Abstract
Sediment suspension in estuarine environment was simulated with particle entrainment simulator device to investigate the phase redistribution characteristics of polycyclic aromatic hydrocarbons (PAHs). The main focus was laid especially on the role of salinity gradient in the static and dynamic release behavior of PAHs via keeping the consistent of other factors (i.e., hydrodynamic condition and sediment component) in the whole experiment. Results showed that salinity facilitated the accumulation of dissolved and particle-bound PAHs in overlying water in sediment pre-standing and suspending period, which certainly increased the adverse ecological effect of PAHs in sediments. The most beneficial value of salinity for PAHs release from sediments was about 20 ‰. Salinity had more significant impact on the release behaviors of 2+3 ring PAHs than other individual PAHs species. Salinity significantly changed the diameter and suspending status of flocs via adsorption and charge neutralization, and thus affected the existing status of PAHs in the overlying water. Based on quantitative calculation, salinity was found to have more significant influence on the release of PAHs than the pre-fixed hydrodynamic force.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediment suspension facilitates multiphase redistribution of pollutants in surface water, and has sparked an interest in research community on potential adverse ecological effects (Tremblay et al. 2005; Manning et al. 2010). The sediments can act as a transient storage compartment for the contaminants, and the sediment suspension will make these compounds release back into the water column and may have the potential to cause adverse toxicity effects on the ecosystems (Hawthorne et al. 2007; Patrolecco et al. 2010).
As a class of well-documented pollutants, polycyclic aromatic hydrocarbon (PAHs) are of great environment concern due to their potential for bioaccumulation, widespread distribution and carcinogenic properties to human and ecosystem health (Li et al. 2012). They often easily bind to suspended particles in surface water and subsequently deposit as typical persistent organic pollutants (POPs) in sediments. Therefore, numerous studies investigated the distribution of PAHs in sediments all over the world in the past decades (Qin et al. 2011), and also PAHs are ideal and typical organic compounds to probe their release behavior in sediment suspension.
Sediment suspension especially happens in estuary due to the natural activities (e.g., runoff and tidal current interaction, salt water and fresh water intersection, wind disturbance) and anthropogenic activities (e.g., shipping and fishing) (Brunk et al. 1997; Xia et al. 2004; Tremblay et al. 2005). In the estuarine area, gradient change of salinity is a typical feature (Tremblay et al. 2005). Under the drive of the “salting-out effect”, high ionic strengths separate hydrophobic compounds from water to other phases (e.g., solid phase) (Jeon et al. 2011). Meanwhile, the salt-induced flocculation influences the deposition and re-suspension of the sediment components (e.g., mineral composition and organic matters), which are important carriers of PAHs in estuaries (Naudin et al. 1997). Therefore, salinity has significant potential for contaminant redistribution between phases in surface water during sediment suspension.
However, according to our knowledge, not all research approved the significant role of salinity in estuaries. In some studies, salt-induced aggregation played a minor role in enhancing the settling of suspended solids (Thill et al. 2001) and salt was even proved to have no obvious effect (Fotopoulou et al. 2011). In sediment suspension, the disruption of sediment cohesion requires sufficient shear stress, and hydrodynamic conditions are often considered as the premise (Evans 1994). Moreover, sediment components are often considered as the main carrier of pollutants and also greatly affect the transportation of contaminants in sediment suspension (Eggleton and Thomas 2004; Mouhri et al. 2008; Wang and Yang 2010). Therefore, obvious controversy still existed on the role of salinity. As for PAHs, even more few studies have yet discussed the impact of salinity on the release behaviors and mechanism of PAHs in sediment suspension (Brunk et al. 1997; Thill et al. 2001; Tremblay et al. 2005; Fotopoulou et al. 2011; Oh et al. 2013).
Therefore, this study sampled raw sediments at Chaoyangnongchang (31°04′49.42″N, 121°53′29.11″E) in the estuary of the Yangtze River (one of the World’s Top 10 Rivers at Risk, World Wide Fund for Nature), and thus kept the consistency of sediment composition and hydrodynamic condition in experiments to probe the impact of salinity gradient on PAHs release behaviors in sediment suspension. The main focus is laid on the impact of salinity on the multiphase redistribution of PAHs. The roles of salinity, hydrodynamic condition and sediment components have also been compared.
Materials and methods
Materials
Sediments were collected from Chaoyangnongchang in the Yangtze estuary using a Van Veen stainless steel grab sampler (Eijkelkamp, Netherlands) and kept at −20 °C prior to laboratory tests. To probe the impact of salinity, artificial seawater of different salinities [5, 20 and 35 ‰ (g g−1)] was synthesized by the prescription in Table 1. The tap water with salinity of ~0 ‰ was used as control water. Sodium azide was added as the microbial inhibitor and the concentration was 100 mg L−1 (Xia et al. 2013; Zhou and Broodbank 2014).
The sixteen US EPA priority PAHs, i.e., naphthalene (Nap), ace-naphthylene (Acy), acenaphthene (Ace), fluorine (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (InP), dibenzo[a,h]anthracene (DahA), and benzo[g,h,i]perylene (BghiP) were analyzed (Li et al. 2012). The standard mixtures [PAH Solution Mix, 0.2 mg mL−1 in CH2Cl2:MeOH(1:1)] were obtained from Beijing J&K Chemical United Win Technology Limited. The working standard solutions (1 mg L−1) were prepared by diluting appropriate volumes of the standard mixture with methanol (HPLC grade, Merck, Germany). Other solvents and reagents included acetone (HPLC grade, Tedia, USA), dichloromethane, n-hexane (HPLC grade, Dikma, USA), silica gel (200–300 mesh) and anhydrous sodium sulfate (analytical grade, Beijing Chemical Reagent Co., China). Silica gel and anhydrous sodium sulfate were baked in a muffle furnace at 450 °C for 4 h, kept in a sealed desiccator prior to use. Water was obtained from a Milli-Q system (Millipore, Bedford, MA, USA) with a specific resistivity of 18.2 MΩ cm. 0.45 µm glass fiber filters (Whatman, England) used to collect suspended particles were cleaned by baking at 450 °C for 4 h. Glass wares were prepared as follows: cleaned in an ultrasonic cleaner (KQ-502B, Kunshan Ultrasonic Instruments, China); then heated at 450 °C in a muffle furnace for 4 h and rinsed with acetone, n-hexane.
Sediment suspension experiments
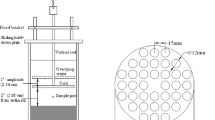
The designed particle entrainment simulator (PES) device, described in detail in our previous study (Feng et al. 2007) illustrated Fig. 1 was used to perform sediment suspension. Before experiment, the sediment (2.43 kg) was fully mixed to maintain the consistence of the physicochemical characteristics throughout the whole experiment. For each group of sediment suspension experiment, 2.43 kg of the homogenized sediment was transferred into the apparatuses. Then, 1.4 L of artificial seawater of certain salinity degree was poured into apparatuses. The organic glass tank was positioned to the PES unit and the perforated grid was lowered into the water. After 20 h pre-standing, 100 mL of overlying water were taken as control sample to measure PAHs release flux from sediment. Afterwards, based on the optimized hydrodynamic condition in our previous study (Feng et al. 2007), the PES was adjusted to 750 r min−1 corresponding to 0.5 N m−1 shear level to make the sediments suspended and the motor was opened to shake for 12 h. The distance of the grid and sediment–water interface was 5 cm. 100 mL of overlying water samples was collected at 0.5, 1.5, 3.5, 6.5 and 12 h. Then 100 mL of water with the same salinity of initial water was added after sampling to maintain volume of the overlying water. Each sample was immediately filtered using 0.45 μm glass fiber filters (Whatman, England) to separate water and particle fraction. 5 mL of water samples was put in clean glass bottle and refrigerated for determination of pH and dissolved organic carbons (DOC). The left 95 mL water was used to determinate the concentration of dissolved PAHs in water phase. Concentrations of total suspended solids (TSS) and PAHs absorbed on the suspended particles were measured via the freeze-dried particles which were filtered out from the water samples.
Analytical methods
PAHs in suspended particles including each glass fiber filter were extracted using hexane and acetone (1:1 v/v) by an accelerated solvent extractor (ASE-300, Dionex, USA) and then were concentrated to 1–2 mL by a rotary evaporator (RV 05, IKA, Germany). The concentrations were further purified by silica gel and anhydrous sodium sulfate, and the detailed progress can be found in our previous study (Li et al. 2012).
PAHs concentrations were analyzed by high-performance liquid chromatography (HPLC) (Dionex Utimate 3000, USA) with UV and fluorescence detectors, which is a classical analytical method to quantify PAHs in environmental matrixes (Gratz et al. 2000; Capelo et al. 2005). The injection volume was 20 μL, and the temperature of the C18 column (Varian, 250 mm × 4.6 mm, 5 μm) was 30 °C. The gradient process was performed by the method of Feng et al. (2007) that 75 % methanol at the beginning and followed by a 17 min linear gradient to 100 % methanol for 30 min. A linear gradient (1 min) at starting conditions followed by a 7 min pre-run to achieve equilibrium to each subsequent run. Acenaphthylene, which was weakly fluorescent, was quantified by UV detector at 229 nm. Sediment particle size and DOC were measured by Laser Particle Size Analyzer (Microtrac S3500, USA) and Total Organic Carbon Analyzer (Elementar, Germany), respectively.
Quality assurance and quality controls
Strict quality control was operated in the experiment. The particle samples and water samples were spiked with the mixed PAHs standard solution, then extracted, purified, and analyzed in the same way as the samples. The six parallel experiments indicated that recoveries for the 16 PAHs were 74.9–120 % from the particles samples and 60–100 % from water samples. The relative standard deviations (RSDs) of the 16 PAHs were all below 10 % for the particles samples and below 7.46 % for water samples. Additionally, the detection limits for the 16 PAHs ranged from 0.098 to 0.191 µg L−1 on water samples, and from 0.34 to 4.05 ng g−1 on a sample dry mass basis, respectively. Each series of experiments was accompanied by two blank experiments and method blanks operation showed no detectable amounts of PAHs.
Results
Physicochemical characteristics of raw sediments and the overlying water
The background contents of the sixteen PAHs in the raw sediment were analyzed and listed in Table 2. The total PAHs content is 544.2 ng g−1 dry weights, which is at the relative low level among estuaries influenced greatly by human activities in the world (Kwach et al. 2009; Garcia et al. 2010). In this study, clay was defined as materials with particles smaller than 2 μm. Silt size particles fall in the range 2–63 μm, and sand from 63 μm to 2 mm (Li et al. 2012). It can be seen that the sediment is mainly composed of silt size particles and the mean diameter (i.e., d 50 size) is 15.7 μm (Table 2).
The concentration changes of suspended solids (TSS) and dissolved organic carbons (DOC) in the overlying water of different salinities with suspension time were analyzed in Fig. 2. The TSS concentrations generally decrease with the increase of salinity and suspension time, though a slight peak concentration is observed under the salinity of 20 ‰ (Fig. 2a). When the salinity increases from 0 to 5 ‰, more significant decreases (about 54.4–75 % for different suspension time) of the TSS concentrations are observed.
Unlike the TSS fractions, the DOC concentration shows an overall increase with salinity in Fig. 2b, although the percent content of TOC in raw sediment is only 0.47 %. However, peak concentrations are also observed under the salinity of 20 ‰. And the concentration of DOC decreased with salinity rise when salinity is higher than 20 ‰, which agrees with previous research (Plavsic et al. 2012).
Dissolved PAHs in the overlying water
Concentration changes of individual (2+3 rings, 4 rings, and 5+6 rings) and total PAHs (ΣPAH) dissolved in the overlying water of different salinities are shown in Fig. 3. It can be inferred that the ranges of ΣPAH concentrations are 1094–2333,1576–3637, 3326–4104 and 1645–3174 ng L−1 for the salinities of 0, 5, 20 and 35 ‰, respectively. The highest concentration is observed under the salinity of 20 ‰. The lowest concentration is found in fresh water (i.e., the salinity of 0 ‰).
In every sediment suspension in Fig. 3, the concentration of ΣPAH increases firstly and then decreases. However, the peak concentrations and the occurrence time of the inflection points are different among the four sediment suspensions. In every sediment suspension experiment, two typical release periods are designed in this study, i.e., the sediment pre-standing period (denoted as the gray rectangles at 0 h in Fig. 3, i.e., static release period) and the following sediment suspension period (denoted as the blank rectangles at 0.5, 1.5, 3.5, 6.5 and 12 h in Fig. 3, i.e., dynamic release). It can also be seen that the mass of the dissolved PAHs released at the former period are approximately 42–85 % of those detected in the later sediment suspension period.
As for the individual PAHs, the concentration change trends are coincident with that of ΣPAH. The 2, 3, 4-rings PAHs constitute the main composition of ΣPAH and play the key role in the concentration changes of ΣPAH due to their high contents in the raw sediments. Relatively, higher concentrations of 2, 3-rings PAHs are observed in the overlying water. The concentrations of 5, 6-rings PAHs are very low and even not detected mostly in Fig. 3.
Particle-bound PAHs in the overlying water
The concentration changes of particle-bound PAHs were also analyzed and described in Fig. 4. The particle-bound ΣPAH concentrations in salt water are generally higher than those in the fresh water. The highest particle-bound ΣPAH concentrations are observed under the salinity of 20 ‰. Very slight increase or decrease trends of particle-bound ΣPAH concentrations with suspension time could also be detected under different salinities.
Concentration changes of different rings and total 16 PAHs bounded on the suspended matters with suspension time in the fresh water (a salinity 0 ‰) and the salt water (b salinity 5 ‰; c salinity 20 ‰; d salinity 35 ‰); the insets represent the concentration multiple times of particle-bound PAHs to those in raw sediment
For individual particle-bound PAH species, 2, 3-rings PAHs also constitute the main composition of ΣPAH. The concentrations of individual PAHs follow the order: 2, 3-rings > 4-rings > 5, 6-rings (Fig. 4).
Discussions
Impact of salinity on PAHs release from sediments
In sediment suspension, PAHs may desorb from sediment component (e.g., mineral composition and organic matter) and entrained into overlying water (Ghosh et al. 2003). This study discussed two typical PAH release patterns, i.e., the static release (at 0 h, after the 20 h pre-standing of sediments) and the following dynamic release (i.e., the hydrodynamic force-driven suspension of sediments). In the static release, no hydrodynamic disturbance existed, but high concentrations of dissolved PAHs were still observed (shown by the gray rectangles in Fig. 3). The main driving force should be the “concentration difference” (Ghosh et al. 2003; Zheng et al. 2013). Ionic competition on particle surface might also be taken into consideration, since seawater contents high concentrations of anions and cations that exercise competition on particle surfaces and lead to removal on some weakly bound inorganic and organic compound from solid particles. As for the dynamic release, hydrodynamic force entrained sediments into the overlying water (Feng et al. 2007), and thus resulted in the changes of physicochemical characteristics of sediment such as organic carbon content and redox state which facilitated the quick increase of the dissolved PAHs concentrations at the initial stage (shown by the blank rectangles in Fig. 3) (Komada and Reimers 2001; Shen 2009). The following decrease means the existence of dynamic equilibrium driven by various factors (e.g., hydrodynamic disturbance, particle size composition and redox potential) in the dynamic release period (Zheng et al. 2013). Phase transfer of PAHs via re-adsorption also occurred simultaneously (Fotopoulou et al. 2011; Jeon et al. 2011), which could be inferred from the comparison of the later decrease of dissolved PAHs concentrations in Fig. 3 with the slight increase of the particle-bound PAHs concentrations in Fig. 4.
In static release, significantly higher concentrations of the dissolved PAHs were observed in saline water (i.e., gray rectangles in Fig. 3b–d) than that in fresh water (i.e., gray rectangles in Fig. 3a). Similar phenomenon also existed in the following dynamic release in Figs. 3 and 4. Either the dissolved (Fig. 3b–d) or the particle-bound PAHs (Fig. 4b–d) have significantly higher concentrations in saline water than those in fresh water (Figs. 3a, 4a, respectively). The high concentration of particle-bound PAHs should be mainly ascribed to the re-adsorption of the dissolved PAHs onto suspended particles. This can be approved by the phenomenon that the concentration of particle-bound PAHs in the overlying water was significantly higher (over ten multiple times, as shown in the insets of Fig. 4) than that of the PAHs in the raw sediment. And higher multiple times were found in salt water.
However, salinity exerted different impact on the release behaviors of individual PAHs species. Higher multiple times were observed for the low-ring PAHs species, which was ascribed to the difference in the binding capacity of particles with different rings PAHs. Generally, high-ring PAHs with big molecular weight are more strongly bound by particles, while low-ring PAHs has higher water solubility which led to less partitioning in particles (Chiou et al. 1998; Chen et al. 2004).
Different salinity also exhibited inequable impact on the release behaviors of PAHs. In the static and dynamic release, peak concentrations of the dissolved and particle-bound PAHs were both observed under the salinity of 20 ‰. Similar distribution was also found for the changes of TSS (Fig. 2a) and DOC (Fig. 2b). The slight increase of DOC in seawater could be explained by the increase in salinity since seawater can encourage the release of organic carbon from sediment (Zhao et al. 2011). The above-mentioned experimental results coincide well with the special phenomenon (i.e., Turbidity Maximum) in the area of the similar salinity (the average salinity of 22.82 ‰) in Yangtze Estuary (Shen et al. 2008), where is the intersection place of fresh water and sea water (Wang et al. 2013).
Action mechanism of salinity
Up to date, no study has proved the direct reaction of saline ions with PAHs. The transportation of PAHs in surface water were closely related to the co-existing organic matters, mineral particles, and so on (Ahrens and Depree 2004; Yang et al. 2008). In this study, relative high correlation of the TSS and DOC fractions, especially the latter, existed with PAHs species. Stronger linear correlations (most R > 0.9) were observed between dissolved PAHs and DOC at the initial stage of sediment suspension. Most likely, salinity changes the physicochemical characteristics of water environment during sediment suspension (Brunk et al. 1997; You et al. 2010), thereby affects the affinity of PAHs to particles and their water solubility (Tremblay et al. 2005).
Generally, particles in raw sediments carry negative charge (Hunter and Liss 1979). Saline ions with positive charges encouraged the aggregation and deposition of the suspended matters via charge neutralization (Liu et al. 2007), and thus resulted in the concentration decrease of TSS in Fig. 2a and particle-bound PAHs in Fig. 4b under the salinity of 5 ‰. Further increase of salinity from 5 to 20 ‰ changed the electrical property of suspended matters via the adsorption of saline ions, and thus inhibited their sedimentation. As a result, more and more organic matters and fine-particles accumulated in the overlying water and formed the active flocs which facilitated the accumulation of PAHs as particle-bound PAHs (Fig. 4c). Thus, peak concentrations of TSS and particle-bound PAHs were all observed under the salinity of 20 ‰. Meanwhile, as shown in Fig. 2b, slight increase of DOC with salinity was also observed. The DOC fraction enhanced the water solubility of PAHs and competed with particles to sorb PAHs and thus keep PAHs in the dissolved phase (Tremblay et al. 2005). Therefore, peak concentrations of DOC and dissolved PAHs were also observed under the salinity of 20 ‰. Similar phenomena were also reported in previous research study (Fotopoulou et al. 2011).
Role of salinity
However, higher salinity (i.e., 35 ‰, excessive charges introduced by salinity) facilitated the deposition of suspended materials again (Stumm 1992). The bulk mass flocs were more beneficial for the deposition of TSS, DOC and PAHs. Therefore, it can be concluded that different salinities may result in different phase redistributions of TSS, DOC, and PAHs in sediment suspension. The inflection point of salinity degree should be approximately 20 ‰. The discrepancy of the roles of salinity among many previous studies should take the salinity degree in their research into consideration (Fotopoulou et al. 2011; Jeon et al. 2011).
If the concentration of the dissolved PAHs at the static release period in fresh water (i.e., the gray rectangle in Fig. 3a) was taken as the base value or background value, it can be inferred from the other blank rectangles in Fig. 3a that the hydrodynamic disturbance resulted in onetime increase of dissolved PAHs concentrations. However, as inferred from the gray rectangles in Fig. 3b–d (i.e., the static release period), salinity can cause 1.5–3.5 multiple times increase of the mean concentrations of dissolved PAHs. From this aspect, salinity exerted more significant impact than hydrodynamic disturbance. Certainly, the higher mean concentrations of particle-bound PAHs (0.1–5 multiple times higher) in salt water in Fig. 4b–d than that in Fig. 4a also proved the conclusion. The dissolved PAHs concentrations at the dynamic release period in Fig. 3b–d (i.e., the blank rectangles) were observed to be 1.5–4 multiple times of the background value in Fig. 3a, which should be ascribed to the coupled effect of the three factors (e.g., salinity, hydrodynamic disturbance, sediment component). Moreover, it can also be easily inferred that the coupled effect was a typical additive effect and salinity facilitates the release of PAHs from sediment into overlying water.
References
Ahrens MJ, Depree CV (2004) Inhomogeneous distribution of polycyclic aromatic hydrocarbons in different size and density fractions of contaminated sediment from Auckland Harbour, New Zealand: an opportunity for mitigation. Mar Pollut Bull 48:341–350
Brunk BK, Jirka GH, Lion LW (1997) Effects of salinity changes and the formation of dissolved organic matter coatings on the sorption of phenanthrene: implications for pollutant trapping in estuaries. Environ Sci Technol 31:119–125
Capelo JL, Galesio MM, Felisberto GM, Vaz C, Pessoa JC (2005) Micro-focused ultrasonic solid–liquid extraction (μfusle) combined with HPLC and fluorescence detection for PAHs determination in sediments: optimization and linking with the analytical minimalism concept. Talanta 66:1272–1280
Chen B, Xuan X, Zhu L, Wang J, Gao Y, Yang K, Shen X, Lou B (2004) Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res 38:3558–3568
Chiou CT, McGroddy SE, Kile DE (1998) Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environ Sci Technol 32:264–269
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980
Evans RD (1994) Empirical evidence of the importance of sediment resuspension in lakes. Hydrobiologia 284:5–12
Feng J, Yang Z, Niu J, Shen Z (2007) Remobilization of polycyclic aromatic hydrocarbons during the resuspension of Yangtze River sediments using a particle entrainment simulator. Environ Pollut 149:193–200
Fotopoulou K, Siavalas G, Karapanagioti HK, Christanis K (2011) Phenanthrene sorption onto heterogeneous sediments containing carbonaceous materials in fresh water and in marine environments: implications for organic pollutant behavior during water mixing. In: Lambrakis Nicolaos et al (eds) Advances in the research of aquatic environment. Springer, Berlin, pp 211–217
Garcia MR, Mirlean N, Baisch PR, Caramão EB (2010) Assessment of polycyclic aromatic hydrocarbon influx and sediment contamination in an urbanized estuary. Environ Monit Assess 168:269–276
Ghosh U, Zimmerman JR, Luthy RG (2003) PCB and PAH speciation among particle types in contaminated harbor sediments and effects on PAH bioavailability. Environ Sci Technol 37:2209–2217
Gratz LD, Bagley ST, Leddy DG, Johnson JH, Chiu C, Stommel P (2000) Interlaboratory comparison of HPLC-fluorescence detection and GC/MS: analysis of PAH compounds present in diesel exhaust. J Hazard Mater 74:37–46
Hawthorne SB, Azzolina NA, Neuhauser EF, Kreitinger JP (2007) Predicting bioavailability of sediment polycyclic aromatic hydrocarbons to hyalella azteca using equilibrium partitioning, supercritical fluid extraction, and pore water concentrations. Environ Sci Technol 41:6297–6304
Hunter KA, Liss PS (1979) The surface charge of suspended particles in estuarine and coastal waters. Nature pp 823–825
Jeon J, Kannan K, Lim BJ, An KG, Kim SD (2011) Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. J Environ Monitor 13:1803–1810
Komada T, Reimers CE (2001) Resuspension-induced partitioning of organic carbon between solid and solution phases from a river-ocean transition. Mar Chem 76:155–174
Kwach OB, Lalah JO, Shem OW (2009) Spatial and seasonal variations in concentrations of polycyclic aromatic hydrocarbons in water and sediment of Kisumu City Bay of Winam Gulf, Lake Victoria-Kenya. Bull of Environ Contam Toxicol 83:722–733
Li B, Feng C, Li X, Chen Y, Niu J, Shen Z (2012) Spatial distribution and source apportionment of PAHs in surficial sediments of the Yangtze Estuary, China. Mar Pollut Bull 64:636–643
Liu Q, Li J, Dai Z, Li D (2007) Flocculation process of fine-grained sediments by the combined effect of salinity and humus in the Changjiang Estuary. Acta Oceanol Sin Engl Edn 26:140–149
Manning AJ, Langston WJ, Jonas P (2010) A review of sediment dynamics in the Severn Estuary: influence of flocculation. Mar Pollut Bull 61:37–51
Mouhri A, Motelay-Massei A, Massei N, Fournier M, Laignel B (2008) Polycyclic aromatic hydrocarbon transport processes on the scale of a flood event in the rural watershed of Le Bebec, France. Chemosphere 73:443–450
Naudin JJ, Cauwet G, Chretiennot-dinet MJ, Deniaux B, Devenon JL, Pauc H (1997) River discharge and wind influence upon particulate transfer at the land–ocean interaction: case study of the Rhône river plume. Estuar Coast Shelf Sci 45:303–316
Oh S, Wang Q, Shin WS, Song DI (2013) Effect of salting out on the desorption-resistance of polycyclic aromatic hydrocarbons (PAHs) in coastal sediment. Chem Eng J 225:84–92
Patrolecco L, Ademollo N, Capri S, Pagnotta R, Polesello S (2010) Occurrence of priority hazardous PAHs in water, suspended particulate matter, sediment and common eels (Anguilla anguilla) in the urban stretch of the River Tiber (Italy). Chemosphere 81:1386–1392
Plavsic M, Strmecki S, Dautovic J, Vojvodic V, Olujic G, Cosovic B (2012) Characterization and distribution of organic matter using specific physico-chemical methods: a case study of the southeast Adriatic continental and shelf slope (Albania). Cont Shelf Res 39–40:41–48
Qin Y, Zheng B, Lei K, Lin T, Hu L, Guo Z (2011) Distribution and mass inventory of polycyclic aromatic hydrocarbons in the sediments of the south Bohai Sea, China. Mar Pollut Bull 62:371–376
Shen J (2009) Transferring and transforming of mercury during resuspension under dynamic disturbing at tidalflat of Yangtze estuary. Dissertation, East China Normal University
Shen Z, Zhou S, Pei S (2008) Transfer and transport of phosphorus and silica in the turbidity maximum zone of the Changjiang estuary. Estuar Coast Shelf S 78:481–492
Stumm W (1992) Chemistry of the solid-water interface: processes at the mineral-water and particle-water interface in natural systems. John Wiley Sons, New York
Thill A, Moustier S, Garnier J, Estournel C, Naudin J, Bottero J (2001) Evolution of particle size and concentration in the Rhine river mixing zone: influence of salt flocculation. Cont Shelf Res 21:2127–2140
Tremblay L, Kohl SD, Rice JA, Gagne JP (2005) Effects of temperature, salinity, and dissolved humic substances on the sorption of polycyclic aromatic hydrocarbons to estuarine particles. Mar Chem 96:21–34
Wang L, Yang Z (2010) Simulation of polycyclic aromatic hydrocarbon remobilization in typical active regions of river system under hydrodynamic conditions. J Soil Sediment 10:1380–1387
Wang D, Feng C, Huang L, Niu J, Shen Z (2013) Historical deposition behaviors of PAHs in the Yangtze River Estuary: role of the sources and water currents. Chemosphere 90:2020–2026
Xia X, Li Y, Yang H, Wu C, Sing TH, Pong HK (2004) Observations on the size and settling velocity distributions of suspended sediment in the Pearl River Estuary, China. Cont Shelf Res 24:1809–1826
Xia X, Zhai Y, Dong J (2013) Contribution ratio of freely to total dissolved concentrations of polycyclic aromatic hydrocarbons in natural river waters. Chemosphere 90:1785–1793
Yang Z, Feng J, Niu J, Shen Z (2008) Release of polycyclic aromatic hydrocarbons from Yangtze River sediment cores during periods of simulated resuspension. Environ Pollut 155:366–374
You C, Jia C, Pan G (2010) Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environ Pollut 158:1343–1347
Zhao J, Bi C, Chen Z, Shen J, Zheng X (2011) Effect of dynamic and salinity conditions on activities of Hg during sediment resuspension event in the Yangtze Estuary. Sci China-Chem 41:105–116
Zheng S, Wang P, Wang C, Hou J, Qian J (2013) Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quatern Int 286:94–102
Zhou J, Broodbank N (2014) Sediment-water interactions of pharmaceutical residues in the river environment. Water Res 48:61–70
Acknowledgments
This work was supported by Beijing Higher Education Yong Elite Teacher Project (YETP0235, 110632111), the National Basic Research Program of China (973 Program, 2010CB429003), and The Nonprofit Environment Protection Specific Project of China (201409040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Funding
This work was supported by Beijing Higher Education Yong Elite Teacher Project (YETP0235,110632111), the National Basic Research Program of China (973 Program, 2010CB429003), and The Nonprofit Environment Protection Specific Project of China (201409040).
Rights and permissions
About this article
Cite this article
Li, R., Feng, C., Wang, D. et al. Role of salinity in the multiphase redistribution of polycyclic aromatic hydrocarbons (PAHs) in sediment suspension. Environ Earth Sci 75, 116 (2016). https://doi.org/10.1007/s12665-015-5014-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5014-2