Abstract
Groundwater is vital to supply residents, livestock and agriculture in Daniudi gasfield area, which is a typical agro-pastoral area. To effectively protect and rationally utilize the groundwater, a hydrochemical investigation of 43 samples from this area was conducted. A hydrogeochemical assessment using a Piper diagram, correlation analysis, ratios of major ions, principle component analysis and saturation index calculations was carried out to detect the hydrochemical characteristics and evolution processes of the groundwater from a Quaternary aquifer (QA) and a Cretaceous aquifer (KA), and fuzzy synthetic evaluation and some water quality indices were applied to assess groundwater suitability for drinking and irrigation. According to the statistic summary, the average abundance of the major ions in groundwater from the QA and KA follows the order: HCO3 − > SO4 2− > Cl− for anions and Ca2+ > Na+ > Mg2+ > K+ for cations. There is evidence that the chemical composition of groundwater in the region has been influenced by human activities. The dominant hydrochemical facies of the groundwater is HCO3–Ca type. Dissolution of carbonate and evaporite minerals and the weathering of silicate minerals are likely to be the sources of major ions in groundwater. Ion exchange is another significant factor affecting the groundwater constituents. Gibbs diagrams suggest that rock weathering is the control process of groundwater chemical composition. As to suitability of groundwater for drinking, single parameter comparison and fuzzy comprehensive evaluation reveal that most of the groundwater in the study area is suitable for drinking under ordinary condition. A US Salinity Laboratory diagram, Wilcox diagram and some irrigation indices indicate that more than 90 % of the groundwater samples are suitable for use in irrigated agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a major source of freshwater, groundwater is widely used for irrigation and domestic purposes in some areas (Odukoya 2015). More than 1.5 billion people worldwide rely on groundwater as their primary source of drinking water (He et al. 2015). Groundwater is much more important for people in arid or semiarid areas, where surface water is of poor quality and scarce (Li et al. 2014a, b). More than 1/3 of China’s mainland has an arid to semiarid climate (principally in the northern and northwestern parts of the country) where groundwater plays a crucial role in sustaining the livelihoods of local communities (Li et al. 2013). However, with the development of economy and society, groundwater in many areas has been affected or polluted by human activities. It is extremely important therefore for groundwater protection, and management measures are urgently implemented to prevent further deterioration of this resource. However, to protect and effectively utilize groundwater resources in arid or semiarid areas, it is necessary to first understand the hydrochemical characteristics and quality of groundwater (Xiao et al. 2014a; Amiri et al. 2015).
The Ordos Basin, which is located in northwestern China, has an arid to semiarid climate and covers an area of 28.2 × 104 km2 (Hou and Zhang 2008; Li et al. 2013). An important energy base with rich resources is located in northern part of the Ordos Basin (Dou 2010). Groundwater plays an important role in the process of energy exploitation. Two important investigations have been conducted by China Geological Survey in 1999–2005 and 2006–2009 to investigate groundwater resources and their exploitation potential in this basin (Cao 2009). In these investigations, the hydrogeological characteristics and the spatial structure of major aquifer system in Ordos Basin and its significance in water supply have been revealed. These investigations have provided information on the origin, recharge mechanisms, flow characteristics and discharge behavior of the groundwater. Moreover, the formation and evolution of the regional groundwater hydrochemistry and the dynamic characteristics of the groundwater had been identified (Hou and Zhang 2008). However, these regional groundwater studies have not been able to provide enough information to characterize groundwater behavior at a local scale. Since then, many scholars have conducted further studies on groundwater characteristics and evolution at small scale (Yin et al. 2009; Li et al. 2013; Liu et al. 2015; Wang et al. 2015), but there are few studies on groundwater geochemistry and suitability evaluation in the agro-pastoral zone of this basin. Hence, it has been necessary to carry out an additional investigation of groundwater resources in this agro-pastoral zone at small scale.
The Daniudi gas field, located in the northeastern part of the Ordos Basin in a transition zone between the Maowusu Desert and the Loess Plateau, is a typical agro-pastoral area. Because of the poor quality of surface water in this gasfield area, groundwater has become the major resource for drinking, agriculture and animal husbandry, and some large groundwater sources supplying urban population exist in this area. Moreover, mining gas inevitably has some influence on groundwater quality. Therefore, groundwater conservation and protection measures must be implemented urgently in this area to protect this resource from gas extraction. Identifying groundwater quality and hydrochemical processes before the development of the gas field favors the protection and rational exploitation of groundwater resources. So far, the hydrogeochemistry and suitability of groundwater for domestic and irrigation uses have not been studied in detail in Daniudi gasfield area.
Principal component analysis (PCA), as a multivariate statistical technique, has been widely used to investigate groundwater geochemistry (Valdes et al. 2007; Tanasković et al. 2012; Re et al. 2014). It can extract a number of independent comprehensive variables from a dataset so as to reduce dimension of original information and simplify it for objective analysis. The use of PCA in conjunction with other hydrogeochemical tools such as a Piper diagram and a Gibbs plot can enable the main factors associated with the hydrochemical variability to be identified (Kudoda and Abdalla 2015).
The present study was undertaken to identify hydrochemical characteristics and the processes controlling groundwater geochemistry by analyzing the hydrochemical data with multiple methods, and to study the groundwater quality for assessing the suitability of shallow groundwater for domestic and irrigation uses, as well as to find out the possible sources of pollution affecting groundwater quality in the study area. The results may provide important information about groundwater hydrochemistry and quality for decision makers and help the rational utilization, protection and scientific management of groundwater resources in agro-pastoral region.
Study area
Location and climate
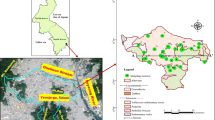
The study area (i.e., Daniudi gasfield area) is located in the convergent area of North China’s Shanxi Province and the Inner Mongolia Autonomous Region. It ranges within longitudes 109°28′6″–110°1′17″E and latitudes 38°43′2″–39°10′16″N, covering about 2112.8 km2 (Fig. 1). The area exhibits a relatively flat topography. The climate of this area is arid and semiarid, and it is characterized by a long, cold, and windy winter, a dust-laden spring, a short, hot, sometimes humid summer and a very short autumn (Li et al. 2013). The annual average temperature of this area is 8.9 °C, and the extremely maximum and minimum temperature is 41.2 and −29.0 °C, respectively. The precipitation is mostly concentrated in summer, ranging from 145.7 to 451.6 mm per year, and average annual precipitation is 356 mm. The annual potential evapotranspiration is 2485.2 mm, and it is most intense in June and July. The largest surface waterbody is Hongjiannao Lake, and no large rivers exist in the area.
Geology and hydrogeology
The study area is tectonically situated in the northeastern Ordos Basin (Fig. 1), which is a Mesozoic sedimentary synclinal basin surrounded by mountains. Quaternary sediments are mainly distributed in the southeast of the area, while Cretaceous formation is mainly exposed in the northwest part (Fig. 1). Older formations, such as the Triassic formation and the Jurassic deposits, are not exposed at the surface and not suitable for groundwater extraction due to great depth. Dolomite, calcite, gypsum, halite, albite, potassium feldspar and illite are major minerals in the Cretaceous and Quaternary aquifers (Hou and Zhang 2008).
The Quaternary porous-medium aquifer (QA), primarily formed by a Pleistocene alluvial lacustrine strata, is mainly composed of medium sand, fine sand and silty sand. Its thickness is greatest in the southern and eastern margins of the region (Fig. 2). The thickness of this aquifer is 22–28 m, 10–85 m and 60–130 m, respectively, revealed by the drill holes in the Menkeqing, Goucha, and Xiaohaotu water source areas in the western, eastern and southern parts of the study area. The depth of water table in this aquifer is usually within 1–3 m of the land surface, but it is more than 3 m at high fixed and semi-fixed sand dune areas and is <1 m deep near lakes. This aquifer is regarded as the main water supply aquifer due to the abundance of water. The Quaternary porous-medium groundwater is mainly recharged from precipitation, regulated by climatic conditions, showing obvious seasonal characters.
Hydrogeologic profile of A–A′ and B–B′ in Fig. 1
The Cretaceous pore-fissure aquifer (KA) of clastic rock partly buried under the Quaternary strata is widely distributed in the area except the southeast corner (Fig. 1). This aquifer is dominated by medium-fine sandstone, fine sandstone and siltstone with cementation and semi-cementation, loose structure, less content of mudstone and unobvious rhythmicity (Hou et al. 2008). The absence of a regionally continuous and stable aquiclude means that there is a close hydraulic connection between the upper phreatic aquifer and the Cretaceous aquifer, so these aquifers behave hydraulically as a unified aquifer system (Fig. 2). Phreatic groundwater occurs in the upper part of the Cretaceous aquifer, and its burial depth is about 12 m on average. The main recharge is from precipitation and upper groundwater. Because of the shallow burial, great thickness (202.0–289.6 m), favorable recharge conditions and local high yielding capacity, this aquifer is viewed as one of the most promising aquifers for supplying groundwater for domestic and agricultural uses. Groundwater in both the Quaternary porous-medium aquifer and the Cretaceous pore-fissure aquifer is of atmospheric origin.
In the study area, land desertification is the most serious environmental problem related to groundwater, while groundwater pollution is slight and local. However, with the exploitation of groundwater in water sources and the development of coal mine and gas field in this area, groundwater, especially shallow groundwater, may face more serious problems of groundwater overdraft and pollution.
Materials and methods
Sample collection and analysis
In the study area, the Quaternary pore groundwater and shallow Cretaceous pore-fissure groundwater (depth <200 m) were sampled in October 2012. A total of 20 Quaternary groundwater samples and 21 Cretaceous groundwater samples were collected from hand-pumped wells and motor-pumped wells. In addition, two surface water samples were taken from Hongjiannao Lake and Chahannao Lake. The sampling locations were set as evenly as possible in the study area, as shown in Fig. 1. Samples were contained in polyethylene bottles. Sample collection, handling and storage follow the standard procedures recommended by the Chinese Ministry of Water Resources (Li et al. 2012). The samples were analyzed by the laboratory of Pony Testing International Group for 15 parameters, including major cations (K+, Na+, Ca2+, Mg2+), major anions (HCO3 −, CO3 2−, SO4 2−, Cl−), fluoride (F−), nitrate nitrogen (NO3 −–N), ammonia nitrogen (NH4 +), total soluble iron (Fe), pH, total hardness (TH) and total dissolved solids (TDS). Among these parameters, Na+ and K+ were determined using a flame photometer, Ca2+, Mg2+ and HCO3 − were analyzed via a titrimetric method, while SO4 2−, Cl−, F−, NO3 −, NH4 + and Fe were determined by spectrophotometry. As electric conductivity (EC) was not measured in the field, it was calculated by AQ·QA (Rock Ware Inc. 2005) for later use (Li et al. 2013).
The quality and suitability of groundwater used for irrigation was assessed by some commonly used indices including the sodium adsorption ratio (SAR), sodium percentage (%Na), residual sodium carbonate (RSC), magnesium ratio (MR), Kelley’s ratio (KR) and permeability index (PI) (Murkute 2014; Amiri et al. 2015). They were calculated by the following equations:
where all ion concentrations are expressed in meq/L. In addition, the Fuzzy Comprehensive Evaluation Method (FCEM) was used to assess groundwater quality and its suitability for domestic and irrigation purposes.
Data analysis and statistics
Statistical analysis was performed through SPSS (version 19.0, SPSS Inc, USA), and correlation coefficients were calculated with it. The hydrochemical facies were obtained from a Piper trilinear diagram drawn by AQ·QA. The geochemical modeling program PHREEQC (version 2.8) was used to calculate the saturation indices (SI) for a suite of minerals, which was commonly used to evaluate the degree of equilibrium between water and the minerals (Aghazadeh and Mogaddam 2010; Lghoul et al. 2014). To reveal ions associations and their origins, principal component analysis (PCA) was performed with SPSS based on selected parameters.
Results and discussion
Chemical characteristics of water
Hydrochemical parameters statistics
A statistical summary of hydrochemical parameters of groundwater samples is presented in Table 1. As shown, the mean concentration of K+ in Quaternary groundwater and Cretaceous groundwater is 1.9 and 2.1 mg/L, while those of Na+ are 14.6 and 44.5 mg/L, respectively. The higher concentration of K+ and Na+ in Cretaceous groundwater may be due to the longer contact between groundwater and the surrounding rock in Cretaceous aquifer and dissolving more minerals containing K+ and Na+. The concentration of Ca2+ in Quaternary groundwater and Cretaceous groundwater ranges from 30.5 to 121.0 mg/L, and 3.7 to 121.0 mg/L, with averages of 56.0 and 48.0 mg/L, respectively, which are presumably derived from carbonate minerals. The average concentration of Mg2+ is 12.3 and 14.9 mg/L for Quaternary groundwater and Cretaceous groundwater, which may be derived from dolomite. As one of the major anions, the concentration of HCO3 − in Quaternary groundwater and Cretaceous groundwater varies from 118.0 to 378.0 mg/L, and 170.8 to 341.0 mg/L, respectively, both with an average more than 200.0 mg/L. SO4 2− generally originates from the dissolution of gypsum or other sulfate, while Cl− is mainly from the dissolution of halite in host rock. The averaged concentrations of SO4 2− and Cl− in Quaternary groundwater and Cretaceous groundwater are 23.1 and 11.3 mg/L, and 32.5 and 24.9 mg/L, respectively. According to Table 1, from the average concentration, the abundance of the major ions in Quaternary groundwater and Cretaceous groundwater is as follows: HCO3 − > SO4 2− > Cl− for anions and Ca2+ > Na+ > Mg2+ > K+ for cations.
As a common element in groundwater, the concentration of F− in this area is somewhat low, with an average of 0.27 and 0.31 mg/L for Quaternary groundwater and Cretaceous groundwater, respectively, which are under the limit value for class I water in the Quality Standard for Groundwater (QSGW) recommended by General Administration of Quality Supervision, Inspection and Quarantine of the PRC. It is concluded that F− in groundwater in the study area is derived from the natural geological environment, not related to human activities. By contrast, elevated concentrations of NH4 + and NO3 −–N usually suggest groundwater contamination due to the use of fertilizer and/or the irrigation of wastewater (Li et al. 2012). The concentrations of NO3 −–N in Quaternary groundwater and Cretaceous groundwater vary from <0.04 to 31.30 mg/L, and <0.04 to 42.80 mg/L (<below the limit of detection), while those of NH4 + are <0.02–1.61 mg/L and <0.02–0.41 mg/L, respectively, both with a maximum concentration over the permitted limits for drinking purposes in the QSGW. Fe in the Quaternary groundwater has higher concentration, with an average of 0.89 mg/L, which is higher than the maximum desirable value.
In addition, the EC values of Quaternary groundwater and Cretaceous groundwater range from 258.3 to 693.9 μS/cm, and 296.9 to 968.4 μS/cm, respectively. The average TH, TDS and pH values of Quaternary and Cretaceous groundwater are 200.6 and 196.4 mg/L, 260.2 and 327.7 mg/L, and 7.7 and 8.1, respectively. Overall, the results of hydrochemical analysis have shown that human activities and natural geological environment had important influence on chemical composition of groundwater in the study area.
Hydrochemical facies
Piper trilinear diagrams have been widely used to understand the hydrogeochemical regime of a study area (Li et al. 2013). Based on the chemical analysis data, all samples were plotted in the Piper diagram by AQ·QA (Rock Ware Inc. 2005) and 50 % increments were then used to define the hydrochemical facies (Ravikumar and Somashekar 2011).
As shown in the diamond part (Fig. 3), all Quaternary groundwater samples fall in zone 5, which suggests that carbonate hardness exceeds 50 % and weak acids exceed strong acids. With respect to cations, all Quaternary groundwater samples are plotted in zone A, indicating the dominance of Ca2+. With respect to anions, all Quaternary groundwater samples are plotted in zone E, suggesting the dominance of HCO3 −. The dominant hydrochemical facies of the Quaternary groundwater is HCO3–Ca type.
Piper diagram of water samples (after Li et al. 2013, red square for groundwater from QA, black circle for groundwater from KA, blue triangle for surface water from lakes)
By contrast to the Quaternary groundwater samples, the Cretaceous groundwater samples of HCO3–Na type and mixed type can be found from the diamond part, but these samples account for only a small proportion of all Cretaceous groundwater samples. Most Cretaceous groundwater samples are distributed in zone 5 of the diamond part, indicating a predominance of calcium bicarbonate type (Li et al. 2012). With respect to cations, Cretaceous groundwater samples are divided into Ca type, Na + K type and mixed type, suggesting that other processes except for dissolution of carbonate minerals, such as ion exchange and weathering of silicate, may take place in the study area. With respect to anions, 95 % of Cretaceous groundwater samples are bicarbonate type, and no samples are SO4 type and Cl type, which suggests that weathering of carbonate minerals is the most important factor controlling groundwater chemistry, and the dissolution of gypsum and evaporite is much less influential (Li et al. 2012).
In addition, surface water samples collected from lakes are sodium chloride type and are not suitable for drinking, indicating strong evaporation and concentration in low-lying areas. Because of active water cycle and far distance of groundwater sampling locations from the lakes, the effect of evaporation on the hydrochemistry of the collected groundwater is not obvious.
Sources of major ions and hydrogeochemical evolution
Correlation analysis
Here Pearson’s correlation coefficients were employed to illustrate and measure associations between major parameters (Table 2). The correlation coefficient >0.7 indicates a strong correlation between two parameters, while the correlation coefficient >0.5 and <0.7 indicates a moderate correlation (Shyu et al. 2011).
As shown in Table 2, the strong correlations between Na+ and Mg2+ (0.84), HCO3 − (0.88) and Cl− (0.86) in the Quaternary groundwater suggest that Na+ is derived from silicate weathering except for the dissolution of halite. The strong correlations between HCO3 − and Ca2+ (0.70), and Mg2+ (0.84), and the moderate correlation between Ca2+ and Mg2+ (0.55), suggest the dissolution of carbonate minerals (calcite and dolomite). The moderate correlations between K+ and Na+ (0.56) and HCO3 − (0.57) show that K+ is mainly derived from silicate weathering. In addition, TDS is correlated with Ca2+, Na+, Mg2+ and HCO3 −, especially the strong correlation between TDS and Ca2+ (0.94), suggesting the continuous increase in these ions concentration along groundwater flow path (Wu et al. 2013). The concentration of SO4 2− is moderately correlated with that of Ca2+ (0.53), suggesting the possible dissolution of gypsum, and the carbonate dissolution/precipitation and ion exchange may account for the lack of significant correlation (Li et al. 2013).
As to the Cretaceous groundwater, Na+ is found to be correlated with SO4 2− (0.68) and Cl− (0.52), indicating that Na+, Cl− and SO4 2− may be derived from mirabilite and halite. The strong correlation between Ca2+ and Mg2+ (0.80), and moderate correlation between Mg2+ and HCO3 − (0.6), suggests that Ca2+ and Mg2+ may be derived from magnesium-rich carbonate. K+ is also correlated with HCO3 − (0.62), indicating that silicate weathering is one of the sources of K+ and HCO3 −. Moderate correlation between K+ and Mg2+ (0.62) indicates a possible silicate source of Mg2+, which is confirmed by the plots shown in Fig. 5b.
Principle component analysis (PCA)
In hydrochemistry, PCA is often used to reveal ion associations and their origins (Zhang et al. 2012; Atanackovic et al. 2013; Xiao et al. 2014b). PCAs for the samples from the Quaternary aquifer and the Cretaceous aquifer were carried out, respectively, and three principle components (PCs) were extracted (Table 3). Significant (i.e., high) principal component loadings were considered for those whose absolute value was >|0.4| (black bold in Table 3), and parameters with high loadings were considered to be the controlling parameters (variables) for the PC (Atanackovic et al. 2013).
For the Quaternary groundwater, the cumulative variance explained by the three PCs is 90.67 %. The PC1, which explains 57.84 % of the total variance, is dominated by the following variables: Na+, Ca2+, Mg2+, HCO3 −, Cl−, TH, TDS and EC. PC2 explains 21.26 % of the variance and has high loadings of K+, Na+, Ca2+, SO4 2− and TH. PC3 explains 11.57 % of the variance and is dominated by pH. The parameters within PC1 suggest that the dominant process leading to the formation of the chemical composition of the Quaternary groundwater is the dissolution of surrounding minerals (carbonate and halite). Theoretically, the dissolution of calcite and dolomite introduces Ca2+, Mg2+ and HCO3 − ions into groundwater at a ratio of between 1:1 and 1:2 (Li et al. 2012; Wu et al. 2013). Figure 4a confirms the dissolution of calcite and dolomite in the Quaternary groundwater. In addition, strong correlation was found between Na+ and Cl− in PC1, indicating the dissolution of halite. The PC2 suggests that weathering and dissolution of sulfur-bearing minerals, such as gypsum, also affect the hydrochemical constitution of the Quaternary groundwater. The PC3 is characterized by high positive pH loadings. The pH of groundwater refers to the form in which CO2 is present. The pH values of the Quaternary groundwater samples range from 7.3 to 8.22, indicating the significant influence of bicarbonate ions (Sethy et al. 2016).
For the Cretaceous groundwater, the cumulative variance explained by the three PCs is 85.84 %. The PC1 explains 50.30 % of the variance and has high loadings of K+, Ca2+, Mg2+, HCO3 −, Cl−, SO4 2−, TH, TDS and EC. PC2 explains 23.70 % of the variance and is dominated by Na+, Ca2+, SO4 2−, Cl− and pH. PC3 has high loadings of K+, HCO3 − and TDS, which explains 11.84 % of the variance. The PC1 suggests that salinization of Cretaceous groundwater is mainly due to the dissolution of carbonate minerals (calcite and dolomite) and evaporite (gypsum and sylvite). Figure 4a shows that the dissolution of calcite and dolomite is an important source of Ca2+ and Mg2+ in the Cretaceous groundwater, but the levels of these ions are also influenced by other factors (Li et al. 2012), such as ion exchange. In PC2, absolute values of loadings for Na+ and Ca2+ are >0.5, but the loading of Ca2+ is negative, which suggests that ion exchange probably has happened, increasing concentration of Na+ and decreasing concentration of Ca2+. Besides, PC2 has high positive loading of pH, which indicates that high pH value has a negative effect on the dissolution of carbonate minerals (Abbas et al. 2015). PC3 suggests that weathering of silicate including K-feldspar is an important source of K+ and HCO3 − and is more important in the groundwater of lower TDS.
Ratios of major ions
Figure 4a shows that the levels of Ca2+, Mg2+ and HCO3 − are not only determined by calcite and dolomite but also influenced by silicate weathering and/or ion exchange (Li et al. 2012; Daniele et al. 2013). A higher Ca/Mg molar ratio (>2) indicates the dissolution of silicate minerals, which contribute Ca2+ and Mg2+ to groundwater (Kumar et al. 2008). Figure 4b suggests that most samples have a silicate source of Ca2+ and Mg2+. The ratio of Na/Cl can reflect the balance and origin of sodium and chloride in groundwater (Salem et al. 2015). In Fig. 4c, the majority of the groundwater samples are distributed above the line with slope one, suggesting that the Na+ is not only derived from dissolution of halite, but also may be derived from other Na-rich minerals and ion exchange.
The Na-normalized Ca versus HCO3 plot (Fig. 5a) and Na-normalized Ca versus Mg plot (Fig. 5b) further illustrate that Cretaceous groundwater samples are influenced by silicate weathering, carbonate dissolution and evaporite dissolution, and Quaternary groundwater samples are influenced mainly by silicate weathering and carbonate dissolution (Mukherjee and Fryar 2008; Xiao et al. 2015; Abbas et al. 2015).
Ion exchange
The excess of Na+ over Cl− in Fig. 4c and the deficit in Ca2+ + Mg2+ in Fig. 4a may be explained by ion exchange. Plot of (Ca2+ + Mg2+)–(SO4 2− + HCO3 −) versus Na+ + K+ − Cl– is widely used to test the possibility that ion exchange significantly affects groundwater compositions (Jalali 2006; Abbas et al. 2015; Amiri et al. 2015). If ion exchange is significant composition-controlling process, the relation between these two parameters should be linear with a slope of −1. Most of the samples are located near the straight line of slope −1 (Fig. 4d), which suggests the existence of ion exchange both in the QA and CA.
The Chloro-alkaline indices (CAI-1 and CAI-2) proposed by Schoeller (1977) can also indicate the ion exchange. Negative CAI-1 and CAI-2 indicate the exchange of Ca2+ and/or Mg2+ in groundwater with Na+ and/or K+ in aquifer materials, while in the instance of a reverse ion exchange, both indices are positive (Chidambaram et al. 2012). The two indices are usually calculated by the following formulas (Li et al. 2012):
where all ions are expressed in meq/L. The Chloro-alkaline indices of all the collected groundwater samples but sample W31, S04 and S08 are less than zero (Fig. 6), which suggests that ion exchange is widespread in the study area. Therefore, ion exchange is also responsible for the excess of Na+ in most groundwater samples.
Geochemical modeling
Saturation index (SI) is commonly used to understand the equilibrium state of the water with respect to a mineral phase (Amiri et al. 2015). It is obtained by the following equation:
where IAP is the ion activity product of the solution, and K S (T) is the equilibrium constant of the reaction considered at temperature T (He et al. 2015). SI values less than zero indicate unsaturation (dissolution), those exactly zero indicate saturation (equilibrium), while values greater than zero suggest oversaturation (precipitation). Plots of saturation indexes of common minerals (calcite, dolomite, gypsum, halite) and related ions are shown in Fig. 7, which illustrate that groundwater in the QA and KA was largely unsaturated with respect to gypsum and halite, indicating the possible dissolution of these minerals. The eventual dissolution of these minerals was supported by the strong positive relationships between SI values and the related ions in Fig. 7a and b (Li et al. 2014b). Quite a few groundwater samples are saturated or oversaturated with respect to dolomite (Fig. 7c) and calcite (Fig. 7d), suggesting that these carbonate mineral phases are present in the corresponding host rock (Jalali 2006). The moderate positive relationships between SI values of calcite and dolomite and some of the ions indicate the dissolution and precipitation of carbonate minerals and ion exchange.
Plots of SI for gypsum, calcite, dolomite and halite versus ions concentration in meq/L (the same legend with Fig. 4)
Groundwater chemistry formation mechanisms
The formation of groundwater chemistry is usually influenced by three factors, including rock weathering, evaporation and crystallization and precipitation. The dominant factor is often determined by Gibbs diagrams (Rao 2002; Jalali 2006; Aghazadeh and Mogaddam 2010; Amiri et al. 2015; Salem et al. 2015). In the present study, all samples fell within the rock-dominance zone of Gibbs diagrams (Fig. 8), indicating that the major ion chemistry of the Quaternary groundwater and the Cretaceous groundwater was primarily controlled by rock-forming mineral weathering (Murkute 2014). Several samples were plotted toward the evaporation-dominance zone, suggesting very secondary evaporation. In addition, 28.6 % of the Cretaceous groundwater samples are distributed in rock-dominance zone with Na+/(Na+ + Ca2+) ratios more than 0.5, and together with relatively constant TDS values in Fig. 8a, which is likely indicative of ion exchange (Li et al. 2012).
Gibbs plots illustrating mechanisms governing groundwater chemistry (after Gibbs 1970)
Groundwater quality assessment
Based on the hydrochemical analysis data, the groundwater quality and its suitability for drinking, livestock and irrigation uses is assessed.
Assessment of groundwater quality for drinking purposes
The permissible limits of WHO and the QSGW(class III) were employed to assess the suitability for drinking purposes. The parameters affecting groundwater quality were compared with the limits (Table 1). The results show that the major parameters of concern are NO3 −–N, NH4 +, Fe, pH and TH (Table 4). With respect to these parameters, most of the groundwater from KA and QA is of a suitable quality for potable use.
Plots of TDS and TH (Fig. 9) illustrate that all groundwater samples are freshwater, and all groundwater samples are in acceptable range of hardness (TH <450) except sample S04 which is not fit for direct drinking due to its high hardness.
Although the quality of groundwater can be assessed by comparing single parameter with limit, it cannot show the combined effects of individual parameters on the over water quality. The fuzzy comprehensive evaluation method (FCEM), which uses membership expressing the degree of each parameter belonging to each water quality category, can address the imprecision of water quality management objectives and the composite effects of the evaluation indices on water quality. So far, this method has become a usual method for evaluating groundwater quality (Dahiya et al. 2007; Singh et al. 2007; Zhang et al. 2012). In this study, FCEM is employed to synthetically assess the groundwater quality and suitability for drinking. A total of eight representative parameters, including Cl−, F−, NO3 −–N, NH4 +, SO4 2−, Fe, TH and TDS, are taken into account in fuzzy comprehensive evaluation. The results of fuzzy comprehensive evaluation according to the QSGW are shown in Table 5.
As shown in Table 5, the Quaternary groundwater samples are divided into four classes. The number of samples within classI, III, IV and V is 13, 2, 4 and 1, respectively, and accounts for 65, 10, 20 and 5 % of Quaternary groundwater, respectively. The Cretaceous groundwater samples are classified into classI, III and IV. The number of samples within classI, III and IV is 15, 2 and 4, respectively, and accounts for 71.4, 9.5 and 19.1 % of Cretaceous groundwater. According to the standards, groundwater samples within class I, II and III are suitable to drink, groundwater samples within class IV can be used as drinking water after proper treatment and directly used for agriculture and industry, while groundwater samples within class V are not suitable to drink or irrigate. In sum, 78.1 % of the total groundwater samples are fit for direct drinking, 19.5 % of the samples are suitable to drink after proper treatment, while 2.4 % of the samples are not fit for drinking or irrigation. This suggests that the quality of groundwater in the study area is generally good.
Samples not suitable to drink under ordinary condition include sample W04, W46, W48, W50, W53, S04, S05, S06 and S09 because the concentrations of NH4 +, Fe and/or NO3 − exceed the allowable values. As these samples are located in the villages of a relatively dense population, where improper disposal of human and animal waste prevails, the contamination of ammonia nitrogen and nitrate indicates the impacts of human activities on the hydrochemical composition of groundwater. The concentration of Fe exceeds the guideline values for drinking water, which is probably caused by the unique geological environment.
Assessment of groundwater quality for irrigation purposes
The area of farmland in the study area is about 230.3 km2, which accounts for 10.9 % of the total area. Groundwater is one of the main sources of the irrigation water in this area. In the present study, the United States Salinity Laboratory (USSL) diagram (1954) and Wilcox diagram (1948) which include EC, and some indices, such as SAR, %Na, RSC, MR, KR and PI, were applied to evaluate the suitability of groundwater for irrigation. Results are shown below (Table 6).
SAR used to measure the sodium (alkali) hazard is an important criterion for determining the suitability of groundwater for irrigation (Amiri et al. 2015). According to the SAR, groundwater can be classified as waters with low (SAR ≤ 10), medium (10 < SAR < 18), high (18 < SAR ≤ 26) and very high (SAR > 26) sodium hazard. The SAR values of groundwater samples from the study area range from 0.2 to 12.8, with an average of 1.4, suggesting that sodium hazard of the samples is low or medium. The plots of sampled groundwater in the US Salinity Laboratory diagram (Fig. 10) shows that all Quaternary groundwater samples and most Cretaceous groundwater samples belong to the category C2S1, while several Cretaceous groundwater samples belong to the category C3S1 and C2S2, suggesting that all groundwater samples are suitable for agricultural purposes with respect to EC and SAR.
High sodium percentage in irrigation water will hinder the growth of plants and reduce the permeability of the soil (Amiri et al. 2015). For irrigation water, %Na <60 is acceptable (Xiao et al. 2014a). The %Na values of sampled groundwater range from 8.4 to 93.2, with an average of 24.2. Samples of %Na >60 account for 7.3 %. According to the Wilcox diagram (Fig. 11), most groundwater samples (87.8 %) are plotted in the excellent to good zone, while samples W19 and S01 fall in the permissible to doubtful zone, and sample W21 is in doubtful to unsuitable zone.
Plot of %Na versus EC (Wilcox 1948)
The suitability of water for irrigation is influenced by the excess sum of carbonate and bicarbonate in water over the sum of calcium and magnesium, which is expressed as RSC. If the RSC value of water is >2.5 meq/L, then the water is unsuitable for irrigation purpose (Murkute 2014). With respect to RSC, 92.7 % of the groundwater samples are suitable for irrigation purpose.
Another index to determine the suitability of groundwater for irrigation purposes is magnesium ratio (MR) (Amiri et al. 2015). The MR values <50 indicate suitability for irrigation (Murkute 2014). 92.7 % of the groundwater samples from the study area are suitable for irrigation with respect to the MR.
Kelly’s ratio (KR) proposed by Kelly (1963) is a significant indicator in evaluation of water quality for irrigation. The KR values of one or less than one indicate good quality of water for irrigation, whereas above one are suggestive of unsuitability for agricultural purpose (Naseem et al. 2010). According to KR values, 90.2 % of the groundwater samples from the study area are suitable for irrigation.
PI is a parameter reflecting the influence of ion content in water on soil permeability. If the PI values are <25, then the water is not suitable for irrigation (Amiri et al. 2015). PI value results show that all the groundwater from the study area is fit for irrigation.
Conclusion
In the study area, the Quaternary groundwater and shallow Cretaceous groundwater contain relative concentrations of major ions in the order of HCO3 − > SO4 2− > Cl− for anions and Ca2+ > Na+ > Mg2+ > K+ for cations. Statistical results of other ions suggest that the groundwater have been polluted by human activities somewhat. In general, the hydrochemical facies are predominantly of HCO3–Ca type, but for shallow Cretaceous groundwater, HCO3–Na type and mixed type exist in small amounts.
According to Gibbs diagram, rock (mineral) weathering and ion exchange are the most important processes controlling groundwater chemistry in this area, while the effect of evaporation is not obvious. Ion exchange is also certified by CAI and ratios of some ions. Based on correlation analysis, PCA and ion ratios, carbonate and silicate minerals are found to be important sources of major hydrochemical constituent, while evaporite are secondary for most samples. The saturation index calculated by geochemical modeling shows a suitable hydrochemical environment for gypsum and halite dissolution (negative saturation index) and carbonate (calcite and dolomite) dissolution/precipitation.
Fuzzy comprehensive evaluation results show that all the groundwater samples from the study area are suitable for drinking, except for the ones polluted by human activities. The USSL diagram, Wilcox diagram and other indices suggest that almost all groundwater samples are suitable for irrigation under ordinary conditions. Thus, before the gas field goes into construction and production, most of the shallow groundwater in this area has good quality for drinking and irrigation.
This study provides detailed information about groundwater chemistry of an agro-pastoral area with multiple methods, which could be a reference for the study of groundwater geochemistry and quality in similar areas, and suggests that combination of traditional hydrochemical analysis and statistical analysis could help achieve a more comprehensive understanding of groundwater hydrochemistry and processes governing that geochemistry.
References
Abbas Z, Su C, Tahira F, Mapoma HWT, Aziz SZ (2015) Quality and hydrochemistry of groundwater used for drinking in Lahore, Pakistan: analysis of source and distributed groundwater. Environ Earth Sci 74:4281–4294. doi:10.1007/s12665-015-4432-5
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area, northwest of Iran. J Environ Prot 01:30–40. doi:10.4236/jep.2010.11005
Amiri V, Sohrabi N, Dadgar MA (2015) Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ Earth Sci 74:6163–6176. doi:10.1007/s12665-015-4638-6
Atanackovic N, Dragisic V, Stojkovic J, Papic P, Zivanovic V (2013) Hydrochemical characteristics of mine waters from abandoned mining sites in Serbia and their impact on surface water quality. Environ Sci Pollut Res Int 20:7615–7626. doi:10.1007/s11356-013-1959-4
Cao Y (2009) Groundwater circulation patterns of typical lake area in Northern Ordos Cretaceous Basin. Dissertation, Jilin University (in Chinese)
Chidambaram S, Anandhan P, Prasanna MV, Srinivasamoorthy K, Vasanthavigar M (2012) Major ion chemistry and identification of hydrogeochemical processes controlling groundwater in and around Neyveli Lignite Mines, Tamil Nadu, South India. Arab J Geosci 6:3451–3467. doi:10.1007/s12517-012-0589-3
Dahiya S, Singh B, Gaur S, Garg VK, Kushwaha HS (2007) Analysis of groundwater quality using fuzzy synthetic evaluation. J Hazard Mater 147:938–946. doi:10.1016/j.jhazmat.2007.01.119
Daniele L, Vallejos Á, Corbella M, Molina L, Pulido-Bosch A (2013) Hydrogeochemistry and geochemical simulations to assess water–rock interactions in complex carbonate aquifers: the case of Aguadulce (SE Spain). Appl Geochem 29:43–54. doi:10.1016/j.apgeochem.2012.11.011
Dou Y (2010) Study on hydrogeochemical evolution and circulation in north of Ordos Cretaceous groundwater basin. Dissertation, Chang’an University (in Chinese)
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
He J, Ma J, Zhao W, Sun S (2015) Groundwater evolution and recharge determination of the Quaternary aquifer in the Shule River basin, Northwest China. Hydrogeol J 23:1745–1759. doi:10.1007/s10040-015-1311-9
Hou G, Zhang MS (2008) Study on groundwater exploration in the Ordos Basin. Geological Publishing House, Beijing, pp 1–20 (in Chinese)
Hou G, Liang Y, Su X (2008) Groundwater systems and resources in the Ordos Basin, China. Acta Geol Sin 82(5):1061–1069
Jalali M (2006) Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol 52:1133–1149. doi:10.1007/s00254-006-0551-3
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95:355–391
Kudoda AM, Abdalla OA (2015) Hydrochemical characterization of the main aquifers in Khartoum, the capital city of Sudan. Environ Earth Sci 74:4771–4786. doi:10.1007/s12665-015-4464-x
Kumar M, Kumari K, Singh UK, Ramanathan AL (2008) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884. doi:10.1007/s00254-008-1367-0
Lghoul M, Maqsoud A, Hakkou R, Kchikach A (2014) Hydrogeochemical behavior around the abandoned Kettara mine site, Morocco. J Geochem Explor 144:456–467. doi:10.1016/j.gexplo.2013.12.003
Li P, Wu J, Qian H (2012) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225. doi:10.1007/s12665-012-2049-5
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32:195–206. doi:10.1007/s10230-013-0234-8
Li P, Wu J, Qian H (2014a) Hydrogeochemistry and Quality assessment of shallow groundwater in the southern part of the Yellow River alluvial plain (Zhongwei section), Northwest China. Earth Sci Res J 18(1):27–38
Li Y, Hu FS, Xue ZQ, Yu YQ, Wu P (2014b) Hydrogeochemical and isotopic characteristics of groundwater in the salt chemical industrial base of Guyuan City, northwestern China. Arab J Geosci 8:3427–3440. doi:10.1007/s12517-014-1442-7
Liu F, Song X, Yang L, Han D, Zhang Y, Ma Y, Bu H (2015) The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci Total Environ 538:327–340. doi:10.1016/j.scitotenv.2015.08.057
Mukherjee A, Fryar AE (2008) Deeper groundwater chemistry and geochemical modeling of the arsenic affected western Bengal basin, West Bengal, India. Appl Geochem 23:863–894. doi:10.1016/j.apgeochem.2007.07.011
Murkute YA (2014) Hydrogeochemical characterization and quality assessment of groundwater around Umrer coal mine area Nagpur District, Maharashtra, India. Environ Earth Sci 72:4059–4073. doi:10.1007/s12665-014-3295-5
Naseem S, Hamaza S, Bashir E (2010) Ground water geochemistry of winder agricultural farms, Balochistan, Pakistan and assessment for irrigation water quality. Eur Water 31:21–32
Odukoya AM (2015) Geochemical and quality assessment of groundwater in some Nigerian basement complex. Int J Environ Sci Technol 12:3643–3656. doi:10.1007/s13762-015-0789-y
Rao NS (2002) geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41:552–562
Ravikumar P, Somashekar RK (2011) Geochemistry of groundwater, Markandeya River Basin, Belgaum district, Karnataka State, India. Chin J Geochem 30:51–74. doi:10.1007/s11631-011-0486-6
Re V, Sacchi E, Mas-Pla J, Mencio´ A, El Amrani N (2014) Identifying the effects of human pressure on groundwater quality to support water management strategies in coastal regions: a multi-tracer and statistical approach (Bou-Areg region, Morocco). Sci Total Environ 500:211–223
Salem ZE, Atwia MG, El-Horiny MM (2015) Hydrogeochemical analysis and evaluation of groundwater in the reclaimed small basin of Abu Mina, Egypt. Hydrogeol J 23:1781–1797. doi:10.1007/s10040-015-1303-9
Schoeller H (1977) Geochemistry of groundwater. Groundwater studies-an international guide for research and practice. UNESCO, Paris, pp 1–18
Sethy SN, Syed TH, Kumar A, Sinha D (2016) Hydrogeochemical characterization and quality assessment of groundwater in parts of Southern Gangetic Plain. Environ Earth Sci 75:232. doi:10.1007/s12665-015-5049-4
Shyu GS, Cheng BY, Chiang CT, Yao PH, Chang TK (2011) Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int J Environ Res Pub Health 8:1084–1109. doi:10.3390/ijerph8041084
Singh B, Dahiya S, Jain S, Garg VK, Kushwaha HS (2007) Use of fuzzy synthetic evaluation for assessment of groundwater quality for drinking usage: a case study of Southern Haryana, India. Environ Geol 54:249–255. doi:10.1007/s00254-007-0812-9
Tanasković I, Golobocanin D, Miljević N (2012) Multivariate statistical analysis of hydrochemical and radiological data of Serbian spa waters. J Geochem Explor 112:226–234. doi:10.1016/j.gexplo.2011.08.014
Valdes D, Dupont J-P, Laignel B, Ogier S, Leboulanger T, Mahler BJ (2007) A spatial analysis of structural controls on Karst groundwater geochemistry at a regional scale. J Hydrol 340(3–4):244–255
Wang H, Jiang X-W, Wan L, Han G, Guo H (2015) Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin. J Hydrol 527:433–441. doi:10.1016/j.jhydrol.2015.04.063
Wilcox LV (1948) The quality of water for irrigation use. US Dept of Agricultural Tech Bull 1962, Washington
Wu J, Li P, Qian H, Duan Z, Zhang X (2013) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7:3973–3982. doi:10.1007/s12517-013-1057-4
Xiao J, Jin Z, Wang J (2014a) Assessment of the hydrogeochemistry and groundwater quality of the Tarim River Basin in an extreme arid region, NW China. Environ Manage 53:135–146. doi:10.1007/s00267-013-0198-2
Xiao J, Jin Z, Wang J (2014b) Geochemistry of trace elements and water quality assessment of natural water within the Tarim River Basin in the extreme arid region, NW China. J Geochem Explor 136:118–126. doi:10.1016/j.gexplo.2013.10.013
Xiao J, Jin ZD, Wang J, Zhang F (2015) Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quatern Int 380–381:237–246. doi:10.1016/j.quaint.2015.01.021
Yin L, Hou G, Dou Y, Tao Z, Li Y (2009) Hydrogeochemical and isotopic study of groundwater in the Habor Lake Basin of the Ordos Plateau, NW China. Environ Earth Sci 64:1575–1584. doi:10.1007/s12665-009-0383-z
Zhang B, Song X, Zhang Y, Han D, Tang C, Yu Y, Ma Y (2012) Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain, Northeast China. Water Res 46:2737–2748. doi:10.1016/j.watres.2012.02.033
Acknowledgments
The research was supported by National Natural Science Foundation of China (No. 41572227), Fundamental Research Funds for the Central Universities (No. 2652015125) and Project supported by Ministry of Land and Resources of China (201511056-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, C., Wu, X., Mu, WP. et al. Hydrogeochemical characterization and suitability assessment of groundwater in an agro-pastoral area, Ordos Basin, NW China. Environ Earth Sci 75, 1356 (2016). https://doi.org/10.1007/s12665-016-6123-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6123-2