Abstract
The aims of the study were (a) the assessment of growth and physiological response of a weed/alternative crop (purslane), an ornamental plant (geranium) and an edible vegetable (lettuce) to Zn- and Cd-contaminated industrial soil and (b) the investigation of the possible exclusion or accumulation process of these plants concerning Zn and Cd, evaluating thus their phytoremediation potential. Both Zn and Cd concentrations increased significantly in all three plant species in the contaminated soil compared to the uncontaminated control. Metal soil-to-plant transfer coefficient was lower in the first soil compared to control, indicating slower metal uptake with increased metal concentrations in soil. Geranium exhibited a growth promotion along with a better photosynthetic performance in the industrial soil. Purslane displayed an altered architecture and a more massive old leaf cohort, but its overall growth remained unaffected by increased [Zn] and [Cd], similarly to lettuce. No effects on PSII photochemical efficiency and photosynthetic pigments of all studied species were recorded. We conclude that metal uptake by plants remained within the limits of favorable growth and metal bioavailability was determined by (a) the fact that metals were deposited over long periods and were thus strongly retained by soil colloidal phases and (b) Cd/Zn antagonism. The results highlight the importance of soil history component in shaping heavy metal behavior, determining thus their bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are trace elements, which have detrimental effects on plants (Ahmadpoor et al. 2010) and humans alike (Cao et al. 2015) when in elevated concentrations. Some of them (e.g., Zn, Fe, Mn, Cu) are essential micronutrients to plants (e.g., Zn, Fe, Mn, Cu), while others (e.g., Cd, Pb, Cr, Ni) do not have any known physiological function to plants (Kabata-Pendias 2001). Although heavy metals may derive in soils from either pedogenetical processes (weathering of metal-containing rock minerals) or due to anthropogenic depositions, high heavy metal concentrations in soil are usually linked to anthropogenic activity. They are typically concentrated around the geographical locality of certain industrial activities due to metal-loaded particles from industrial plant fumes being deposited onto soils or due to the disposing or the application of metal-borne wastes (e.g., sewage sludge or wastewater) to soils. For example, Huong et al. (2010) found increasing metal concentrations in wastewater-treated soil samples as the distance decreased to a wastewater canal. Thus, contaminated soils are rather expected to be found in countries with heavy industrial activity. However, elevated heavy metal concentrations are also likely to be found in areas with unregulated industries or with insufficient law enforcement concerning waste management regulations.
Heavy-metal-contaminated soils are difficult to remediate, because inorganic substances cannot be decomposed neither can they change in nature over time. A cost-effective, environment-friendly restoration strategy is phyto-remediation, i.e., the introduction of certain plants that tolerate and (hyper) accumulate high metal concentrations in their body thus cleaning the soil over time, provided they do not enter in any way the human food chain (Chaney et al. 2007; Ali et al. 2013). Alternatively, metal excluder plants could also be introduced, which restrict the movement of metals from their roots and prevent their translocation to the aerial parts over a broad range of metal concentration in soil (Baker 1981; Alvarenga et al. 2014). In the latter case, the selection of suitable species would allow the commercial exploitation of contaminated areas.
Accurate risk assessment and management of polluted soils require integration of all aspects modulating contaminant transfer to plants. Intensive experimental efforts have been devoted to the identification of the underlying physical, chemical, and biological factors that ultimately govern this transfer (Orcutt and Nilsen 2000; Kabata-Pendias 2001; Valerio et al. 2007; Cherif et al. 2011). Although many aspects are still to be elucidated, the bioavailability concept that has recently been proposed integrates the multi-level key processes involved (Chojnaka et al. 2005; Peijnenburg et al. 2007; Van Gestel 2008; Pauget et al. 2015). Except for the obvious role of soil properties and plant physiological characteristics, especially complex processes regulating homeostasis, the bioavailability approach defines the effective exposure time as a crucial factor. Therefore, using this conceptual framework in designing studies of heavy metal effects on plants requires biomonitoring in realistic conditions in terms of substrate and time. The overall approach allows a “physiologically defined” evaluation of metal bioavailability (Pauget et al. 2015).
It has long been recognized that leafy vegetables (e.g., lettuce), especially those which may be consumed as raw salads, are more sensitive to elevated heavy metal concentrations (Valerio et al. 2007). Although there have been research efforts dealing with such plants, there is a void in the literature concerning “alternative” species (Ho et al. 2013). The latter could include leafy vegetables which are not widely traded, those traded not on a weight basis but rather on other characteristics, such as content in medicinal substances and essential oils, or non-edible species, such as ornamental plants. Thus, there is scarce information on the potential of use of such species in contaminated soils, and it is also unknown whether they would exhibit metal accumulation or metal exclusion properties.
The present study aims at addressing the need for exploring alternative species potential for cultivation in contaminated soils. Purslane and geranium were selected on the basis of their tolerance and adaptability under various biotic and abiotic stress conditions (Sánchez-Blanco et al. 2009; Kale et al. 2015). Purslane (Portulaca oleracea) is an annual and succulent weed, which is a widespread, fast growing, successful invasive species. In several parts of the world, it is a commercially cultivated vegetable, because it is a rich source of essential fatty acids and of high nutritive value, having a high potential to be used as a functional food (Gharneh and Hassandokht 2012; Kale et al. 2015). Horseshoe geranium (Pelargonium zonale) is an ornamental species, important part of urban green areas. Together with the weed and ornamental species, a common edible vegetable, lettuce (Lactuca sativa), was examined. A heavily contaminated soil with Zn and Cd from the industrial area of Elefsina and an uncontaminated rural soil were used for a long experimental period (2 months) in order to (a) assess the three plant species growth and physiological response to elevated heavy metal concentrations and (b) investigate the possible exclusion and accumulation processes of these plants concerning Cd and Zn, thus evaluating their phytoremediation potential.
Materials and methods
Growth conditions
Soil samples were obtained from an agricultural area with low metal content, Velestino (soil A thereafter), and from an area with a history in heavy industrial activity, Elefsina (soil B thereafter), in Central Greece. Soils were selected so that they may differ only in heavy metal content, but had similar pH (soil A = 8.82, soil B = 8.50), organic matter (soil A = 2.30 %, soil B = 5.94 %), and texture (soil A = sandy clay loam, soil B = sandy loam), and they both were calcareous (CaCO3 in soil A = 9.6 %, and in soil B = 33.1 %). Each soil was air-dried, sieved through a 5-mm sieve, and placed in 1-L pots, which were sown with purslane (Portulaca oleracea), horseshoe geranium (Pelargonium zonale), and lettuce (Lactuca sativa). The resultant 60 pots (2 soils × 3 species × 10 replicates) were placed into a greenhouse at 21 ± 2 °C, and plants were grown for 60 days under conditions of sufficient water supply.
Measurements during experimental period
During the growth period, various plant physiological and morphological characteristics were measured. Gas-exchange measurements were performed with a portable photosynthesis system (LCpro+, ADC BioScientific Ltd, Hoddesdon, UK) under natural sunlight and in ambient concentrations of CO2 and H2O. The climate control capability of the instrument was used for keeping the leaf chamber temperature in accordance with ambient temperature ±1 °C. Chlorophyll, a in vivo fluorescence, was measured with a PAM-2100 (Walz, Effeltuch, Germany) chlorophyll fluorometer, and PSII photochemical efficiency was determined as light-adapted PSII yields via Fv/Fm and ETR.
Chlorophyll content was measured with a portable chlorophyll meter (Minolta, SPAD-502). The SPAD values were transformed into actual chlorophyll concentrations by using a reference curve. For this purpose, leaves from plants not used in the experiment and having various SPAD values were extracted with 80 % acetone, and chlorophylls were estimated using the equations of Lichtenthaler and Wellburn (1983).
Concerning morphological parameters, total leaf area and leaf number at plant level were recorded throughout the experimental period. At the same dates, leaf thickness measurements were performed using a leaf micrometer (Mitutoyo, UK).
Final harvest and soil analysis
At final harvest, plants were divided into leaves, stems, and roots. Leaves were further divided into different cohorts to reveal age-dependent changes and classified as new, mature, and old cohorts depending on their size and position in the stem. The aerial plant parts were weighed for fresh weight, and then leaf number and area of each cohort were recorded. The dry mass of plant parts was measured after drying at 80 °C for 24 h. Leaf specific mass (LSM) was calculated from mass and area measurements.
In the dry aerial biomass, Zn and Cd were extracted by dry ashing (ashed at 500 °C for 5 h and then extracted with 20 mL 20 % HCl). Soils after the 60-day period were air-dried, passed through a 2-mm sieve, and extracted with DTPA (diethylene-triamine-penta-acetic acid, representing the extractable metal fraction) for Zn and Cd. The extracting solution was prepared by dissolving 1.967 g of DTPA, 1.48 g of CaCl2·2H2O, and 14.9 g of triethanolamine (TEA) per L of solution and was adjusted to pH 7.3. Extraction was then performed in a 1-to-2 soil-to-extracting solution ratio for 2 h (Lindsay and Norvell 1978). In a separate extraction procedure, soils were digested with aqua regia (mixture of 1:3 concentrated HCl:HNO3, representing the “total” metal concentrations) for Zn and Cd. Metals in both plant and soil were analyzed in an atomic absorption spectrophotometer (PerkinElmer 3300). Based on metal concentrations, we also calculated metal transfer coefficient (TC = metal concentration in plant/metal concentration in soil).
Since the soil used in this study originated from an industrial area, we proceeded to the assessment of all heavy metal concentrations, according to the protocols described above. The other heavy metals determined were Cr = 118.32 mg kg−1, Ni = 147.16 mg kg−1, Cu = 93.79 mg kg−1, and Pb = 189.28 mg kg−1. Thus, we decided to study Cd and Zn because they were the most elevated of those initially determined relative to the heavy metal limits in the EU Directive 86/278/EEC (EU 1986).
Statistics
The data were analyzed for statistical significance with one-way ANOVA, while regression analysis was performed between metal levels in soils and metal concentrations in plants using the statistical package IBM SPSS Statistics version 21.0 for Windows (IBM corp.).
Results
During the experimental period
Growth response, photosynthesis, and chlorophyll content were recorded on a biweekly basis during the experimental period. Growth profile was obviously species-specific, and the same was true for the differences between plants growing in the control (soil A) versus contaminated soil (soil B, Fig. 1). Purslane plants growing in Soil B gradually increased their total leaf area until nearly the end of the experimental period, while plants of soil A suffered a massive leaf loss at that time (Fig. 1a). Nevertheless, at the final measurement, no differences of growth parameters were recorded in plants of the two treatments. Geranium seemed to benefit from the contaminated soil, since plants of soil B exhibited better growth early in the experimental period, which continued until the end (Fig. 1b). Completely identical growth curves were recorded in lettuce (Fig. 1c).
Photosynthetic rates of purslane and lettuce plants in soil A were always higher compared with soil B plants throughout the experimental period, although the differences were not statistically significant for purslane and lettuce (Fig. 2a, c). Opposite was the picture of photosynthesis in geranium, where plants growing in the contaminated soil exhibited elevated photosynthetic rates reaching considerable and statistically significant differences at the end of the experimental period (Fig. 2b). Concerning PSII photochemical efficiency, no differences were recorded in all three species (data not shown). Also total chlorophyll content remained unaffected by contaminated soil in all three tested species throughout the experimental period (data not shown).
Soil analysis and metal accumulation in plant tissues
Both DTPA- and aqua regia-extracted metals were significantly higher in soil B compared to uncontaminated soil A (Table 1). Both Cd and Zn “total” concentrations were higher than the permissible levels according to the EU Directive (maximum regulation limits for agricultural soils are 3 mg Cd kg−1 and 300 mg Zn kg−1, EU Directive 86/278/EC). Likewise, Zn concentration in the aboveground biomass of all three plant species was significantly higher in soil B compared to soil A, with values close to 100 mg kg−1 and percentage differences ranging from 74 to 270 % (Table 2). As for Cd concentration in lettuce, it was significantly higher in soil B (100 % increase), but no difference was found in purslane and geranium.
In order to further investigate this effect, we calculated metal soil-to-plant transfer coefficient (TC). Although metal concentrations in soil and plant were higher in soil B compared to soil A, as for metal TC, we noted a decreasing trend in soil B compared to soil A (Table 3). This indicates slower metal uptake with increased metal concentrations in soil; in other words, the rate of metal uptake decreases with augmented metal concentrations in soil. However, this trend was significant only for Zn in geranium and lettuce. In all other cases, although there was a decreasing trend from soil A to soil B, differences were not significant. In contaminated soil, Cd TC was significantly higher in all three plant species compared to Zn TC, while in control soil, Cd TC was higher than Zn TC in geranium and lettuce (Table 3).
The fact that Zn in all plants had clearer differences between soils than Cd was also confirmed by the regression analyses we performed between metal levels in soils (DTPA and aqua regia) and metal concentrations in plants (Table 4). DTPA-Cd was correlated with Cd content in purslane, while DTPA-extracted and aqua regia-digested Zn were correlated significantly with lettuce metal content. Metal levels in geranium showed no correlation with metals in soils.
Plant characteristics at final harvest
The results of the above-described metal movements and accumulation on plant growth and morphological characteristics were evaluated at final harvest. An attempt was made to reveal age-dependent changes in foliage growth by dividing leaves into different cohorts. Growing in the contaminated soil (soil B) caused an increase in senescent leaves cohort in purslane plants (Fig. 3a). Thus, the contribution of aged leaves to the total leaf area of these plants was significantly enhanced compared to the control plants. However, new and mature leaf cohorts as well as total leaf area remained unaffected. The beneficial effects of soil B to geranium growth were evident in the final harvest with the significant enhancement of total leaf area, which reflected more massive mature and new leaf cohorts, the former being significant (Fig. 3b). Different treatments did not influence any aspect of lettuce growth as was obvious at final harvest (Fig. 3c). Similar to the aforementioned results was the picture of biomass accumulation in the leaf cohorts of the tested species. In Fig. 4, the differences in dry weight of all aboveground plant parts of geranium are presented. Geranium plants grown in contaminated soil exhibited significant increase in biomass investment to new and mature leaves, while senescent leaves were comparable to the respective control group.
Purslane mature leaves of soil B were thinner than control, and this was true for the average leaf thickness of soil B plants (noted as “total” in Fig. 5a) as well. On the contrary, geranium of soil B possessed significantly thicker new leaves, while the other leaf cohorts remained unresponsive concerning this trait (Fig. 5b). Finally, the only significant response of lettuce plants to contaminated soil was the decrease in leaf thickness of new and mature leaf cohorts (Fig. 5c).
Finally, as shown in Fig. 6, contaminated soil resulted in altered plant architecture in purslane compared to control plants. Indeed, plants grown in soil B were more branched as judged by the remarkably extensive lateral stems, while the main stem was comparable to soil A plants. Lengthier lateral stems in soil A resulted in a significant increment of total stem length (Fig. 6).
Discussion
The weed purslane, the ornamental geranium, and the edible vegetable lettuce were grown in a contaminated, industrial soil rich in Zn and Cd. Both Zn and Cd concentrations were higher than the permissible levels according to the EU Directive (maximum regulation limits for agricultural soils are 3 mg Cd kg−1 and 300 mg Zn kg−1, EU Directive 86/278/EC). [Zn] and [Cd] in the industrial soil were 16.5-fold and 4.8-fold higher, compared to the uncontaminated soil used as control. This loading is the effect of a long history of heavy metal depositions due to intensive industrial activity in Elefsina. High metal availability induced an increased Zn concentration in plant tissues growing in the contaminated soil (reaching almost 100 mg kg−1 DW) when compared with plants of the uncontaminated rural soil (28–51 mg kg−1 DW). This was evident for all three plant species: we found a 270 % increase in purslane, 129 % in lettuce, and 74 % in geranium with all differences being significant. According to Marschner (2012), the typical leaf Zn concentration ([Zn]leaf) required for adequate growth approximates 15–20 mg Zn kg−1 DW, while toxicity symptoms usually become visible at [Zn]leaf > 300 mg Zn kg−1 DW, although some crops show toxicity symptoms at [Zn]leaf < 100 mg Zn kg−1 DW. Concerning Cd concentration in our experimental plant tissues, it was significantly higher in soil B only in lettuce, whereas no differences were recorded in geranium and purslane. The recorded values were much higher than the median concentrations in plants for Cd (0.23 mg kg−1) reported by Pauget et al. (2015) for plants developed in industrial contaminated soils. Nevertheless, the toxic level of Cd in leaves of plants which is 5–30 mg kg−1 DW (Orcutt and Nilsen 2000) was never exceeded. Zinc and Cd toxicity thresholds are highly variable among species and within the same species (Broadley et al. 2007). It is well documented that the presence of metals in excess usually affects adversely plant health. Enhanced Cd and Zn levels in plants interfere with and inhibit various physiological processes such as plant–water relationships, chlorophyll biosynthesis, transpiration rates, enzyme activities, nutrient uptake, biomass production, and growth (Broadley et al. 2007; Dresler et al. 2014; Fernàndez-Martínez et al. 2014). Apparently, all three species tested in the present study developed no visible signs of metal toxicity. On the contrary, the experimental data revealed a promotion of geranium growth in the contaminated soil and the unresponsiveness of purslane and lettuce growth characteristics. Geranium seemed to benefit from the enhanced plant Zn concentration which probably ameliorated photosynthetic rates and subsequently growth throughout the experimental period (Figs. 1b, 2b). At final harvest, higher total leaf area resulting from a more massive mature leaf cohort in combination with augmented biomass accumulation was evident for geranium developed in contaminated soil compared to control (Figs. 3b, 4). The biochemical and metabolic significances of Zn have been reviewed thoroughly (Broadley et al. 2007; Hänsch and Mendel 2009; Sharma et al. 2013). Briefly, the essentiality of this micronutrient includes its special role as a component of enzymes for protein synthesis and energy production, in the maintenance of the structural integrity of biomembranes and ultimately in the promotion of growth and tolerance to stress. Results obtained for geranium imply that [Zn] in aboveground tissues did not exceed the threshold limit of optimal concentrations for favorable growth. The observed insignificant Cd uptake probably complements geranium growth advancement.
Purslane grown in the contaminated soil remained unaffected in terms of productivity and photosynthetic performance. Despite the absence of an obvious functional impairment, two interesting results were recorded, i.e., more massive senescent leaf cohort and altered plant architecture. Thus, in the contaminated soil, the contribution of aged leaves to the total leaf area of purslane plants was significantly enhanced compared to the control. Noteworthy is that plants in soil B accumulated 270 % more Zn (reaching 104 mg kg−1) than control plant tissues. The combination of these two features leads to the hypothesis that this [Zn] increase is based on Zn accumulation in old leaves, or going further, that Zn accumulation to mature purslane leaves accelerated their senescence. The latter is an assumption that cannot be confirmed by the results of the present study. Nevertheless, the connection of excess Zn with the generation of oxidative stress is well documented and may be involved in aging/senescence (Wang et al. 2009; Li et al. 2013; Fernàndez-Martínez et al. 2014). Additionally, the negative effect of increased [Zn] in endogenous plant cytokinin content, a phytohormone with a key role in retarding senescence (Xu et al. 2013; Pavlíková et al. 2014), corroborates the hypothesis of Zn induced aging of purslane leaves. Regarding the first hypothesis, i.e., the accumulation of Zn in old purslane leaves, it is supported by metal localization studies which confirmed the compartmentalization of Zn in aged tissues (Di Baccio et al. 2009; Durand et al. 2011). Working with poplar, Durand et al. (2011) argued that the control of the Zn homeostasis is an inducible mechanism based on the compartmentalization of the metal ions in old leaves. Thereby, the probable connection of purslane massive old leaf cohort with the significant increase in [Zn] compared to control along with plant growth unresponsiveness may imply an efficient metal homeostasis. Altered plant architecture in purslane of contaminated soil with respect to control corresponds to more branched plants with remarkably extensive lateral stems. Higher in number and lengthier lateral stems resulted in sparse plant foliage in soil B, since leaf number per plant was similar in the two soils. The optimization of radiation penetration is the apparent advantage of this modification, with long-term beneficial effects on plant productivity if other factors remain favorable. The opposite result, i.e., a reduction in number and diameter of secondary branches of coriander, has been reported as a component of a general impairment of plant growth under Zn excess (Marichali et al. 2014).
Lettuce was the only examined species that exhibited an enhanced [Cd] in leaf tissues compared to control plants (100 % increase), accompanied by an increase in [Zn] which reached 105 mg kg−1. This doubling of metal concentrations caused no effect on measured growth parameters, while photosynthetic profiles appeared identical between treated and control plants. The only feature affected was leaf thickness, which showed a significant decrease, a finding in contrast to other studies with different species (Di Baccio et al. 2009; Marichali et al. 2014). Recently, a comprehensive work on lettuce proteome response to Zn stress gave new insights in our results that indicated no Zn effect on growth (Lucini and Benardo 2015); they reported that lettuce grown in excess Zn did not show notable decrease in biomass accumulation. On the contrary, the analysis of proteins exhibiting a minimum of a threefold change revealed the involvement of photosynthesis, photophosphorylation, hormone profile, and oxidative stress compounds, as an early response to stress. The proteomic response of lettuce throughout the experiment indicated that the plant actually exhibited an adaptation to stress over time, which became obvious after 30 days. Consequently, lettuce in our experiment either did not undergo a Zn (and Cd) stress by controlling metal uptake or the 2-month experimental period was long enough for the conclusion of the adaptation procedure before the final harvest.
Metal transfer coefficient is an index of metal availability to a plant: higher TC means higher metal uptake by a plant, thus accumulation, while low TC indicates exclusion (Uka et al. 2013). Moreover, TC may serve as a means of comparing either the mobility of various metals (higher values indicating higher mobility) or the behavior of studied plants concerning metal absorption. Metal TC values were lower in the contaminated soil (B) than in the control soil (A). This effect may be attributed to the fact that a plant has a higher ability to absorb a soil-added element when additions are at low levels and absorption becomes slower with increasing soil-added element (as observed by Sandras and Lemaire (2014) for nutrient N, and Guo et al. (2014) for heavy metals such as Zn, Cd, and Pb). However, this trend was significant only for Zn in geranium and lettuce. In all other cases, although there was a decreasing trend from soil A to soil B, differences were not significant. Similarly, Kachenko and Singh (2006) found Zn TC values decreasing from 7.2 for 50 mg kg−1 in soil to 1.3 for 1000 mg kg−1 in soil, while Cd TC values were rather unaffected by soil levels in that work. In soil B, Cd TC was significantly higher in all three plants compared to Zn TC, and in soil A, Cd TC was higher than Zn TC in lettuce and geranium. This shows that Cd is a more mobile metal in soil and it has higher plant availability than Zn (as also agreed by Alvarez-Ayuso et al. 2013).
The relationship between the elevated metal levels in soil and metal concentrations in plants was assessed through regression analyses, which exhibited significance only in three cases: Cd-DTPA versus Cd in purslane and DTPA-extracted Zn and aqua regia-digested Zn versus Zn in lettuce (Table 4). This relative lack of relationship between soil and plant levels of Zn and Cd seems to be rather contrary to the reported trend in the literature (e.g., Chojnaka et al. 2005). A plausible explanation of the above-mentioned finding we assume to be twofold. First, we investigated contamination in a situation where metals have been deposited over decades. It is known that over time deposited metals tend to drift into non-mobile residual soil pools (as was also shown in a 3-year experiment reported by Peruzzi et al. 2011), and thus, although total and even DTPA extractions are marked as high, plants are not necessarily affected. Indeed, soil pH, organic matter, calcium concentration, and presence of other metals are important factors affecting metal bioavailability in the soil (Sanitá di Toppi and Gabbrielli 1999). The transfer of metals from the readily available to less-available soil phases is significantly influenced by the competition for surface exchange sites by other cations and by the presence of binding colloids such as the organic matter (Manousaki and Kalogerakis 2009). In their review revisiting the bioavailability concept, Peijnenburg et al. (2007) also noted that “[metal] contaminant levels are not necessarily indicative of actually occurring [plant] adverse effects,” although they concluded that this does not nullify ecological risks. Second, we dealt with a multi-metal contaminated site, as is always the case in real-life situations where metals are not purposefully being spiked. Zinc and Cd, the two most elevated metals in the studied contaminated soil, seemed to have had antagonistic behavior, which decelerated their phyto-availability. The two metals, known antagonists in both soil level (addition of the one in the presence of the other leads to reduced mobility, as per Feng et al. 2013) and plant level (i.e., Zn diminishes Cd-induced stress to plants, as suggested by Aravind et al. 2009), have been added to the soil with slow deposition processes over a period of nearly a century, and thus this antagonistic behavior has been reflected in our study.
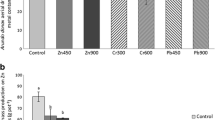
In order to investigate the possible Cd/Zn antagonism, we further explored differences among plant behavior, by plotting metal TC values against aqua regia-extractable concentrations in soil. As indicated earlier, in such a plot, a line with decreasing (i.e., negative) slope should be expected. In interpreting the line slope of this graph, there are two extremes:
-
Plants exclude metals: When the slope becomes more negative, the tested plant retains stable metal concentrations with increasing soil metal levels; in this case, metal exclusion mechanisms prevail in the plant.
-
Plants accumulate metals: A negative slope approaching zero is generated (thus getting close to a horizontal line); this shows that metal concentrations in plant increase with enhanced soil metal concentrations. If the slope becomes zero, the metal concentration increase in the plant is linearly proportional to increases in metal levels in soil. In this case, plants tend to accumulate metals.
As noted in Fig. 7 (which concerns contaminated soil only), line slopes were higher (closer to zero) for Zn than for Cd, in all three plant species. This shows that plants tend to absorb the essential metal (i.e., Zn) to the expense (and thus causing the exclusion) of the nonessential Cd, even in this multi-element contamination situation such as ours. Similar findings were also reported by Cherif et al. (2011), who found that elevated Zn concentrations in soil decreased Cd in tomato plants, while soil Cd levels were kept stable. In other similar cases, Zn has been reported as being taken up preferentially over Cd, when plants are exposed to both elements (Aravind et al. 2009; Saifullah et al. 2014). This is probably a major reason behind the fact that our tested plants exhibited remarkable tolerance to Cd and Zn exposure and even gained a growth advantage as was evident in geranium.
Conclusions
The ornamental geranium exhibited a growth promotion when grown in industrial soil with elevated Cd and Zn concentrations, while the weed purslane displayed an altered architecture, but its growth remained unaffected, similarly to lettuce. Thus, our plants did not seem to suffer a metal stress. We conclude that this was caused due to the fact that (a) metals were deposited over long periods and were thus strongly retained by soil colloidal phases, especially in our calcareous soil, and (b) there has been a well-established Cd/Zn antagonism. Also, in none of the studied plants have we found a behavior of metal accumulation alone or of metal exclusion alone; however, in all three plants, Zn was absorbed in favor of Cd, irrespective of the fact that Cd was more mobile than Zn (judged by metal TC values). Concerning phytoremediation potential, none of the tested species exhibited ideal properties, although geranium could be proposed for cultivation in contaminated areas, enabling their economic exploitation. Performed under the bioavailability conceptual framework, the present study highlights the complementarity of soil history aspect to the “physiologically tuned” approach of biomonitoring to assess bioavailability and bioaccessibility of heavy metals in contaminated soils.
References
Ahmadpoor P, Navvi AM, Abdu A, Abdul-Hamid H, Singh DK, Hassan A, Majid NM, Jusop S (2010) Uptake of heavy metals by Jatropa curcas L. planted in soils containing sewage sludge. Am J Appl Sci 7:1291–1299
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Alvarenga P, Simões I, Palma P, Amaral O, Matos JX (2014) Field study on the accumulation of trace elements by vegetables produced in the vicinity of abandoned pyrite mines. Sci Total Environ 470–471:1233–1242
Alvarez-Ayuso E, Otones V, Murciego A, Garcia-Sanchez A, Santa Regina I (2013) Zinc, cadmium and thallium distribution in soils and plants of an area impacted by sphalerite-bearing mine wastes. Geoderma 207–208:25–34
Aravind P, Prasad MNV, Malec P, Waloszek A, Strzalka K (2009) Zinc protects Ceratophyllum demersum L. (free-floating hydrophyte) against reactive oxygen species induced by cadmium. J Trace Elem Med Biol 23:50–60
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal(loid)s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ Pollut 200:16–23
Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36:1429–1443
Cherif J, Mediouni C, Ben AW, Jemal F (2011) Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum). J Environ Sci 23:837–844
Chojnaka K, Chojnaki A, Gorecka H, Gorecki H (2005) Bioavailability of heavy metals from polluted soils to plants. Sci Total Environ 337:175–182
Di Baccio D, Tognetti R, Minnocci A, Sebastiani L (2009) Responses of the Populus x euramericana clone I-214 to excess zinc: carbon assimilation, structural modification, metal distribution and cellular localization. Environ Exp Bot 67:153–163
Dresler S, Bednarek W, Wójcik M (2014) Effect of cadmium on selected physiological and morphological parameters in metallicolous and non-metallicolous populations of Echium vulgare L. Ecotoxicol Environ Saf 104:332–338
Durand TC, Baillif P, Albéric P, Carpin S, Label P, Hausman J-F, Morabito D (2011) Cadmium and zinc are differentially distributed in Populus tremula x P. alba exposed to metal excess. Plant Biosyst 145(2):397–405
EU (European Union) L 181 04/07/1986 (1986) Council Directive 86/278/EEC of 12 June 1986 on the “protection of the environment and in particular of the soil, when sewage sludge is used in agriculture.” http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31986L0278:ES:HTML
Feng R, Qiu W, Lian F, Yu Z, Yang Y, Song Z (2013) Field evaluation of in situ remediation of Cd-contaminated soil using four additives, two foliar fertilisers and two varieties of pakchoi. J Environ Manag 124:17–24
Fernàndez-Martínez J, Zacchini M, Fernández-Marín B, García-Plazaola JI, Fleck I (2014) Gas-exchange, photo- and antioxidant protection, and metal accumulation in I-214 and Eridano populus sp. clones subjected to elevated zinc concentrations. Environ Exp Bot 107:144–153
Gharneh HAA, Hassandokht MR (2012) Chemical composition of some Iranian purslane (Portulaca oleracea) as a leafy vegetable in south parts of Iran. Acta Hortic 944(26):41–44
Guo J, Feng R, Ding Y, Wang R (2014) Applying carbon dioxide, plant-growth promoting rhizobacterium and EDTA can enhance the phytoremediation efficiency of ryegrass in a soil polluted with zinc, arsenic, cadmium, and lead. J Environ Manag 141:1–8
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Ho C-P, Hseu Z-Y, Chen N-C, Tsai C-C (2013) Evaluating heavy metal concentration of plants on a serpentine site for phytoremediation applications. Environ Earth Sci 70:191–199
Huong NTL, Ohtsubo M, Li L, Higashi T, Kanayama M (2010) Heavy-metal contamination of soil and vegetables in wastewater-irrigated agricultural soil in suburban area of Hanoi, Vietnam. Commun Soil Sci Plant Anal 41:390–407
Kabata-Pendias A (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kachenko AG, Singh B (2006) Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut 169:101–123
Kale RA, Lokhande VH, Ade AB (2015) Investigation of chromium phytoremediation and tolerance capacity of a weed, Portulaca oleracea L. in a hydroponic system. Water Environ J 29:236–242
Li X, Yang Y, Jia L, Chen H, Wie X (2013) Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf 89:150–157
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lindsay WL, Norvell WA (1978) Development of DTPA test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Lucini L, Benardo L (2015) Comparison of proteome response to saline and zinc stress in lettuce. Front Plant Sci 6:1–12
Manousaki E, Kalogerakis N (2009) Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res 16:844–854
Marichali A, Dallali S, Ouerghemmi S, Sebei H, Hosni K (2014) Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind Crop Prod 55:248–257
Marschner H (2012) Marschner’s mineral nutrition of higher plants. Academic Press, London
Orcutt DM, Nilsen ET (2000) Phytotoxicity and soil pollution: heavy metals and xenobiotics. In: Orcutt DM, Nilsen ET (eds) The physiology of plants under stress, soil and biotic factors. Wiley, New York, pp 481–517
Pauget B, Faure O, Conord C, Crini N, de Vaufleury A (2015) In situ assessment of phyto and zooavailability of trace elements: a complementary approach to chemical extraction procedures. Sci Total Environ 521–522:400–410
Pavlíková D, Zemanová V, Procházková D, Pavlík M, Száková J, Wilhelmová N (2014) The long-term effect of zinc soil contamination on selected free amino acids playing an important role in plant adaptation to stress and senescence. Ecotoxicol Environ Saf 100:166–170
Peijnenburg WJGM, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67:163–179
Peruzzi E, Masciandaro G, Macci C, Doni S, Ravelo SGM, Peruzzi P, Ceccanti P (2011) Heavy metal fractionation and organic matter stabilization in sewage sludge treatment wetlands. Ecol Eng 37:771–778
Saifullah SN, Bibi S, Ahmad M, Ok YS (2014) Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ Earth Sci 71:1663–1672
Sánchez-Blanco MJ, Álvarez S, Navarro A, Bañón S (2009) Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J Plant Physiol 166(5):467–476
Sandras VO, Lemaire G (2014) Quantifying crop nitrogen status for comparisons of agronomic practices and genotypes. Field Crop Res 164:54–64
Sanitá di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Sharma A, Patni B, Shankhdhar D, Shankhdhar SC (2013) Zinc—an indispensable micronutrient. Physiol Mol Biol Plants 19:11–20
Uka UN, Chukwuka KS, Afoke C (2013) Heavy metal accumulation by Telfairia occidentalis Hook f. grown on waste dumpsites in South-eastern Nigeria. Res J Environ Toxicol 7:47–53
Valerio ME, Garcia JF, Peinado FM (2007) Determination of phytotoxicity of soluble elements in soils, based on a bioassay with lettuce (Lactuca sativa L.). Sci Total Environ 378:63–66
Van Gestel CAM (2008) Physico-chemical and biological parameters determine metal bioavailability in soils. Sci Total Environ 406:385–395
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476
Xu QS, Chu WY, Qiu H, Fu YY, Cai SJ, Sha S (2013) Responses of Hydrilla verticillata (L.f.) Royle to zinc: in situ localization, subcellular distribution and physiological and ultrastructural modifications. Plant Physiol Biochem 69:43–48
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levizou, E., Antoniadis, V. & Papatheodorou, S. Without exceeding the limits: industrial soil rich in Zn and Cd has no effect on purslane and lettuce but promotes geranium growth. Environ Earth Sci 75, 1256 (2016). https://doi.org/10.1007/s12665-016-6070-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6070-y