Abstract
Production water collected from the “Nafoora” oil water pit, located near the Gallo Oasis, Libya, was used for the screening of obligate hydrocarbonoclastic bacteria that can degrade crude oil. Bacterial strain NAF1 was isolated and characterized after enrichment on crude oil at pH 7 and 30 °C. Solid Waste Dates (SWD) and Corn Steep Liquor (CSL) were usedas to enhance biodegradation of crude oil. Strain NAF1 was able to utilize a number of aliphatic and aromatic hydrocarbons. During growth on natural products, high emulsifying activity in the presence of cells was also observed, which indicated the production of biosurfactant by strain NAF1. The new isolated strain NAF1 produced a yellow-green pigment. The bacterial strain showed removal efficiency of 91 and 97 % of crude oil in 28 days when cultivated with >0.5 % (w/v) of pretreated CSL and SWD, respectively. Alignment of the 16S rRNA gene sequences of NAF1 with sequences obtained by Basic local Alignment search tool (BLAST) search revealed 98 % similarity to P. aeruginosa PAO1. Therefore, the crude oil-metabolizing bacterium could secrete surfactants using agro-industrial products as substrates, which further enhanced hydrocarbon degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum and its derivatives are important energy resources used in industries and in our daily lives. However, petroleum and its derivatives have become major pollutants in marine environments because of refuse from coastal oil refineries, offshore oil production, shipping activities, accidental spills, and so on (Gentili et al. 2006; Federici et al. 2012). Furthermore, oil spill is one of the highest potential environmental threats in Mediterranean Sea due to high number of oil extracting and refining sites along the basin coasts, and the intense maritime traffic of oil tankers with the southern coast of the Mediterranean, which includes the Libyan coastline (Daffonchio et al. 2013). So it has become prone to the increasing of oil pollutants incidences. In Libya, five oil terminal facilities and many other operating companies are discharging high rate of effluents. Oil pollution is severely hazardous to ecosystems along parts of the Libyan Marine Coast where oil industries are located. Given the focus on the protection of environment and pollution control in Libya, most terminal facilities with conventional ballast water processes are likely to face serious threats in the coming years. Thus, a safe environmental friendly process that eliminates the use of discharging ballast water into the sea is necessary. Bioremediation, the use of bacteria to remove pollutants, is recently received an attention as the most suitable method for bioremediation of crude oil because it is cost-effective and produce harmless by-products, to the environment (Bharti and Irafan 2011; Fuentes et al. 2014). Various microbial populations, such as bacteria, fungi, and algae, can act in crude oil biodegradation. Most researchers consider bacteria to be the most important group of petroleum-degrading organisms because it has rapid and Numerous metabolic rates of organic biodegradation (Singh and Lin 2010; Maciel et al. 2009). About 175 bacterial types can efficiently oxidized petroleum hydrocarbons. These genera include Pseudomonas, Aeromonas, Bacillus, Flavobacterium, Corynebacterium, and Micrococcus (Prince et al. 2010). Previous literature reported that Pseudomonas aeruginosa is the most efficient in crude oil biodegradation. (Lal and Khanna 1996; Saadoun 2002; Banat et al. 2000). The use of biosurfactants has been found to increase the bioavailability of hydrocarbon resulting in enhanced growth and degradation of contaminants by hydrocarbon-degrading bacteria present in polluted water and change the properties of the bacterial cell surface. (Pacwa-Płociniczak et al. 2011). Among various surfactants, rhamnolipids are considered to be the most effective in degrading hydrocarbons, thereby increasing the mass transfer rate by making hydrophobic pollutants more bioavailable for microorganisms (Itoh and Suzuki 1972; Andrä et al. 2006; Gunther NW 2007).
Biodegradation is one of the accepted and approved techniques by environmental authorities in cleaning marine pollutions. Corn steep liquor (CSL) and solid waste date (SWD) as the agro-industrial waste, recently received attention in enhancing biodegradation of crude oil. The performance of CSL and SWD in improving biodegradation of high concentrated crude oil is still limited (Silva et al. 2014). Most literatures used consortium bacterial group for crude oil biodegradation. Consequently, the effectiveness of employing CSL and SWD to enhance the capability of single strain for crude oil biodegradation has not been well investigated. The design criteria are not sufficiently established as well. Biodegradation efficiency under different operating conditions (i.e., pH, initial crude oil concentration and incubation time) remains not well established. As the insolubility of hydrocarbons decreases the efficiency and rate of degradation, this limitation can be overcome by either the addition of a surface active compound (surfactant) to the growth medium, thereby making hydrocarbons more soluble in water and available for cells to degrade, or by the addition of degrading microorganisms.
Pseudomonas aeruginosa is a typical strain for rhamnolipid production. It can utilize crude oil as the sole carbon source. Pseudomonas aeruginosa can also tolerate and grow in high concentrations (up to 50 % v/v) of crude oil and utilize compounds, such as aliphatic and monoaromatic hydrocarbons and alcohols, as substrates (Zhang et al. 2005). However, the success of rhamnolipid production depends on the increase in yield, development of economical biotechnology processes, and use of low-cost effective renewable agro-industrial substrates. This study aimed characterized the isolated an bacterium from an on-shore oil field in Libya and its application for the biodegradation as a combined of biostimulation and bioaugmentation strategy for oil bioremediation in polluted seawater.
Materials and methods
Sampling
In the current study, oil-contaminated water samples were collected from Nafoora oil field in Libya (Fig. 1), where large amounts of crude oil are produced by Arabian Gulf Oil Co. Company (AGOCO). Simple sampling technique was used to collect samples. Care was taken in handling and immediately transferred to the laboratory and stored in cold room at 4 °C to avoid biochemical reaction. Seawater was used for culturing the isolated microorganisms during bioremediation process.
Preparation of media
Liquid media
Basal salt mineral (BSM) medium according to Piddington et al. (1995) was prepared by dissolving 2.44 g of KH2PO4, 5.57 g of NaHPO4. 2.0 g of NH4Cl, 0.2 g of MgCl2.6H2O, 0.001 g of FeCl3.6H2O, and 0.001 g of CaCl2.2H2O in 1 L of sea water. The pH was adjusted to 7.0 using 10 % NaOH, blended with >0.5 % (w/v) Sarir crude oil to serve as a carbon source, and autoclaved for 20 min at 120 °C.
Tryptone glucose yeast extract (TGY) medium (Benson 1994) was prepared by dissolving 5.0 g of tryptone, 3 g of yeast extract, and 1 g of glucose in 1 L of double distilled water. The medium was then autoclaved. For total viable count, the Ph of each medium was adjusted to 7.0 using 10 % NaOH.
Solid media
For isolation and enumeration of total viable cells, BSM with >0.5 % (w/v) crude oil and TGY were solidified by 2 % agar and then plated. Each medium was autoclaved for 20 min at 120 °C.
Nutrients
Throughout this section, we will use the word “solid waste date” to refer to the fleshy part of the fruit. Date, which is very sweet, comprises about 50–88 % of the total weight according to cultivar, stage of ripening, and water content. Local pretreated solid waste date (SWD) was purchased from the Benghazi local market in Libya. According to the manufacturer, local SWD contained the following components (for each 100 g): moisture, 13–16 %; protein, 1.0–2.3 %; ash, 1.4–1.8 %; total sugar, 75–76 % (glucose: 38 %; fructose: 36 %); pectin, 0.1–0.2 %; tannin, 0.2–0.3 %; pH, 4.5–5.3; and TDS, 74–75 %. Corn steep liquor (CSL) used in this study was provided from El-Nasr Pharmaceutical Company. The carbon: nitrogen: phosphorus content of CSL was 6.8: 1.06: 2.14 (% w w−1).
Hydrocarbons
Thephysical parameters of Sarir crude oil included a kinematic viscosityat 40 °C of 13.563 mm2 s−1, API gravity of 35.64°, and densityat 40 °C of 0.829 g cm−3. Details of crude oil characterization have been reported earlier (Alghanduri et al. 2010).
Enrichment and isolation
Enrichment is a technique used to isolate microorganisms from their natural environment. It involves inoculating natural sources of bacteria into selective media and then growing under physiological conditions optimum for the desired organisms (Kaplan and Kitts 2004).
This method was used for the enrichment, detection, and assessment of the size of indigenous crude oil/biodegrading microorganisms (BDM) in the samples used for isolation. Enrichment was conducted based on Duarte et al. (2001) and Davoodi-Dehaghani et al. (2010), but with slight modification. In the current study, 10 mL of each sample was mixed with 90 mL of enrichment medium. Samples were incubatedin flasks at 30 °C during 168 h at 150 rpm shaking speed. About 10 mL from each flask was withdrawn and added to 90 mL of enrichment media. Serial dilutions (10−1) of each transferred samples were injected in TGY medium plates to enumerate the total colony-forming unit (CFU) and onto BSM-crude oil plates to count BDM and incubated at 30 °C. Separate colonies from BSM-crude oil plates were selected and purified.
Identification and characterization of bacteria
Biochemical characterization
To identify and characterize the bacterial isolates, colonies of the designed strain NAF1 were characterized following primary and secondary identification. Morphological, physiological, and biochemical characteristics of pure isolates were examined according to the Bergey’s Manual of Determinative Bacteriology (Cappuccino and Sherman 2004). Representative colonies of strain NAF1 that appeared on plates were checked for purity through microscopy. Pure isolates were streaked on slants of BSM-crude oil medium on which they developed during isolation and then stored at 4 °C for further investigation. For secondary identification, bacterial isolates were characterized by 16S rRNA gene sequencing (Schattner et al. 2005).
Molecular identification
DNA isolation and amplification were performed in Sigma (Giza, Egypt).
(a) DNA isolation: The most efficient biodegrading bacterial isolate was incubated separately in TGY for 48 h at 30 °C on a rotatory shaker (150 rpm). Genomic DNA was extracted using a Gene JET Genomic DNA Purification Kit K0729 (Fermentas, USA).
(b) Polymerase chain reaction (PCR) amplification of 16S rDNA regions: Two sets of primers were used to amplify regions specific for almost all eubacterial 16S rDNA sequences. The 16SrDNA gene was amplified by PCR using forward primer (5′-ACGTGAGTAACCTGCCCTTG-3′) and Reverse primer (5′-GCCTTGGTGAGCCATTACCT-3′).
The reaction was performedusing 0.5 µL of DreamTaq (5 U/µL; Fermentas, USA), 5 µL of 10×DreamTaqbuffer, 5 µL of target DNA, 1 µL of each dNTP(20 mM), 1 µL of each appropriate primer (10 pmol/µL), and 36.5 µL of dH2O. ThePCR products were visualized using 5 μL of the suspension electrophoresed on 1 % agarose gels in 1×Tris-acetateEDTA buffer. The gels were then stained with ethidium bromide and examined under UV light. Bands were excised, and DNA was purified from gel slices using a QIAquick Gel Extraction Kit (Cat. No. 28704, Qiagen, USA).
(c) Sequencing: The purified PCR products were sequenced with the same primer that was used in the amplification of the target sequence. Sequencing was conducted by an ABI 3730 XL automatic DNA sequencer (Sigma, Giza, Egypt).
(d) Phylogenetic analysis and sequence analyses: The 16S rDNA sequences (Query sequence) were initially analyzed at National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.org) using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and corresponding sequences from the database were downloaded. Evolutionary history was inferred using the neighbor-joining method (Dhanve et al. 2009). The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distance used to infer the phylogenetic tree.
Crude oil-biodegradability assay
Biodegradation of Libyan light crude oil (CO35) (API gravity, 35) by bacterial strain Pseudomonas sp. NAF1 was studied. Biodegradation assays were performed in liquid culture to examine the ability of isolated microorganisms to utilize crude oil as a sole energy source (Ismail et al. 2013). In these assays, cells were incubated at 30 °C in TGY for 24 h in a shaking incubator (150 rpm). Cells were pelleted by centrifugation at 5000 rpm for 15 min and then washed three times with Basal salt Media (BSM). To test the ability of isolates to degrade crude oil (crude oil-biodegradability assay), washed cells were inoculated into BSM containing crude oil as a sole energy source.
The inoculum was adjusted so that the initial absorbance was A 600 = 0.3. The cultures were incubated at 30 °C for 7 day in a shaking incubator (150 rpm). Growth was monitored at the prescribed time intervals by analyzing the optical density at 600 nm using a UV/Vis spectrophotometer (JASCO, V570, USA).Non-inoculated BSM was used as a blank. The pH of the cultures was determined using a pHmeter (DIGMEDDM-22, Brazil).
The residual oil content in the culture flask was extracted using a 1:1 proportion of organic solvent (n-hexane: BSM), followed by evaporating the solvent phase and measuring the weight of the dry extract. All the experiments were carried out in duplicate, and the mean values were considered.
Analysis of variance (ANOVA) for the treatment techniques was used for graphical analyses of the data to obtain the relations between the process variables and the responses. The quality of the fit polynomial model was expressed by the value of coefficient of determination (R 2), and its statistical significance was checked by the F-test in the same program. Model terms were evaluated by the p value (probability) with 95 % confidence level. In this section, CCD and RSM were applied to optimize and assess the relationship among three significant independent variables: (1) crude oil concentration (CO), (2) SWD and CSL concentration, and (3) incubation period.
Hydrocarbon analysis
The Total petroleum hydrocarbons (TPHs) were measured according to the Environmental Protection Agency (EPA Method-1664; US-EPA 1999). Analysis of TPH from water samples revealed that the TPH concentration was 10–181.4 mg/L. It can be considered as oil-polluted water according to the Egyptian Environment Law Number 4 of 1994. Malaysia’s Environmental Quality (Sewage and Industrial Effluents) Regulations (1979) set a standard of 10 mg/L for effluent from land-based sources that are not discharged into water courses used for drinking water. The extracts were dried using granular anhydrous sodium sulphate (Merck, Darmstadt, Germany).The solvent was distilled from the extract, and the n-hexane extractable material (HEM) was dried, weighed and then re-dissolved in n-hexane. Polar materials is removed by adding Silica gel into HEM solution. The solution was filtered to remove silica gel, and the solvent was distilled. Calibration verification and analysis of blanks were performed daily. Percent recovery (S) was calculated using Eq. (1):
where n is the number of samples and x is the percent of recovery in each sample.
Moreover, matrix spike (MS) was tested to ensure the accuracy of the analysis.
The relative percent difference (RPD) between the matrix spike and matrix spike duplicated (MSD) were calculated following Eq. (2):
where D 1 is the concentration of hexane extractable material in the sample and D 2 is the concentration of hexane extractable material in the duplicate sample.
Gas chromatography (GC)
Results were verified by GC (US-EPA 1999) using a GC 2000 series equipped with a flame ionization detector (Fisons Instruments, Milan, Italy). A DB-5 capillary column (J & W Scientific, Folsom, CA, USA) (60 m × 0.25 mm ID, film thickness of 0.25 µm) was used. The operating conditions were as follows: injector temperature, 300 °C; detector temperature, 300 °C; carrier gas, helium 99.999 % (35 mL/s); air flow, 350 mL/s; and make-up gas, nitrogen at 30 mL/s. The oven temperature program was as follows: 1 min at 60 °C, followed by a temperature increase of 10 °C/min up to 160 °C, followed by 10 min at 160 °C, followed by a temperature increase of 4 °C/min up to 300 °C, and finally, 10 min at 300 °C. Splitless mode injections were carried out with the splitless time at 0.8 min. The chromatographic data were analyzed using Chrom-Card data system version 2.1 software (Thermo Electron, Rodano, Italy).
Results and discussion
Isolation and identification of the bacteria
Twenty bacterial strains were isolated from enrichment cultures, which were maintained at 30 °C for 4 weeks. One isolated strain that showed higher growth rate on crude oil were selected from among the 20 isolates for further study. The selection of strain was based on their high capacity to degrade crude oil (>0.5 % w/v). However, Xu et al. (2014) reported no decrease in the CFU of strain SY23 during degradation, even at high concentrations of crude oil. These results indicated that the bacterial strain NAF1 supported growth even at high concentrations. An enrichment culture initiated in basal medium containing >0.5 % (w/v) crude oil as the carbon and energy source became turbid, and the dark layer of crude oil became clear, suggesting the degradation of this substrate. Enrichment culture showed the presence of aerobic bacteria with diverse cellular morphologies, which were isolated in solid media to obtain pure bacterial strains. One colony was chosen, and a pure culture designed NAF1 was selected. This culturing technique was based on the principle that viable microorganism will develop into a colony when material-containing microorganisms are cultured (Aneja 2004).
The strain was identified as Pseudomonas sp. NAF1, and water-soluble pigments that gave it a characteristic yellow-green color are shown in Fig. 1. Strain selection was based on the strain’s high capacity to degrade crude oil in solid and liquid media. Strain NAF1 was used for further characterization. This strain was first identified using classical biochemical morphological characteristics. The results are shown in Table 1.
Molecular identification of isolates was performed by amplifying and sequencing the 16S rRNA gene, and comparing the sequences to the database of known 16S rRNA sequences. The nucleotide sequence of the 1098 bp fragment containing Pseudomonas sp. NAF1 and 16S rDNA structural genes were deposited in Genbank (accession number: NR-074828.1). Phylogenetic analysis suggested that the strain belonged to P. aeruginosa. The 16S rRNA gene sequence formed a stable clade with typical strains of the genus Pseudomonas, and showed a high 16S rRNA gene sequence similarity of 98 % with P. aeruginosa strain PAO1 (Fig. 2).
Gram staining, morphological analyses and 16SrDNA sequence studies revealed that this isolate belonged to P.aeruginosaPAO1. Therefore, Zhang et al. (2012) reported that naturally occurring crude oil-degrading bacteria with a capacity to yield biosurfactants should be isolated and identified.
Crude oil biodegradation
By the end of 28 day culture flasks containing crude oil exhibited 91 and 97 % supplemented with CSL and SWD were degraded respectively, as shown in Fig. 6. Subsequently the final growth rate of 2.3 × 1011, and 1.1 × 1014 TCFU/mL-1 (CFU: colony forming unit) were measured. Thus, it can be concluded that the presence of visual emulsification was found to be growth related or associated in strain NAF1, where a parallel relationship exists between growth substrate utilization and emulsion activity.
The bacterial strain NAF1 was grown in >0.5 % (w/v) light crude oil for 4 weeks with shaking. After the incubation time, the levels of microbial growth and crude oil biodegradation were analyzed using spectrometry-based methods. NAF1 could degrade crude oil in basal liquid and solid media in the presence of 0.5 % (w/v) CSL and SWD. CFUs were measured for crude oil, and continued to grow until the end of the experiment. The presence of visual emulsification activity was found to be associated with growth of strain NAF1, thereby suggesting a parallel relationship between growth substrate utilization and emulsification activity. The displacement of oily substances from water molecules increased with emulsification, which resulted in increased bioaccessibility and subsequent biodegradation of the hydrocarbon (Singh and Cameotra 2004; Cameotra et al. 2010). NAF1 strain produced a yellow-green pigment, as shown in Fig. 5.
These results indicate that the P. aeruginosa is more effective in the biodegradation of crude oil compounds as compared with other species of bacteria. The results were close to the results of previous study (Tavassoli et al. 2012). Significant biomass growth and difference in oil concentration suggest that crude oil is easily degraded by the strain NAF1 under optimized culture conditions. It might be due to the higher API gravity which made the microbes more susceptibility and successfully degraded. Crude oil has emulsified in a very short time in bacterial culture flasks and tolerates relatively high concentrations of degraded petroleum compounds.
The growth of the strain on crude oil was followed by measuring the TPH. GC/MS analysis showed that strain NAF1 was active with the total aliphatic hydrocarbons present in crude oil (C11–C30) after 28 day of incubation. The bacterial strain NAF1 from the culture of enrichment was found the most successful in degrading alkanes to a greater extent and long chain too from n-C15 to n-C35 present in the crude oil in a quantity of 99 % of crude oil were incubated aerobically for a period of up to 28 days. Hamzah et al. (2011) has reported that the species Pseudomonas carries the alkane hydroxylase gene, which is responsible for hydroxylation of alkanes, a major component of crude oil, and naphthalene dioxygenase, which is responsible for the initial attack on aromatic hydrocarbon degradation.
Marín et al. (2003) has reported that Pseudomonas aeruginosa strains RR1 and PAO1contain double alkane hydroxylases, AlkB1 and AlkB2. As minimum in strain PAO1, the substrate variety of these two enzymes overlaps significantly because AlkB1 oxidizes C16–C24 n-alkanes while AlkB2 is active on C12–C20n-alkanes. On the other hand, Beilen et al. (2003) have concluded that P. aeruginosa PAO1 has three AlkBs. Furthermore, Hou et al. (2013) has reported that Alkanes are common environmental pollutants constituting 20–50 % of crude oil, and alkane-degrading microorganisms are widely distributed in nature.
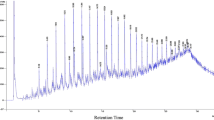
Figures 3, 4 shows the GC profile of control sample and residual crude oil for Pseudomonas sp. NAF1 conducted after a 28 days of incubation period.
This result was confirmed by diminution or total disappearance of the correspondent peak of each compound, as shown in Fig. 5. Strain NAF1 degraded 97 % of crude oil present in the culture after 4 weeks of incubation as shown in Fig. 6.
The cultures were grown aerobically at 28 °C for 28 days with constant shaking (120 rpm). During the experiment, the inoculated culture gradually became turbid because of mixing crude oil with water and emulsification. Any changes in the physical condition of the medium were not observed in the control experiment, and the medium remained clear.
Data shown in Table 2 demonstrated that oil removal by natural attenuation without nutrient supplementation or bacterial addition was much slower than that by bioaugmentation. After 28 d, only about 38 % of >0.5 % (w/v) crude oil hydrocarbon was removed by natural attenuation. The oil concentration was reduced by 66 % when NAF1 was used without enhancement. During the same period, 91 and 97 % of oil were removed by NAF1 enhanced with CSL and SWD, respectively, as shown in Fig. 6.
The addition of CSL and SWD significantly accelerated crude oil degradation. The experimental results showed that the stationary phase possessed excellent selectivity and good recognition ability toward these organic compounds, especially solid waste dates. Strain NAF1 was not directly involved in the degradation process, but possibly played a key role by producing micronutrients or surface-active agents for the solubilization of aromatic hydrocarbons. Silva et al. (2014) reported that biosurfactants could be synthesized from inexpensive substrates, such as molasses and CSL, for use in environmental applications. The addition of both a biosurfactant and/or bacterial cells of P. cepacia favored the biodegradation of hydrophobic organic compounds. The results clearly showed that the biosurfactant alone and its producer species were both capable of stimulating biodegradation to a large extent. Similar results were reported by Aparna et al. (2011), who reported that Pseudomonas spp. show a significant reduction in crude oil concentration. In their study, the bacterium degraded around 52 % of crude oil by the 7th day and 87 % degradation of the crude oil was observed on the 21st day by adding glucose only. Patowary et al. (2014) reported that Bacillus pumilus shows maximum TPH degradation (80.45 %) at the fourth week of incubation and minimum TPH degradation (51.95 %) at the first week of incubation by adding 2 % (w/v) glucose. In the present study, SWD and CSL were added to increase the rate of degradation of the hydrocarbons in crude oil to 97 %, which was observed on the 28th day. Summary results of biodegradation enhanced with CSL and SWD are presented in Tables 3 and 4, respectively.
Tables 5 and 6 present the ANOVA of regression parameters for the predicted response surface quadratic models and other statistical parameters for the removal of crude using CSL and SWD, respectively. The data given in these tables demonstrate that the model was significant at 5 % confidence level, given that p values were less than 0.05. The value of the correlation coefficient obtained in the present study for CO removals using CSL and SWD (R 2 = 0.9893 and 0.9375) were greater than 0.80. For a model with good fit, the minimum correlation coefficient must be 0.80 (Nikolopoulou et al. 2013; Kostka et al. 2011) (Table 6).
The results showed that the presence of Pseudomonas sp. in t oil-contaminated water, thereby suggesting that Pseudomonas sp. exhibited biodegrading efficiency of crude oil that could. Nikolopoulou et al. (2013) and Jyothi et al. (2012) reported that identifying the key organisms that play important roles in different bioremediation treatments is essential for understanding, evaluating, and deciding the optimum in situ bioremediation strategy. Furthermore, the culture medium is a key factor in maximizing heterologous protein expression in strain NAF1.
To reduce experimental costs, corn steep liquor solid and waste dates, as by-product of corn wet milling for starch, are extensively used in bioremediation as components of microbial culture media. These ingredients provide a rich but economical source of nutrients, rich sugars, amino acids, organic acids, vitamins, and minerals (Ye et al. 2010; Al-Bahry et al. 2013; El Mahdi et al. 2014).
Conclusion
Bacterial strain NAF1 belonging to the genus Pseudomonas was isolated and characterized according to its 16S rDNA and biochemical characteristics. DNA–DNA relatedness indicated that strain NAF1 was a member of the same genomic species. The Gram-negative bacterium had large, opaque, shiny colonies with serrated edges that occurred in pairs and sometimes in chains or groups. It was aerobic, oxidase-positive, and catalase-positive. Pseudomonas sp. strain NAF1 could utilize crude oil hydrocarbons as the sole carbon source. Strain NAF1 demonstrated high ability to degrade crude oil at pH 7 and 30 °C. The degradation efficiencies were examined by GC/MS, and the results showed that the isolate could remove 91 and 97 % in 28 d when cultivated with 0.5 % (w/v) CSL and SWD, respectively.
For the isolate, Gram staining, morphology analyses and the 16S rDNA sequence studies revealed, that the isolate belonged to the Pseudomonas, based on literature available on crude oil-degrading and emulsifying have not been reported before as isolate from oil-polluted sites in Libya, enhanced by corn steep liquor and solid waste dates in particularly. This study strongly suggested that pretreated solid waste date addition would accelerate and greatly enhance the biodegradation rate. The bacterial isolate is appropriate candidate for practical field application for effective in situ bioremediation of hydrocarbon-contaminated sites.
References
Al-Bahry N, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ, Al-Makhmari HS, Al-SulaimaniH S (2013) Biosurfactant production by Bacillus subtilisB20 using date molasses and its possible application in enhanced oil recovery. Int Biodeter Biodegrad 81:141–146
Alghanduri LM, Elgarni MM, Daridon JL, Coutinho JA (2010) Characterization of Libyan waxy crude oils. Energy Fuels 24(5):3101–3107
Andrä J, Rademann J, Howe J, Koch M, Heine H, Zähringer U, Brandenburg K (2006) Endotoxin-like properties of a rhamnolipidexotoxin from Burkholderia (Pseudomonas) plantarii: immunecell stimulation and biophysical characterization. BiolChem 301:310–387
Aneja KR (2004) Experiments in microbiology, plant pathology and biotechnology. New Age International (P) Ltd, New Delhi
Aparna A, Srinikethan G, Hegde S (2011( Effect of addition of biosurfactant produced by Pseudomonas sps. on biodegradation of crude oil. In: 2011 2nd International Conference on Environmental Science and Technology IPCBEE.
Banat JM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Benson HJ (1994) Microbiological applications, 6th edn. Wm. Brown and Co., New York
Bharti P, Irafan M (2011) Pseudomonas aeruginosa is present in crude oil contaminated sites of Barmer Region (India). J Bioremed Biodegrad 2(5):1–2
Cameotra SS, Makkar RS, Kaur J, MehtaS )2010( Synthesis of biosurfactants and their advantages to microorganisms and mankind, Biosurfaces 672
Cappuccino JG, Sherman N (2004) Microbiology—a laboratory manual. Pearson Education, Singapore
Daffonchio D, Ferrer M, Mapelli F, Cherif A, Lafraya Á, Malkawi HI, Golyshin PN (2013) Bioremediation of Southern Mediterranean oil polluted sites comes of age. New Biotechnol 30(6):743–748
Davoodi-Dehaghani F, Vosoughi M, Ziaee AA (2010) Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis strain. Bioresource Technol 101:1102–1105
Dhanve RS, Kalyani DC, Phugare SS, Jadhav JP (2009) Coordinate action of exiguo bacterial oxido reductive enzymes in biodegradation of reactive yellow 84A dye. Biodegradation 20:245–255
Duarte GF, Rosado AS, Seldin L, de Araujo W, van Elsas JD (2001) Analysis of bacterial community structure in sulfurous-oil-containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl Environ Microbiol 67:1052–1062
El Mahdi AM, Aziz HA, El-Gendy NS, Amr SSA, Nassar HN (2014) Optimization of Libyan crude oil biodegradation by using solid waste date as a natural low-cost material. J Bioremed Biodeg 5(252):2
Federici E, Giubilei M, Santi G, Zanaroli G, Negroni A, Fava F, D’Annibale A (2012) Bioaugmentation of a historically contaminated soil by polychlorinated biphenyls with Lentinustigrinus. Microb Cell Factories 11:35
Fuentes S, Méndez V, Aguila P, Seeger M (2014) Bioremediation of petroleum hydrocarbons: catabolic genes, microbial communities, and applications. Appl Microbiol Biotechnol 98(11):4781–4794
Gentili AR, Cubittoa MA, Ferrero M, Rodrigue MS (2006) Bioremediation of crude oil polluted seawater by a hydrocarbon—degrading bacterial strain immobilized on chitin and chitosan flakes. Int Biodeter Biodegr 57:222–228
Gunther NW (2007( Processes for the production of rhamnolipids. US Patent, 7,202,063 B1
Hamzah A, Tavakoli A, Rabu A (2011) Detection of toluene degradation in bacteria isolated from oil contaminated soils. Sains Malaysiana 40(11):1231–1235
Hou D, Shi Z, Shen X, He Y, Sun M, Wang Q (2013) Isolation, identification and alkane hydroxylase genes detection of a marine diesel-degrading bacterial strain (F9). African J Microbiol Res 7(22):2794–2802
Ismail W, Alhamad NA, El-Sayed WS, El Nayal AM, Chiang YR, Hamzah RY (2013) Bacterial degradation of the saturate fraction of arabian light crude oil: biosurfactant production and the effect of ZnO nanoparticles. J Petrol Environ Biotechnol. doi:10.4172/2157-7463.1000163
Itoh S, Suzuki T (1972) Effect of rhamnolipids on growth of Pseudomonas aeruginosa mutant deficient in n-paraffin-utilizing ability. Agric Biol Chem 36:2233–2235
Jyothi K, Babu KS, Clara NK, Kumar A (2012) Identification and isolation of hydrocarbon degrading Bacteria by molecular characterization. Helix 2:105–111
Kaplan CW, Kitts CL (2004) Bacterial succession in a petroleum land treatment unit. Appl Environ Microbiol 70(3):1777–1786
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huette M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico Beach sands impacted by the deepwater horizon oil spil. Appl Environ Microbiol 77(22):7962–7974
Lal B, Khanna S (1996) Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenesodorans. J Appl Bacteriol 81:355–362
Maciel BM, Santos ACF, Dias JCT, Vidal RO, Dias RJC, Gross E, Rezende RP (2009) Simple DNA extraction protocol for a 16S rDNA study of bacterial diversity in tropical landfarm soil used for bioremediation of oil waste. Genet Mol Res 8(1):375–388
Marín MM, Yuste L, Rojo F (2003) Differential expression of the components of the two alkane hydroxylases from Pseudomonas aeruginosa. J Bacteriol 10:3232–3237
Nikolopoulou M, Pasadakis N, Norf H, Kalogerakis N (2013) Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Marine Pollution Bull 77(1):37–44
Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12(1):633–654
Patowary K, Saikia RR, Kalita MC, Deka S (2014) Degradation of polyaromatic hydrocarbons employing biosurfactant-producing Bacillus pumilus KS2. Ann Microbial. doi:10.1007/s13213-014-0854-7
Piddington CS, Kovacevich BR, Rambosek J (1995) Sequence and molecular characterization of aDNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol 61:468–475
Prince RC, Gramain A, McGenityTJ (2010) Prokaryotic hydrocarbon degraders. Timmis KN, McGenity TJ, van der Meer JR, de Lorenzo V (eds), In: Handbook of hydrocarbon and lipid microbiology. Berlin Heidelberg, 8: 1671–1692
Saadoun I (2002) Isolation and characterisation of bacteria from crude petroleum oil contaminated soil and their potential to degrade diesel fuel. J Basic Microbiol 42:420–428
Schattner P, Brooks AN, Lowe TM (2005) The tRNAscan-SE, snoscan and snoGPSweb servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33:686–689
Silva EJ, Rochae NMP, Rufino RD, Luna JM, Silva RO, Sarubbo LA (2014) Characterization of a biosurfactant produced by Pseudomonas cepacia CCT6659 in the presence of industrial wastes and its application in the biodegradation of hydrophobic compounds in soil. Colloids Surf B 117:36–41
Singh P, Cameotra SS (2004) Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol 22:142–146
Singh C, Lin J (2010) Bioagumentation efficiency of diesel degradation by Bacillus pumilus JLB and Acinatobacter calcoacetics LT1 in contanated soils. African J Biotechnol 9(41):6881–6888
Tavassoli M, Javadi S, Firozi R, Rezaei F, Khezri AR, Hadian M (2012) Hair contamination of sheep dog and pet dogs with Toxocara cani’s eggs. Iran J Parasitol 7(4):110–115
US-EPA (1999) Method 1664, Revision A: N-hexane extractable material (HEM; Oil and Grease) and silica gel treated N-hexane extractable material (SGT-HEM; non-polar material) by extraction and gravimetry. United States Environmental Protection Agency, Cincinnati
Van Beilen JB, Li Z, Duetz WA, Smits THM, Witholt B (2003) Diversity of alkane hydroxylase systems in the environment. Oil Gas Sci Technol 58:427–440
Xu J, Deng H, Huang T, Song S (2014) Enhanced biodegradation of crude oil in contaminated soil by inoculation of hydrocarbon-degraders. Desalination Water Treat 52:5126–5135
Ye Q, Li X, Yan M, Cao H, Xu L, Zhang Y, Ying H (2010) CHigh-level production of heterologous proteins using untreated cane molasses and corn steep liquor in Escherichia coli medium. Appl Microbiol Biotechnol 87(2):517–525
Zhang GL, Wu YT, QianXP Meng Q (2005) Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J. Zhejiang. Univ. SCI. 6B:725–730
Zhang X, Xu D, Zhu C, Lundaa T, Scherr KE (2012) Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa strains. Chem Eng J 209:138–146
Acknowledgments
Special thanks are due to AGOCO committee, for always being around and support. This work was funded by AGOCO, Libya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Mahdi, A.M., Aziz, H.A., Abu Amr, S.S. et al. Isolation and characterization of Pseudomonas sp. NAF1 and its application in biodegradation of crude oil. Environ Earth Sci 75, 380 (2016). https://doi.org/10.1007/s12665-016-5296-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5296-z