Abstract
A multivariate statistical technique was used to determine the major hydrochemical processes that control the groundwater quality variations during “aquifer storage and recovery” (ASR) operations. Principal component analysis (PCA) was applied on chemical and isotopic data of 83 groundwater samples. It has allowed the reduction of the 14 variables to four significant PCs (factors F) that explain 82.2 % of the total variance. F1 (54.1 %) represents the mineralization and groundwater nitrates pollution, whereas F2 (13.2 %) exhibits the isotopic enrichment by evaporation of waters components. Factor score of F1–F2 contains four groups: Injected freshwater (II) distinguished by low mineralization. Native groundwater characterized by relatively high mineralization. Mixing groundwater has intermediate mineralization. The shifting of native groundwater to injected water is related to the mixing ratio between these two components. Thus, the quality of groundwater samples situated close to the injected water is enhanced. Surface water (QF) is enriched by δ18O and δ2H compared to the other components due to evaporation. The spatial pattern of iso-factor scores maps is generally similar to the pattern of EC, Cl−, NO3 −, and mixing ratio maps performed for the same experiment dates. Consequently, under the applied conditions of ASR process, the effective diameter is 250 m and 3 months of recovery time after complete injection. The results of this study clearly demonstrate the usefulness of multivariate statistical analysis as (PCA) in the ASR process investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artificial recharge is regarded as groundwater management technique and generally termed “Management of Aquifer Recharge” (MAR). This term describes the augmentation of groundwater resources by artificial means including various forms of surface infiltration and direct well injection into the aquifers, stored there and recovered to meet the requirements of increasing water demand. It is widespread technique applied to cover different aspects of water management such as groundwater decline, saltwater intrusion and wastewater treatment (Dillon et al. 2006; Guillaume and Xanthoulis 1996) and restore the groundwater balance (Dillon 2005). In arid and semiarid areas, rainwater harvesting is considered as MAR method and experienced for thousands of years (Gal et al. 2002). Different engineering systems such as check dams and gravel pits are used to increase groundwater recharge in water scarcity areas (Martin-Rosales et al. 2007). Groundwater artificial recharge is used to manage seawater intrusion (Shammas 2008). Common recharge techniques consists of riverbank filtration to improving source water quality were carried out in many countries (Ray et al. 2002; Regli et al. 2003).
Artificial storage and recovery (ASR) is considered as one of the MAR methods and applied in the worldwide at specially designed wells as open wells which are appropriate for very high injection rate for storage and recovery for reuse at demand. ASR is generally applied for different time scales, of which seasonal storage is the most frequently used to cover the water shortage during low season (Herczeg et al. 2004). Many studies have dealt with the feasibility of ASR (Pyne 1998; Pavelic et al. 2002). The major source of water recharge used in aquifer storage and recovery (ASR) experiments is generally of good quality water particularly for potable water replenishment (Dillon 2005). However, potable water or safe drinking water as defined by WHO Guidelines (WHO 2008, 2011) is good quality water suitable for human consumption (drinking, food and personal hygiene) and does not represent any significant risk to human health. Generally, the injection of high-quality water into adequate aquifers is used to enhance quantity and quality of groundwater reservoir. MAR processes are considered as a tool for sustainable water resources management to challenge many economic and environmental problems, e.g., mitigation the effects of climate changes (floods and droughts; Pyne 1995). Generally, in arid and semiarid areas, water demand surpasses the water amount from renewable resources (e.g., the Middle East and Gulf region), and the excess water produced in desalination operation is used in ASR to restore groundwater reservoir and improve water quality (Mukhopadhyay and Al-Sulaimi 1998).
Geochemical and isotope techniques are used in the investigation of MAR processes (Clark et al. 2004). The environmental isotope methods are used in ASR when there is no significant geochemical difference between native groundwater and injected water (contrast) during injection and recovery processes. Consequently, environmental stable isotopes such as δ18O and δ2H are commonly used to identify mixing processes in the aquifers (Muir and Coplan 1981; Ma and Spalding 1996; Dillon et al. 2002; Le Gal La Salle et al. 2005). Abou Zakhem and Hafez (2012) have used chemical and stable isotope techniques to investigate the effectiveness of ASR, compute the mixing ratios between water components (injected and native groundwater) and delineate its effective diameter and recovery time.
The aim of this study is using multivariate statistical technique in the investigation of artificial recharge to determine the major hydrochemical processes that control the variations of groundwater quality during ASR, to examine the correlations matrix among different variables and delineate the temporal and spatial variations of artificial recharge efficiency in the studied area. The multivariate statistical has the advantage over single tracers’ analysis. Principal component analysis (PCA) was applied on the data from the same experimental site of ASR and will be compared with the interpretation of previous results.
Study area description
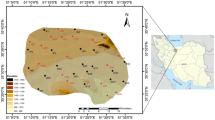
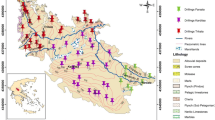
The lower part of Damascus Basin or the so-called Barada and Awaj basins is mainly formed by Damascus Oasis of about 1200 km2. The mean elevation of the Oasis is 650 m above sea level (a.s.l.). Damascus city is located in the northwestern part of the Oasis, while Oteibeh and Hijaneh ephemeral lakes are situated in the lower Damascus Basin and constitute the natural drainage of Barada and Awaj rivers, respectively (Fig. 1; Abou Zakhem and Hafez 2012). The study is under arid and semiarid Mediterranean climate type, which is characterized by rainy cold winters (November–May) and hot dry summer (June–September), with warm relatively short spring and autumn seasons (Soumi and Chayeb 1989). The average annual precipitation is between 221 mm measured in Mazzeh and 136 mm in Damascus international airport gauges (Homsi et al. 1989). Damascus Oasis is a quaternary depression filled with detrital and lacustrine deposits, which are varied from pebble, gravel and conglomerates in the western part to fine sand and silty soils in the center of Damascus Oasis and change to loam, silt and clay of recent lacustrine formations surrounding Oteibeh and Hijaneh lakes (Ponikarov 1966). The catchment area of Barada and Awaj rivers is situated in highlands of the Anti-Lebanon Range in the northwest of the study area. These rivers are discharged during flood period into Oteibeh and Hijaneh, respectively. They form the major elements of the natural drainage system in Damascus Basin. The Barada River is mainly supplied by both Barada Spring (3.12 m3/s) and Fijeh Cretaceous Karstic Spring (7.7 m3/s) main annual discharges (Selkhozpromexport 1986). Figeh Spring is mainly used to supply Damascus city for drinking and domestic purposes. Quaternary alluvial aquifer system of 400–450 m thickness is mainly composed of pebble, gravel, sand, silt and clay lacustrine deposits. This aquifer compiles alluvial sediments complex including several sub-aquifers (multi layers) which are differentiated by clay content. The aquifer is generally assumed to be unconfined, becoming locally semi-confined related to the clay intercalations. Alluvial–proluvial mainly of loam and clay sediments constitutes the top of the aquifer (10–20 m). This aquifer is characterized by hydraulic conductivity varying between 3.7 and 142 m/day, while the transmissivity ranges from 165 to 3700 m2/day (Selkhozpromexport 1986). The aquifer system is dramatically being exploited throughout the several thousands of wells drilled within the Damascus Oasis (>25,000) for drinking and irrigation purposes. Groundwater levels are continually dropped caused by over-exploitation of groundwater mainly for irrigation. During the last three decades, the groundwater levels have dropped to 40–50 m depth in the west and 100–120 m depth east of Damascus Oasis. Generally, the groundwater flow paths are from west to east. This direction of groundwater movement is parallel to that of the Barada and Aawaj rivers flow. The hydraulic head decreases from 650 m a.s.l. in the west to less than 500 m a.s.l. in the vicinity of Oteibeh Lake with hydraulic gradient of 0.004 ± 0.002 (Abou Zakhem and Hafez 2001). The quality of groundwater is good (TDS < 0.9 g/l); however, socioeconomic and agricultural activities caused high pollution load since the 1990s. Consequently, the nitrate concentrations surpass the maximum concentration limit of Syrian standard (MCL: 50 mg/l) for drinking water in many areas (Abou Zakhem and Hafez 2009, 2015).
Aquifer storage and recovery application
Aquifer storage and recovery (ASR) is applied in Mazraha pumping field during two hydrological cycles (2007 and 2008). Mazraha field is located at 36°18′E and 33°31′N, and it includes 24 pumping wells used for drinking water supply (Fig. 1). Groundwater level map (Fig. 1) shows that flow directions are from NW to SE controlled by average gradient of 0.003. Subsequent to the sever decreases in groundwater level during the late 1990s, two open wells (I and II) were drilled and specially designed for very high injection rate. These wells are characterized by large diameter of about 3 and 30 m depth, 50 cm of perforated screen casing combined with 25 cm of gravel horde to enhance infiltration and 6 aeration tubes of 30 m depth to avoid gas clogging during injection processes (Fig. 2). The aquifer is mainly composed of conglomerate, gravel and sand alternated with clay lenses overlaid by 8–10 m of loam and clay. The scheme of the ASR operation is to inject the excess of Fijeh springwater directly through the injection wells (I and II) during flood (February–May), for later extraction via 24 pumping wells during the dry season (July–October). Consequently, the ASR contributes to restore groundwater reservoir and enhance water quality, since water quality of Fijeh Spring is good. The injection process started in 6 March and finished in May 7, 2007 (62 days; Fig. 3). The average injection rate was 7400 m3/day corresponding to the total injected water (TIW) of 461.8 × 103 m3. Pumping rate was about 19,500 m3/day, between June 18 and August 31, 2007, and decreases gradually to 11,600 m3/day until March 5, 2008, forming a total pumped water (TPW) of 4.1 × 106 m3 during 261 days. The injection stage was between March 8 and April 23, 2008 (47 days), with constant injection rate of 8300 m3/day corresponding to 391.8 × 103 m3 of TIW. Pumping phase of about 10,137 m3/day, between May 31 and June 2008, it increases to 15,500 m3/day in July and decreased gradually to 11,177 m3/day until December 2008 (215 days) forming 2.7 × 106 m3 of TPW. The TIW during ASR experiments represents only about 10–15 % of the TPW in Mazraha field (Abou Zakhem and Hafez 2012).
Schematic injection well (Abou Zakhem and Hafez 2012)
Water balance of artificial recharge (Abou Zakhem and Hafez 2012)
Sampling and analyses methodology
Thirteen groundwater samples were selected from active wells based on hydrogeology conditions (P2, P4, P10, P12, P16, P19, P20, P21, P25, Z1, LS, 9T and 11T), one injection well (II), and one sample representing surface water of Mazraha canal (QR) situated at 500 m north of injection center was sampled every 15 days during injection phase and once per month for recovery period during two hydrological cycles (2006–2007 and 2007–2008; Fig. 1). Groundwater samples were taken from active wells pumped continuously or pumped for a significant amount of time to get a representative sample. The samples were collected in 1- and 0.5-l new polyethylene bottles for chemical and isotopic analyses, respectively. All bottles had been rinsed three times with groundwater before filling it to capacity and then labeled accordingly. Prior to analysis in the laboratory, the samples were stored at a temperature below 4 °C. Physical parameters such as electrical conductivity (EC), temperature, pH and alkalinity were measured in the field. Stable isotope as (δ18O and δ2H) were analyzed using Finnigan Mat Mass Spectrometer DELTA plus, after applying CO2—water equilibration standard method (Epstein and Mayeda 1953) with measurement accuracy of ±0.1 and ±1 ‰, respectively. The tritium (3H) values of water samples were measured by a liquid scintillation counter (Quantulus 1220) after electrolysis enrichment with an accuracy of ±0.5 TU. Chemical analyses of major ions as Ca2+, Mg2+, Na+, K+, Cl−, SO4 2−, HCO3 − and NO3 − were analyzed using Chromatograph (Dionex 120). The analytical precision for the measurements of cations and anions was based on the ionic balance error (IBE). Generally, the precision of chemical analysis was about ±5 %. Chemical and isotopic analyses were carried out in the laboratories of geology department (AECS). Reference materials and quality analysis are under the control of the National Quality Control Office in cooperation with the International Atomic Energy Agency (IAEA).
Principal component analysis (PCA)
PCA is considered as multivariate statistical technique that analyzes the data table including several observations and inter-correlated quantitative variables (I rows of observations and J columns of variables). The purpose of this analyzes is to extract the significant information from the data table and transform it to new orthogonal set called principal components (PC). The new representation condenses the data by maintaining only the high significant information, simplify the explanation and analyze the composition and structure of the data table (Abdi and Williams 2010).
Standardization of the original variables tends to remove the influence of measurement units rendering data dimensionless as expressed below:
where Z i is the standardized value, X i is the original value of parameter i, μ is the mean, and σ is the standard deviation of the data set (Davis 1973; Hamzaoui-Azaza et al. 2009).
The PCA method converts an original set of variables into a new orthogonal and uncorrelated set of principal components. Precisely, it decomposes the original matrix X (I observations, J variables) into scores and loadings matrices, as follows:
where each column of the matrix X is standardized; T (I, J) represents the matrix of J principal components scores (each column of matrix T refers to a principal components); P′ refers to the transpose of original data; and E, the residual matrix. Generally, the first principal components R are selected with high percentage of the total variance in the original matrix X (R < J), and thus, data reduction is realized (Iyer et al. 2003). The PCs with eigenvalues ≥1 are rotated using varimax rotation to augment the participation of high significant variables and reduce the less significant variables based on there loadings and to generate a simple components structure (Kleinbaum et al. 1988; Al-Tamir 2008).
Correlation matrix compiles the linear correlations coefficients (−1 to 1) between variables. High correlation coefficients reflect the significance of the relationship between two parameters. A positive coefficient demonstrates harmony and similarity between the correlated parameters. A negative coefficient exhibits opposite characters and evolution of these variables (Hamzaoui-Azaza et al. 2009).
Results and discussion
Groundwater hydrochemistry
Chemical water quality is the major factor that determines its adequacy for drinking and public health purposes by comparing the water analysis with guideline values. The analytical results of physical and chemical parameters (EC, pH, T, Ca2+, Mg2+, Na+, K+, Cl−, SO4 2−, HCO3 − and NO3 −) of water samples were matched with the guideline values of World Health Organization (WHO 2004, 2011) and the Syrian standard (Ministry of Environment 1994) for drinking water. Physical and chemical parameters, field measurements and isotopic data with mean (m) and standard deviation (SD), are presented in Table 1. Native groundwater has generally low mineralization, where water mineralization is defined as the water salinity measured by the concentrations of dissolved solid in water (cations and anions) or by electrical conductivity (EC), whereas the water is not valid for drinking purposes caused by the high nitrate concentration (mean ± standard deviation; 68.7 ± 10.5 mg/l) which surpass the maximum concentration limit (MCL: 50 mg/l) in Syrian standards for drinking water (Abou Zakhem and Hafez 2009). Native groundwater is characterized by relatively high EC and nitrate concentration 843 ± 59 µS/cm and 68.7 ± 10.5 mg/l compared to the injected freshwater which has low EC and nitrate concentration 338 ± 28 µS/cm and 7.6 ± 0.8 mg/l, respectively. Consequently, the quality of mixed groundwater subsequent to injection operation is proportionally enhanced according to the mixing rate. Furthermore, the nitrate concentration has decreased below MCL of 50 mg/l (38.8 ± 16.5 mg/l) during the efficient period of ASR. Groundwater mean (m) temperatures and standard deviation (SD) values are 17.8 and 1 °C, respectively, whereas injected water m ± SD are 14.9 ± 1.1 °C and mixing groundwater are 17.4 ± 1.3 °C. Groundwater mean pH and standard deviation values are 7.2 ± 0.2, while injected water mean pH and standard deviation are 7.8 ± 0.3. This relatively high pH is explained by the importance of recent waters derived from the fast flow component of Fijeh karstic spring during the flood period (Al-Charideh 2012; UN-ESCWA and BGR 2013), characterized by low mineralization and moderate alkaline water, whereas native groundwater is characterized by relatively lower pH related to high mineral concentrations. This difference is further emphasized by PCAs.
Injected water and native groundwater have similar calcium bicarbonate (Ca-HCO3) category. Consequently, mixing groundwater has the same calcium bicarbonate type as well. The abundance of the major ions in native and mixing groundwater is in the following order: Ca2+ > Mg2+ > Na+ > K+ = HCO3 − > Cl− > SO4 2− (Fig. 4). Generally, the concentrations of cations and anions of groundwater samples are below the maximum acceptable level for dinking waters.
Principal component analysis (PCA)
PCA method allows reducing a large number of variables (measured physical parameters, major and minor elements in water samples), to smaller number of new orthogonal factors illustrating the correlation matrix, without missing much information (Jackson 1991; Meglen 1992; Cloutier et al. 2008). PCA enable to generate eigenvectors of a variance covariance or a correlation matrix from a raw matrix (I observations and J variables; Hamzaoui-Azaza et al. 2009; Davis 1986).
PCA was applied to physico-chemical and isotopic data of 80 groundwater and 3 surface water samples from Mazraha station to extract the principal factors corresponding to the different processes that control water chemistry and sources of variation in the data during artificial recharge operations. Consequently, the 14 original variables produce 14 orthogonal factors called principal component (PC). As shown in Table 3, factor loadings control the contribution of the original variables in the PC, whereas factor scores measure the converted observations.
This technique was extensively used in hydrogeochemical characteristics and groundwater quality studies (Helena et al. 2000; Adams et al. 2001; Stamatis et al. 2011). XLSTAT software was used for PCA, and the 14 variables are temperature (T), pH, EC, δ18O, δ2H, 3H, Ca2+, Mg2+, K+, Na+, Cl−, SO4 2−, NO3 − and HCO3 −.
Correlation matrix
PCA generates a correlation matrix including correlation coefficient (r) between variables. Correlation coefficient of 0.5 and above is considered significant and highlighted in bold (Table 2). High positive correlation coefficient is observed among HCO3 − and Ca2+ (r = 0.53), Mg2+ and SO4 2− (r = 0.73), Na+ and Cl− gives (r = 0.87) and Na+ and SO4 2− (r = 0.81). The positive and strong correlation among EC and Ca2+, Mg2+, Na+, Cl− and SO4 2− is expected. These relationships reflect the lithological formation of the aquifer. Nevertheless, the correlations of NO3 − with EC give (r = 0.91), Ca2+ (r = 0.71) and pH (r = −0.56) which indicate to anthropogenic pollution.
The PCA was carried out by a diagonalization of the correlation matrix, so the problems arising from different measurement scales and numerical ranges of the original variables are avoided, since all variables are automatically auto-scaled to mean zero and variance unit.
Factor loadings
Table 3 summarizes the PCA results including the loadings (participation of the original variables in the new ones) and the eigenvalue of each PC. The amount of variance (i.e., information) covered by each PC (also shown in Table 3) depends on the relative value of its eigenvalue with respect to the total sum of eigenvalues. There are several criteria to identify the number of PCs (factors) to be retained in order to understand the underlying data structure (Jackson 1991). A plot of variance % of factors was used, which shows a change of slope after the fourth eigenvalue. Four factors were chosen based on their percentage contribution on the total cumulative variance (82.16 %; (Fig. 5). Table 3 includes factor loadings of 14 variables on these four PCs.
F1 explains 54.11 % of the variance and provide a good correlation with variables Ca2+, Mg2+, Na+, Cl−, SO4 2−, NO3 −, HCO3 −, T and EC, and negatively with pH as demonstrated by factor loadings (Table 3). This factor demonstrates groundwater nitrates pollution correlated to high Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations, high EC and relatively low pH values. F2 expresses 13.21 % of the total variance and is mainly correlated with δ18O and δ2H, and negatively with temperatures. Based on factor loadings, this factor exhibits the isotopic enrichments by evaporation of waters components. F3 explains 8.45 % of the variance and contributed by the variable as 3H. This factor reveals the transit time of groundwater and the tritium age of Fijeh springwater. F4 explains 6.39 % of the variance and involves variables as K+. The potassium is usually used in the detergent and can be considered as indicator of industrial pollution. This factor divulges the anthropogenic contamination. Factor loadings of F1 and F2 (67.3 %) of 14 variables are shown in Fig. 6.
Factor scores
Factor score connected to F1 and F2 includes four water groups (Fig. 7):
Group 1 has negative correlation with F1 and represents samples of injected water from Fijeh Spring (II). This group is distinguished by high pH and low NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and low EC and T values. The water of this group is Ca-HCO3 type.
Group 2 has positive correlation with F1 and involves samples of native groundwater before injection processes (P12, P16, 9T, LS and Z1). It is characterized by relatively low pH and high NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and high EC and T values. The water of this group is Ca-HCO3 type.
Group 3 is related to F1 and represents mixing groundwater between native groundwater (group 2) and injected water (group 1). The shifting of native groundwater to injected water is related to the mixing ratio between these two components. Thus, the groundwater quality of the samples located toward the injected water samples is improved. This group is characterized by relatively high pH and low NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and low EC and T values. The water of mixing groundwater is Ca-HCO3 type as well.
Group 4 is positively correlated to F2, where samples of surface water (QF) are enriched by δ18O and δ2H compared to native groundwater and injected water. The isotopic enrichment is considered as a strong indicator of evaporation effect on surface water that is clearly explained by F2. Consequently, surface water samples are located above the mixing line (F1) between these two components indicating no direct hydraulic connection between surface water and groundwater.
Factor scores F1 distribution maps
To examine the spatial variation of multivariate integrated physico-chemical parameters and for a better interpretation and display of the PCA results in space and time, iso-factor maps were established. These maps were drawn using the values of factor scores (F1) of the observations (2 injections and 10 monitoring wells) between March 19 and July 31, 2007.
Samples with low negative factor scores correlated to F1 represent injected water from Fijeh Spring (wells I and II). These samples are characterized by high pH and low NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and low EC and T values. The water of these samples is Ca-HCO3 type. Samples with high positive factor scores correlated to F1 represent native groundwater before injection processes (P12, P16, 9T, LS and Z1). These samples are characterized by relatively low pH and high NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and high EC and T values. The water of these samples is Ca-HCO3 type.
Mixing groundwater between native groundwater and injected water is related to F1. The shifting of native groundwater to injected water depends on the mixing ratio between these two components and expressed by factor scores. These samples are characterized by relatively high pH and low NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and low EC and T values, while the samples with positive factor scores are characterized by relatively low pH and high NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2−, HCO3 − concentrations and high EC and T values. The water of mixing samples is Ca-HCO3 type as well.
To examine the spatial and temporal effectiveness of the artificial recharge operation by multivariate integrated parameters, we constructed iso-factor scores distribution maps of F1 during the injection and after the complete achievement of the injection process.
Factor scores distribution map of F1 (19-3-2007)
Iso-factor scores distribution map of F1 was performed after 13 days of injection (March 19, 2007; Fig. 8). Positive anomalies (high factor scores) in the maps mean high EC, NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2− and HCO3 − concentrations, while negative anomalies (low factor scores) mean the opposite (i.e., low values of these chemical parameters). Figure 11 shows that the factor scores tend to remarkably decrease down-gradient toward the injection center and very low factor scores are observed near two wells (I and II). The groundwater quality of the samples (P10, P21 and P25) having negative factor scores is enhanced. It is noteworthy that the spatial pattern observed in Fig. 8 is generally similar with the patterns of EC, Cl−, NO3 −, and mixing ratio maps performed in March 19, 2007 (i.e., Fig. 9 spatial distribution of EC value maps is performed for several dates). The mixing ratios between first end member (injected water) and second end member (native groundwater) are computed based on chloride mass balance which is considered as conservative element (Herczeg and Edmunds 2000; Pavelic et al. 2005; Abou Zakhem and Hafez 2012). Chemical equilibrium and the mixing ratios are computed using Hydrowin software.
Spatial and temporal variation of EC value maps (Abou Zakhem and Hafez 2012)
Factor scores distribution map of F1 (15-5-2007)
Iso-factor scores distribution map of F1 was constructed after 62 days of injection in May 15, 2007 (Fig. 10). Low factor scores in the maps mean low values of EC, NO3 −, Ca2+, Mg2+, Na+, Cl−, SO4 2− and HCO3 − concentrations. Figure 10 shows the propagation of freshwater in the groundwater. Therefore, it is clear that negative factor scores cover almost the major part of the maps including I, II, P2, P10, P12, P20, P21, P19 and P25. Consequently, the groundwater quality of these samples is improved and demonstrates the efficiency of the aquifer storage and recovery process.
Factor scores distribution map of F1 (26-6-2007)
Iso-factor scores map of F1 was performed after 50 days of the completion of injection process (Fig. 11). According to this map, the groundwater dilutions plume progress to the south toward P2 and P21 including I, II P25, P10, P20 and P19 with negative factor scores. Thus, groundwater quality of these samples is improved by mixing and dilution process.
Factor scores distribution of F1 (31-7-2007)
After 85 days of complete injection process, iso-factor scores map of F1 was constructed (Fig. 12). This map shows that the dilution plume centre is shifted to P2 including P19 and P21 characterized by negative factor scores. The displacement of the dilution plume is completely related to the hydrodynamic setting of the native groundwater and the application of artificial recharge operation conditions. It is clear that the spatial pattern of iso-factor scores maps observed in Figs. 8, 10, 11 and 12 is generally similar with the patterns of EC (Fig. 10), Cl−, NO3 − and mixing ratio maps, performed for the same experiment dates (Abou Zakhem and Hafez 2012). Consequently, under the applied conditions of aquifer storage and recovery process such as injected water amount, groundwater flow direction and its gradient and the boundary conditions of the aquifer, the estimated effective diameter is about 250 m.
Iso-factor scores maps performed at several time intervals approve that the efficient time appropriate for recovery phase of the ASR is about 3 months subsequent to injection stage. Comparing with previous interpretation based on a single tracer. The mixing ratios were based on the chloride mass balance. The advantage of this new interpretation is mainly dealing with multiples chemical and isotopic tracers in addition to physical parameters, where the mixing between native and injected water is expressed by F1. This interpretation is based on maximizing the important information and reducing the less important (e.g., the stable isotopes cannot be used as relevant tracers for mixing computation). In this case, PCA marginalizes the stable isotopes represented by F2 to differentiate between surface water and groundwater. Thus, the new factor score mapping is more substantial rigorous and global resuming all single trace maps. The results of this study clearly demonstrate the usefulness of multivariate statistical analysis as PCA in the interpretations of aquifer storage and recovery (ASR) process.
Conclusions
Principal component analysis (PCA) provide useful information not available at first glance. PCA was applied on chemical and isotopic data of 80 groundwater and 3 surface water samples (observations) and allowed the reduction of the 14 variables to four significant PCs (factors F) that explain 82.2 % of the variance (i.e., information) of the original data set.
F1 (54.1 %) provide a positive correlation with Ca2+, Mg2+, Na+, Cl−, SO4 2−, NO3 −, HCO3 − T, and EC, and negative correlation with pH. This factor demonstrates groundwater mineralization and nitrates pollution, whereas F2 (13.2 %) is related to δ18O, δ2H and negatively with temperatures. This factor exhibits the isotopic enrichments by evaporation of waters components. F3 (8.5 %) contributed by 3H and reveals the transit time or the tritium age of groundwater. F4 (8.4 %) involve variables as K+ related to the anthropogenic contamination.
Factor score of observations values linked to F1 and F2 includes four groups: Group 1 has negative correlation with F1 and represents injected water samples from Fijeh Spring (I and II). This group is distinguished by high pH and low mineralization, EC and T values. Group 2 has positive correlation with F1 and involves samples of native groundwater before injection processes. It is characterized by relatively low pH and high mineralization, EC and T values. Group 3 is related to F1 and represents mixing groundwater between injected water (group 1) and native groundwater (group 2). The shifting of native groundwater to injected water is related to the mixing ratio between these two components. Accordingly, the quality of groundwater samples placed close to the injected water is enhanced. Group 4 is positively correlated to F2 representing the surface water (QF). The isotopic enrichment by δ18O and δ2H is considered as a strong indicator of evaporation effect on surface water that is clearly explained by F2. Consequently, surface water samples are located above the mixing line (F1) between these two components indicating no direct hydraulic connection between surface water and groundwater.
It is clear that the spatial pattern of iso-factor scores maps is generally similar with the patterns of EC, Cl−, NO3 − and mixing ratio maps performed for the same experiment dates. Consequently, under the applied conditions of aquifer storage and recovery process, the effective diameter is 250 m and 3 months suitable for recovery time after complete injection process.
The results of this study clearly demonstrate the usefulness of multivariate statistical analysis as (PCA) in the investigations of aquifer storage and recovery (ASR) process. Such approach is recommended as a helpful tool for sustainable water quality management and demonstrates the efficiency of the artificial recharge methods.
References
Abdi H, Williams LJ (2010) Principal component analysis. WIREs Comp Stat 2:433–459. doi:10.1002/wics.101
Abou Zakhem B, Hafez R (2001) General hydro-isotopic study of direct infiltration and evaporation process through the unsaturated zone in Damascus Oasis, Syria. IAEA-TECDOC-1246, pp 131–170
Abou Zakhem B, Hafez R (2009) Chemical and isotopic study of pollutants transport through unsaturated zone in Damascus Oasis (Syria). IAEA-TECDOC-1618, pp 169–193
Abou Zakhem B, Hafez R (2012) Chemical and isotopic methods for management of artificial recharge in Mazraha Station (Damascus Basin, Syria). Hydrol Process 26:3712–3724
Abou Zakhem B, Hafez R (2015) Hydrochemical, isotopic and statistical characteristics of groundwater nitrate pollution in Damascus Oasis (Syria). Environ Earth Sci. doi:10.1007/s12665-015-4258-1
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Al-Charideh A (2012) Recharge rate estimation in the mountain karst aquifer system of Figeh Spring, Syria. Environ Earth Sci 65(4):1169–1178
Al-Tamir MA (2008) Interpretation of ground water quality data variation in Erbil city, Northern Iraq. Al-Rafidain Eng 16(2):24–30
Clark JF, Hudson GB, Davisson ML, Woodside G, Herndon R (2004) Geochemical imaging of flow near an artificial recharge facility, Orange County, California. Ground Water 42(2):167–174
Cloutier V, Lefebvre RR, Therrien M, Savard M (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Davis JC (1973) Statistics and data analysis in geology. Wiley, New York
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Dillon P (2005) Future management of aquifer recharge. Hydrogeol J 13:313–316
Dillon P, Herczeg A, Rattray K, Pavelic P, Barry KE (2002) Storage of stormwater in aquifers via injection wells—evaluation of fate and subsurface processes using geochemical and isotopic tracers. IHP-IAH Workshop on Evaluation of Recharge Enhancement Projects in Arid and Semi-arid Areas, 21–22 September 2002
Dillon P, Pavelic P, Tozea T, Rinck-Pfeiffer S, Martin R, Knapton A, Pidsley D (2006) Role of aquifer storage in water reuse. Desalination 188:123–134
Epstein S, Mayeda TK (1953) Variations of the 18O/16O ratio in natural waters. Geochim Cosmochim Acta 4:213–224
Gal I, Neumann R, Calow R, Moench M (2002) The effectiveness of artificial recharge of groundwater: a review. British Geological Survey, commercial report CR/02/108 N, Keyworth, Nottingham
Guillaume P, Xanthoulis D (1996) Irrigation of vegetable crops as a means of recycling wastewater: applied to Hesbaye Frost. Water Sci Technol 33:317–326
Hamzaoui-Azaza F, Bouhlila R, Gueddari M (2009) Geochemistry of fluoride and major ion in the groundwater samples of triassic aquifer (South Eastern Tunisia), through multivariate and hydrochemical techniques. J Appl Sci Res 5(11):1941–1951
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of ground water composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34:807–816
Herczeg AL, Edmunds WM (2000) Inorganic ions as tracers. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Publishers, Boston, pp 31–77
Herczeg AL, Rattray KJ, Dillon PJ, Pavelic P, Barry KE (2004) Mineral–solution interactions during 5 years of aquifer storage and recovery in a confined carbonate aquifer. Ground Water 42(3):438–445
Homsi M, Bouni M, Kurdi A (1989) Damascus basin climate and its role in economical plans and the climatic changes. National Meteorology report, p 35
Iyer CS, Sindhu M, Kulkarni SG, Tambe SS, Kulkarni BD (2003) Statistical analysis of the physico-chemical data on the coastal waters of Cochin. J Environ Monit 5:324–327
Jackson JE (1991) A user’s guide to principal components. Wiley, New York
Kleinbaum DG, Kupper LL, Muller KE (1988) Applied regression analysis and other multivariable methods, 2nd edn. PWS-KENT Publishing, Boston, p 718
Le Gal La Salle C, Vanderzalm J, Hutson J, Dillon P, Pavelic P, Martin R (2005) Isotope evolution and contribution to geochemical investigations in aquifer storage and recovery: a case study using reclaimed water at Bolivar, South Australia. Hydrol Process 19:3395–3411
Ma L, Spalding RF (1996) Stable isotope characterization of the impacts of artificial ground water recharge. Water Resour Bull 32(6):1273–1282
Martin-Rosales W, Gisbert J, Pulido-Bosch A, Vallejos A, Fernandez-Cortes A (2007) Estimating groundwater recharge induced by engineering systems in a semiarid area (southeastern Spain). Environ Geol 52:985–995
Meglen RR (1992) Examining large databases: a chemo-metric approach using principal component analysis. Mar Chem 39:217–237
Ministry of Environment (1994) Drinking water quality standards. Higher council for Environment and safety. Damascus, Syria (Arabic)
Muir KS, Coplan TP (1981) Tracing groundwater movement by using the stable isotopes of oxygen and hydrogen, Upper Penitencia Creek alluvial fan, Santa Clara Valley, California. U.S.G.S. Water-Supply, Paper 2075
Mukhopadhyay A, Al-Sulaimi J (1998) Creation of potable water reserve in Kuwait through artificial recharge. In: Peters JH et al (eds) Artificial recharge of ground water. Balkema, Rotterdam, Netherlands, Amsterdam, pp 175–180
Pavelic P, Dillon PJ, Simmons CT (2002) Lumped parameter estimation of initial recovery efficiency during aquifer storage and recovery. In: Dillon PJ (ed) Management of aquifer recharge for sustainability. Proceedings of the 4th international symposium on artificial recharge (ISAR4), Adelaide September 22–26, 2002, Swets & Zeitlinger, Lisse, pp 285–290
Pavelic P, Nicholson BC, Dillon PJ, Barry KE (2005) Fate of disinfection by-products in groundwater during aquifer storage and recovery with reclaimed water. J Contam Hydrol 77:351–373
Ponikarov VP (1966) Explanatory notes on the geological map of Syria, scale 1/200.000, sheet VII (Damascus) “Techno-export”
Pyne RDG (1995) Groundwater recharge and wells: a guide to aquifer storage recovery. Lewis Publishers, Baco Raton
Pyne RDG (1998) Aquifer storage recovery: recent developments in the United States. In: Peters JH (ed) Artificial recharge of groundwater. Proceedings third international symposium on artificial recharge of groundwater—TISAR, Amsterdam 21–25 September, Balkema Publishers, Rotterdam, pp 257–261
Ray C, Melin G, Linsky RB (2002) Riverbank filtration—improving source-water quality. Kluwer Academic Publishers, Dordrecht
Regli C, Rauber M, Huggenberger P (2003) Analysis of aquifer heterogeneity within a well capture zone, comparison of model data with field experiments: a case study from the river Wiese, Switzerland. Aquat Sci 65:111–128
Selkhozpromexport (1986) Water resources use in Barada and Auvage basins for irrigation of crops, volume II, book 2, USSR, Moscow, p 484
Shammas MI (2008) The effectiveness of artificial recharge in combating seawater intrusion in Salalah coastal aquifer, Oman. Environ Geol 55:191–204
Soumi G, Chayeb R (1989) Water need and plant irrigation techniques. Rep. UNDP, FAO, SYR/86/015, agriculture ministry, p 33
Stamatis G, Parpodis K, Filintas A, Zagana E (2011) Groundwater quality, nitrate pollution and irrigation environmental management in the Neogene sediments of an agricultural region in central Thessaly (Greece). Environ Earth Sci 64:1081–1105
UN-ESCWA and BGR (2013) (United Nations Economic and Social Commission for Western Asia; Bundesanstalt für Geowissenschaften und Rohstoffe) 2013. Inventory of Shared Water Resources in Western Asia. Beirut
WHO (2004) Guidelines for drinking water quality, Vol. 1 recommendations, 3rd edn. WHO, Geneva
WHO (2008) Guidelines for drinking water quality, Vol. 1 recommendations, 3rd edn. WHO, Geneva
WHO (2011) Guidelines for drinking water quality, Vol. 1 recommendations, 4th edn. WHO, Geneva
Acknowledgments
The author is thankful to Prof. Dr. I. Othman, Director General of Atomic Energy Commission of Syria (AECS), for his encouragement. Thanks to Dr. B. Newman technical officer of the RP, RAS/8/103 (IAEA). The laboratory work performed by the colleagues in Geology Department (AECS) is highly appreciated. This study was carried out under the framework of regional project no RAS/8/103 in cooperation with the International Atomic Energy Agency (IAEA) entitled: “The use of isotopes and geochemical techniques in the study of artificial recharge”. The author would like to thank the Editor Prof. James W. LaMoreaux and reviewers for their constructive and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou Zakhem, B. Using principal component analysis (PCA) in the investigation of aquifer storage and recovery (ASR) in Damascus Basin (Syria). Environ Earth Sci 75, 1123 (2016). https://doi.org/10.1007/s12665-016-5923-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5923-8