACOST-RIO: Estudo da Intrusão Marinha em Aqüíferos Costeiros entre os Municípios de Niterói e Rio das Ostras—RJ. PADCT-FINEP. Technical Report 154 p. UFRJ, Rio de Janeiro
Silva-Filho EV, Marques ED, Vilaça M, Gomes OVO, Sanders CJ, Kutter VT (2014) Distribution of trace metals in stream sediments along the Trans-Amazonian Federal Highway, Pará State, Brazil. J South Am Earth Sci 54:182–195
Suguio K (2003) Tópicos de Geociências para o Desenvolvimento Sustentável: as Regiões Litorâneas. Revista do instituto de Geociências- USP. Série Didática
UNESCO—International Hydrological Programme (1998) World water resources at the beginning of the 21st century. Cambridge University Press, Cambridge
Valladares CS (1996) Evolução geológica do Complexo Paraíba do Sul no segmento central da Faixa Ribeira com base em estudos de geoquímica e de geocronologia U-Pb. Ph.D. Thesis, IG/USP, São Paulo
Van der Weijden CH, Pacheco FAL (2006) Hydrochemistry in the Vouga River basin (central Portugal): pollution and chemical weathering. Appl Geochem 21:580–613
Warren LA, Haack EA (2001) Biogeochemical controls on metal behavior in freshwater environments. Earth Sci Rev 54(4):261–320
Acknowledgements
The authors would like to thank CAPES (PVE Project 88881.068131/2014-01) and INCT-TMCOcean 573-601/2008-9 for financial support. Emmanoel V. Silva-Filho is senior researcher from the National Council for Research and Development (CNPq, Brazil) and of the Foundation for Research Support of the Rio de Janeiro (FAPERJ, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marques, E.D., Silva-Filho, E.V., Souza, G.V.C. et al. Seasonal variations of water quality in a highly populated drainage basin, SE Brazil: water chemistry assessment and geochemical modeling approaches. Environ Earth Sci 75, 1498 (2016). https://doi.org/10.1007/s12665-016-6297-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6297-7