Abstract
The sorption behaviors of pollutants affected by hydrodynamic conditions were confirmed in natural water environment. The effects of hydrodynamic conditions on the sorption behaviors of aniline on sediment with coexistence of nitrobenzene were investigated. The particle entrainment simulator (PES) was used to simulate varied bottom shear stresses. The batch equilibrium method was applied to the experiments with the stress levels and the action time controlled at 0.2–0.5 N/m2 and 24 h, respectively. The findings indicated that apparent partition coefficient of aniline on sediment increased with the shear stress significantly, while decreased with nitrobenzene concentration. On the contrary, both the sorption amount of aniline on suspended particulate matter (Q s) and the effect of nitrobenzene concentration on Q s declined as the shear stress increased. The sorption kinetic results showed that the sorption process followed the pseudo-second-order kinetics equation, and the process included two stages: fast sorption stage and slow sorption stage, among which the average sorption rate of fast stage was 7.5–9.5 times that of slow one. The effect of shear stress on the average sorption rate of aniline was enhanced with the increase of nitrobenzene concentration. And shear stress weakened the disturbance of cosolute on main solute sorption process. In addition, experiment results of sorption kinetic show that only the initial sorption rate was affected by shear stress and cosolute concentration. In the first 5 min, shear stress had positive effects on the sorption rate. After that, the sorption rate barely changed with shear stress and cosolute concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediment is an important part of the aquatic ecosystem that plays a role as the carrier, fate, and reservoir of transport and transformation process of pollutants in aquatic environment (Blom and Winkels 1998). As there are so many disturbance factors will affect hydrodynamic conditions, such as current, wave, dredging, ship traffic, and bioturbation, it cannot measure the level of external disturbance comprehensively only using current velocity, wave height, wave period, etc. Recent studies (Gabel et al. 2012; Wang et al. 2013a; Reardon et al. 2014; Wu and Hua 2014) suggested that bottom shear stresses in water bodies can be expressed as the functions of many hydrodynamic processes. These bottom disturbances entrain large amount of sediments into the water column, in which pollutants can be transported between suspended sediments and aqueous phase through sorption–desorption behavior, and affect the distribution of pollutants in sediment–water interface. Therefore, investigation on the sorption behavior of pollutants on sediments under the varied hydrodynamic conditions is significant for understanding the migration, transformation, and fate of pollutants in aquatic ecosystem.
The organic matter in sediments and water also play an important role in the transport and bioavailability of contaminants in the aquatic environment. On the one hand, sediment organic matter (SOM) has been shown to be the most important component in the adsorption of hydrophobic organic pollutants (Chiou et al. 1979). The adsorption of organic pollutants by SOM often has a stronger affinity compared to the adsorption by mineral fractions, especially in SOM-rich soil or sediment (Cornelissen et al. 2005; Zhao et al. 2010). On the other hand, dissolved organic matter (DOM) has been shown to interact with organic contaminants and can alter their mobility, bioavailability, and degradation in the environment. Contaminants in aquatic environments can be bound to DOM, preventing them from being captured and taken up by organisms or from escaping into the atmosphere (Hassett 2006). Heringa and Hermens (2003) clearly pointed out that the interaction of organic contaminants with DOM decreases the freely dissolved concentrations and the bioavailability of organic contaminants.
Since aniline (AN) and nitrobenzene (NB) are highly toxic chemicals which can lead to carcinogenesis, teratogenesis, and mutagenesis, they have been listed as priority pollutants by the US EPA, EU, and China EPA. Additionally, as important industrial chemicals and intermediates of dyestuff, pesticide, and pharmaceutical, the widely use of AN and NB has caused serious pollution on environment. AN and NB are typically found in the same contaminated site because NB is the primary starting material for the manufacture of AN, which leads to the co-occurrence of these compounds in an accidental spill (Liu et al. 2013). For this reason, it can provide the theoretical basis for water pollution control, risk assessment of AN pollution by studying on the sorption of AN to sediments with the coexistence of NB.
In addition to sorption, the fate of aniline and nitrobenzene in aquatic sediments is also governed by biodegradation (see Kurt et al. 2012) and abiotic reactions facilitated by reduced sulfur compounds (e.g., bisulfide and polysulfides) in sediment pore waters (see Zeng et al. 2012 and 2011). Kurt et al. (2012) suggested that robust biodegradation of toxic compounds migrating across the sediment/water interface and the biodegradation capacities were sufficient to eliminate transport of the contaminants to the overlying water. Zeng et al. (2012 and 2011) revealed that the reduced sulfur species in the porewaters readily transformed the target pesticides into sulfur-substituted products which was also an important removal pathway for pesticides. For instance, nitrobenzene likely undergoes reduction of the nitro group by sulfides to form aniline, which also explains the co-occurrence of AN and NB in contaminated sites.
In the last couple of decades, a large number of studies have been performed on endogenous release caused by the hydrodynamic inducement of sediment resuspension, which mainly focused on the nutrients release of nitrogen and phosphorus (Blackburn 1997; Kim et al. 2003; Wang et al. 2008, 2009, 2013b; Hu et al. 2011; Wu and Hua 2014). In addition, previous major researches about organic pollutants sorption behavior focused on the effects of sediment physicochemical properties and solution characteristics (Hari et al. 2005; Ma and Zhu 2006; Conkle et al. 2010; Zhang et al. 2014). However, the effects of hydrodynamic conditions on the sorption behavior of organic pollutants are poorly understood so far. Particularly, little was known about the relationship between hydrodynamic forces and the distribution characteristics of organic pollutants in the water and sediments.
Traditional sorption models based on octanol–water partitioning are usually applied to predict the adsorbance of organic pollutants and the partition coefficient (Chiou et al. 1979). Nevertheless, it has become more and more obvious that the differences between the results of classical models and those of in situ tests (Arp et al. 2009; Folberth et al. 2009; Miller and Hornbuckle 2010) are very huge. The reasons of this gap may vary. For example, the concentrations of organic pollutants are usually at a very low level in natural water environment, and the sediment–water partition coefficient at such a low level could not be reliably predicted by traditional models (Arp et al. 2009). Hawthorne et al. (2006) established a large, consistent, and comprehensive data set of log K TOC values of various native polycyclic aromatic hydrocarbons (PAHs) for over 114 highly varying impacted sediments. From this they were clearly able to distinguish that PAH-specific log K TOC values are distributed over 3 orders of magnitude. Further they noted that traditional log K TOC − log K Tow relationships correspond to only the lowest range of measured K TOC values. Furthermore, the sediment–water partition coefficients at laboratory scales are equilibrium values only under fully oscillation conditions. While, in most cases the hydrodynamic forces change frequently in field site, so the partition coefficients could be apparent values under a certain hydrodynamic condition.
In this paper, a series of simulation experiments were carried out to study the sorption behavior of AN on sediment under varied hydrodynamic conditions and coexistence of NB. The effect of hydrodynamic conditions on sorption isotherms and kinetics of AN were revealed, and the relationships between shear stresses and the apparent values of partition coefficients were determined, as well as sorption rates. The results will help us improve ability of predicting the temporal and spatial distribution characteristics and evaluating the release risk of organic pollutants from sediments in natural water bodies.
Materials and methods
Sediment
The sediment sample was collected from the surface layer of sediments (0–20 cm depths) in Meiliang Bay of Taihu Lake, China (31°25′21″N, 120°07′41″E). Impurities were removed from the collected sediment sample. The sample was air-dried and pulverized to pass through a 100-mesh sieve. Then, the sample was stored in sealed containers at 4 °C.
Taihu Lake, located in the southern part of the Yangtze River Delta, is the third largest freshwater lake in China, with an area of 2,338 km2 and an average depth of 1.89 m. As one of the most developed and densely populated areas in China, the Taihu Lake Basin covers only 0.4 % of the total territory of the country, but contributes 10.8 % of the total GDP.
The main component of the sediment sample was silt and clay, which consisting 13.8 % of coarse silt (0.1–1 mm), 70.5 % of fine silt (0.01–0.1 mm), and 15.7 % of clay (<0.01 mm). The median grain size of the sediment was 0.018 mm. Sediment pH was measured to be 7.48 at sediment to solution ratio of 1:2.5 (M/V) in the CaCl2 solution (0.01 M). The moisture content was measured to be 44.81 %. The organic carbon content (f oc) of the sediment (1.68 %) was determined by a wet oxidation with K2Cr2O7 in acidic environment. The cation exchange capacity (CEC) was determined to be 14.4 cmol/kg.
Reagents
Aniline, nitrobenzene, and calcium chloride are all of analytical grade obtained from Shanghai Chemical Reagent Co. in China. The physicochemical properties of organic compounds are given in Table 1.
Shear stresses calibration
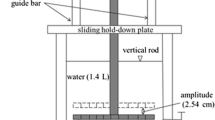
In this study, a particle entrainment simulator (PES) was used to simulate varied bottom equivalent shear stresses. The PES, as shown in Fig. 1, is a portable device, which has been widely used to study the characteristics of estuarine, coastal, and lake sediment resuspension as well as the transportation of pollutants between solid and liquid phases (Latimer et al. 1999; Cantwell et al. 2002; Cantwell and Burgess 2004; Feng et al. 2007; Wang et al. 2013a, b) in recent decades.
This apparatus consists of a 12.7-cm-diameter vertical acrylic cylinder and an oscillating perforated grid which was driven by a variable speed motor. A cam on the motor shaft allowed the grid to oscillate up and down with a vertical amplitude of 2.54 cm. The oscillation rate can be changed by adjusting the motor speed, simulating varied shear stresses on the surface of sediment.
Tsai and Lick (1986) empirically calibrated the PES by comparing the total amount of resuspended sediment in the water column at different oscillation speeds against those from an annular flume whose shear stress levels were known, and discovered the most common distribution range of shear stress was from 0.2 to 0.5 N/m2. The relationship between shear stress and the oscillation speed of a digital water-bathing constant temperature shaker was calibrated. The principle of calibration is that when the shaker and PES produce the same concentration of resuspended sediments, the stresses needed to produce these resuspended sediments are equivalent. In this study shear stress levels of 0.2, 0.3, 0.4, and 0.5 N/m2 corresponded to 113, 116, 125, and 137 rpm of the water-bathing constant temperature shaker, respectively.
Experiments procedures
Sorption isotherm experiment
The batch equilibrium method was used in a single component sorption experiment. The amount of 2 g sediments was filled in 50-mL stoppered erlenmeyer flasks with 20 mL AN solutions, whose concentrations were 5, 10, 20, 50, 100, 200, and 400 mg/L. The erlenmeyer flasks were shaken at 25 °C for 24 h, and the shear stresses were controlled at the level of 0.2, 0.3, 0.4, and 0.5 N/m2. Then the solutions were centrifuged at 3,500 rpm for 15 min by using a benchtop high speed refrigerated centrifuge and the supernatants were collected for further analysis.
The multicomponent sorption experiment was performed by using the same procedure as the single component sorption experiment. The concentrations of NB coexisting in the solutions were 50, 300, and 600 mg/L, respectively. All the solutions contained 0.005 mol/L CaCl2 and 0.01 % NaN3. CaCl2 was used to maintain the appropriate ionic strength for the solutions, and NaN3 was used to inhibit the microbial activity.
Sorption kinetics experiment
The procedure of sorption kinetics experiment was similar to the single component sorption experiment. The initial concentration of AN was 200 mg/L, and the concentrations of NB were 0, 50, and 600 mg/L. The flasks with solution were shaken under different shear stresses at 25 °C for 5, 15, 30, 60, 120, 240, 480, and 1,440 min. Then the solutions were centrifuged at 3,500 rpm for 15 min and the supernatants were collected.
A negative control experiment with no sediment was performed which indicated that the adsorption loss of AN on the glassware was negligible. Control experiments with no solute were also carried out in order to avoid the interference of the organic matter released from the sediment. Each experiment was duplicated under the same conditions.
Analytical methods
A series of AN solution with known concentrations were prepared, then absorbance of these solution were determined by using a ultraviolet–visible spectrophotometer (DR5000, Hach) at the wavelength of 230 nm (Hu et al. 2015) and standard curve which showed the relationship between absorbance and concentration was obtained. The AN residual concentrations of sorption experiments were analyzed using external standard method.
Data analysis
All calculations of sorption coefficients were carried out according to OECD guideline 106 (OECD 2000). The difference of the initial and equilibrium concentration of AN in the liquid phase was the sorption amount of the sediment. It was calculated according to Eq. (1).
Where Q e is the sorption amount of AN on bulk sediment (in milligram per kilogram), C 0 and C e are the concentration of AN initially and residually (in milligram per liter), V is the volume of the solution (in milliliter), W is the mass of bulk sediment (in gram).
The amount of AN adsorbed on the suspended particle was calculated by Eq. (2).
Where Q s is the sorption amount of AN adsorbed on the suspended particle (in milligram per gram), C 0 and C e are the concentration of AN initially and residually (in milligram per liter), C SS is the concentration of the suspended particulate matter (in gram per liter).
The adsorption rate of AN at different sorption stages were calculated via Eqs. (3), (4), and (5).
-
a.
The average adsorption rate at the fast sorption stage
$$ {K}_{\mathrm{fs}}=\frac{Q_{\mathrm{fs}}}{t_{\mathrm{fs}}} $$(3)Where K fs is the average adsorption rate at the fast sorption stage (in milligram per kilogram minute), Q fs is the amount of AN adsorbed on bulk sediment at this stage (in milligram per kilogram), t fs is the duration time of this stage (in minute).
-
b.
The average adsorption rate at slow sorption stage
$$ {K}_{ss}=\frac{Q_{ss}}{t_{ss}} $$(4)Where K ss is the average adsorption rate at the slow sorption stage (in milligram per kilogram minute), Q ss is the amount of AN adsorbed on bulk sediment at the slow sorption stage (in milligram per kilogram), t ss is the duration time of the stage (in minute).
-
c.
The average adsorption rate in each time period
$$ {K}_r=\frac{\varDelta Q}{\varDelta t} $$(5)Where K r is the average adsorption rate during each time period (in milligram per kilogram minute), ∆Q is the incremental adsorption amount during each time period (in milligram per kilogram), ∆t is the duration time of each period (in minute).
Results and discussion
The preliminary results showed that the sorption equilibrium was reached under the shear stress level of 0.5 N/m2 within 24 h, but not for the shear stress level of 0.2, 0.3, 0.4 N/m2. Thus, the adsorption parameters obtained under 0.2, 0.3, 0.4 N/m2 stress level were defined as apparent values, and those obtained under 0.5 N/m2 were equilibrium values.
The correlation between shear stress and the amount of adsorbed AN and sorption rate were discussed, as well as the effects and interaction of shear stress and cosolute NB on AN sorption behaviors.
Effect of shear stress on apparent partitioning coefficient
Figure 2 showed the sorption isotherms of AN in the presence of NB under different shear stress levels. It was observed that the experimental data fitted linear equation as follow by using the regression analysis.
Where Q e is the apparent sorption amount of AN (in milligram per kilogram), C e is the concentration of AN in solution phase (milligram per liter), K p is the apparent partitioning coefficient (in liter per kilogram).
Previous studies suggested that the organic carbon content (f oc) played a dominant role in the sediment–water partition behavior of organic compounds, therefore the sediment–water partition coefficients which were normalized to f oc, were referred as K oc value. It has been well accepted that the K oc value of the same organic compound on different sediments is constant and has no correlation with the sediment types. Thus early environmental chemists established empirical formulas of K oc and K ow : log K oc = a · log K ow + b, where both a and b were parameters, and mainly depended on the type of compound. According to the empirical formula of K oc and K ow , log K oc = 0.52 · log K ow + 0.88 (Briggs 1981), the K oc value of NB is higher than that of AN, which means NB’s sorption capacity is stronger on sediment than that of AN, which indicates that the reduction of sorption capacity for AN was attributed to the presence of high NB concentration (Conkle et al. 2010; Jadhav and Srivastava 2013).
By normalizing the apparent K p value to f oc, the work evidenced a series of apparent log K oc value of AN under different shear stress levels. Then the relationship between apparent log K oc values and shear stresses was established. The apparent log K oc value showed a significant positive correlation with shear stress, and the fitting equations and correlation coefficients were summarized in Table 2. The relation curve between log K oc and shear stress was shown in Fig. 3 which showed that the apparent log K oc value increased with the increasing shear stress level linearly. It indicated that shear stress could increase the apparent adsorption capacity of AN on sediment which mainly attributed to the raise of the sediment resuspension flux.
Previously, Wang et al. (2010) showed that shear stress played a significant role in promoting the sediment resuspension potential, the time-average value of sediment resuspension flux presented exponential increased with shear stress, and the concentration of suspended particles in water phase also increased. Therefore, the more possibility of collisions between suspended particles and AN will happen.
To investigate the role of sediment resuspension flux on the apparent sorption amount of AN, we calculated the amount of AN which was adsorbed on suspended particle per unit mass (Q s) by using Eq. (2). And the relation curve of C e and Q s was shown in Fig. 4. Regression analysis showed that C e and Q s had an obvious linear relation, and the correlation coefficient was higher than 0.996. In contrast, Q s which has an association with the sediment particle size distribution under different shear stress levels was negatively correlated with shear stress. It may be attributed to the selective transport of fine particles (Grundtner et al. 2014). Sediment particles in a smaller size had a larger proportion rate than those in a bigger size in overlying water under a low shear stress level. In addition, fine sediment particles had a relatively large specific surface area, so they accordingly had more space to accommodate AN. In agreement to this conclusion, with the shear stress increasing, the fraction of larger size suspended particles was getting higher and their specific surface area was becoming smaller. Therefore, Q s under a lower shear stress level was larger than that under a higher one.
Effect of shear stress on sorption rate
In the recent studies, the adsorption rates of AN were determined by testing the pseudo-first-order sorption kinetics model or pseudo-second-order sorption kinetics model. Pseudo-first-order sorption kinetics model is shown below:
Pseudo-second-order sorption kinetics model is:
Where q t is the sorption quantity at time t (in milligram per kilogram), q e is the maximum adsorbed amount (in milligram per gram), k 1 is the first-order rate constant (in per minute), k 2 is the second-order rate constant (in kilogram per milligram minute), t is the adsorption time.
According to the first-order and second-order kinetics models, the kinetics fitting curves under different shear stress levels were shown in Fig. 5. It was clear that the pseudo-second-order model fitted better than the pseudo-first-order model for the adsorption kinetics of AN. The maximum adsorbed amounts (q e) under varied shear stress levels were obtained by Eq. (8), and then compared with the experimental data. The relative errors were between 0.27 and 1.91 %, which further proved the better explanation of adsorption kinetics of AN with the pseudo-second-order model.
Previous researches showed that the sorption kinetic process of organic pollutants could be divided into two stages (Fang et al. 2014 ). It was defined that the first 2 h was the fast sorption stage and the period from 2 to 24 h was the slow one according to the experiment data. The average rates of the two stages were shown in Table 3 which indicated the fast stage rates were 7.5–9.5 times of the slow stage rates.
Comparing the average rates under shear stress level of 0.5 and 0.2 N/m2, both the fast sorption rates and slow sorption rates grew with the increase of NB concentration (Fig. 6). It indicated that the effect of shear stress on the average sorption rate of the main solute AN became more marked along with the cosolute NB concentration increasing. The probable explanation was that shear stress enhanced the sediment resuspension flux, promoted the sorption process, and relatively weakened the interference of cosolute to main solute at the same time. Further studies should be conducted to address this.
In order to analyze the response of sheer stress to sorption rate under different period further, eight continuous time periods within 24 h were selected, and corresponding average adsorption rates of each time periods were calculated by using Eq. (5).
Figure 7 showed the average rate (K r) variation processes of the eight time periods under different shear stress levels in the presence of 0, 50, and 600 mg/L NB. As shown in Fig. 7, only in the initial phase did the shear stress have a significant effect on the average rates. The average rate of the first 5 min had a positive correlation with shear stress, whereas it had a negative correlation with NB concentration under the same shear stress level.
However, the average rates of the next seven periods barely changed with hydrodynamic conditions. The rates were almost insusceptible to shear stress and cosolute NB concentration. A possible reason was that the solution system changed from stable status to disturbed status in the initial sorption stage, and a sudden increase of the suspended particle came into contact with solute molecules which appeared to be obviously rising in the sorption rate. Then the concentration of suspended particle tended to be equilibrium, and contact between solute and suspended particles began to stabilize, therefore the sorption rates remained the same.
Conclusion
In this study, the effects of shear stress levels and action time on the sorption of AN was investigated. We reached the following conclusions:
-
1.
Partition effect played a leading role in the sorption of AN on sediment, and the linear model fitted well for AN sorption isotherm. The apparent sorption capacity of AN was enhanced along with the increase of shear stress, and decreased with the increase of NB concentration.
-
2.
Both the sorption amount of AN on suspended particulate matter per unit mass and the effect of NB concentration on Q s decreased with the increase of shear stress.
-
3.
The adsorption process followed pseudo-second-order kinetics equation, and the process included two stages: fast sorption stage and slow sorption stage, among which the average sorption rate of fast stage was 7.5–9.5 times that of slow one. Shear stress affected the average sorption rate of AN more significantly with the increase of NB concentration. In addition, shear stress weakened the disturbance of cosolute on main solute sorption process.
-
4.
Both shear stress and cosolute concentration mainly influenced the initial sorption stage. The average sorption rates during fast stage were positively correlated with shear stress, while were negatively correlated with cosolute concentration. Whereas the average sorption rates of slow stage were barely affected by shear stress and cosolute concentration.
References
Arp HPH, Breedveld GD, Cornelissen G (2009) Estimating the in situ sediment—porewater distribution of PAHs and chlorinated aromatic hydrocarbons in anthropogenic impacted sediments. Environ Sci Technol 43:5576–5585
Blackburn TH (1997) Release of nitrogen compounds following resuspension of sediment: model predictions. J Mar Syst 11:343–352
Blom G, Winkels HJ (1998) Modelling sediment accumulation and dispersion of contaminants in Lake Ijsselmeer (The Netherlands). Water Sci Technol 37:17–24
Briggs GG (1981) Theoretical and experimental relationships between soil adsorption, octanol–water partition coefficients, water solubilities, bioconcentration factors, and the parachor. J Agric Food Chem 29:1050–1059
Cantwell MG, Burgess RM (2004) Variability of parameters measured during the resuspension of sediments with a particle entrainment simulator. Chemosphere 56:51–58
Cantwell MG, Burgess RM, Kester DR (2002) Release and phase partitioning of metals from anoxic estuarine sediments during periods of simulated resuspension. Environ Sci Technol 36:5328–5334
Chiou CT, Peters LJ, Freed VH (1979) A physical concept of soil–water equilibria for nonionic organic compounds. Science 206:831–832
Conkle JL, Lattao C, White JR, Cook RL (2010) Competitive sorption and desorption behavior for three fluoroquinolone antibiotics in a wastewater treatment wetland soil. Chemosphere 80:1353–1359
Cornelissen G, Gustafsson O, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895
Fang CR, Long YY, Shen DS (2014) Sorption behavior of dibutyl phthalate and dioctyl phthalate by aged refuse. Environ Sci Pollut R 21:7641–7649
Feng JL, Yang ZF, Niu JF, Shen ZY (2007) Remobilization of polycyclic aromatic hydrocarbons during the resuspension of Yangtze River sediments using a particle entrainment simulator. Environ Pollut 149:193–200
Folberth C, Suhadolc M, Scherb H, Munch JC, Schroll R (2009) Batch experiments versus soil pore water extraction—What makes the difference in isoproturon (bio-)availability? Chemosphere 77:756–763
Gabel F, Garcia XF, Schnauder I, Pusch MT (2012) Effects of ship-induced waves on littoral benthic invertebrates. Freshw Biol 57:2425–2435
Grundtner A, Gupta S, Bloom P (2014) River bank materials as a source and as carriers of phosphorus to Lake Pepin. J Environ Qual 43:1991–2001
Hari AC, Paruchuri RA, Sabatini DA, Kibbey TC (2005) Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ Sci Technol 39:2592–2598
Hassett JP (2006) Chemistry. Dissolved natural organic matter as a microreactor. Science 311:1723–1724
Hawthorne SB, Grabanski CB, Miller DJ (2006) Measured partitioning coefficients for parent and alkyl polycyclic aromatic hydrocarbons in 114 historically contaminated sediments: part 1. K-OC values. Environ Toxicol Chem 25:2901–2911
Heringa MB, Hermens JLM (2003) Measurement of free concentrations using negligible depletion-solid phase microextraction (nd-SPME). Trac Trend Anal Chem 22:575–587
Hu KM, Pang Y, Wang H, Wang X, Wu X, Bao K, Liu Q (2011) Simulation study on water quality based on sediment release flume experiment in Lake Taihu, China. Ecol Eng 37:607–615
Hu R, Wang X, Dai S, Shao D, Hayat T, Alsaedi A (2015) Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem Eng J 260:469–477
Jadhav AJ, Srivastava VC (2013) Adsorbed solution theory based modeling of binary adsorption of nitrobenzene, aniline and phenol onto granulated activated carbon. Chem Eng J 299:450–459
Kim LH, Choi E, Stenstrom MK (2003) Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 50:53–61
Kurt Z, Shin K, Spain JC (2012) Biodegradation of chlorobenzene and nitrobenzene at interfaces between sediment and water. Environ Sci Technol 46:11829–11835
Latimer JS, Davis WR, Keith DJ (1999) Mobilization of PAHs and PCBs from in-place contaminated marine sediments during simulated resuspension events. Estuar Coast Shelf Sci 49:577–595
Liu N, Li H, Ding F, Xiu Z, Liu P, Yu Y (2013) Analysis of biodegradation by-products of nitrobenzene and aniline mixture by a cold-tolerant microbial consortium. J Hazard Mater 260:323–329
Ma J, Zhu L (2006) Simultaneous sorption of phosphate and phenanthrene to inorgano–organo-bentonite from water. J Hazard Mater 136:982–988
Miller SM, Hornbuckle KC (2010) Spatial and temporal variations of persistent organic pollutants impacted by episodic sediment resuspension in southern Lake Michigan. J Great Lakes Res 36:256–266
OECD (2000) OECD guideline 106 - OECD guideline for the testing of chemicals: adsorption–desorption using a batch equilibrium method
Reardon KE, Bombardelli FA, Moreno-Casas PA, Rueda FJ, Schladow SG (2014) Wind-driven nearshore sediment resuspension in a deep lake during winter. Water Resour Res 50:8826–8844
Tsai CH, Lick W (1986) A portable device for measuring sediment resuspension. J Great Lakes Res 12:314–321
Wang S, Jin X, Bu Q, Jiao L, Wu F (2008) Effects of dissolved oxygen supply level on phosphorus release from lake sediments. Colloids Surf A 316:245–252
Wang S, Jin X, Zhao H, Wu F (2009) Phosphorus release characteristics of different trophic lake sediments under simulative disturbing conditions. J Hazard Mater 161:1551–1559
Wang P, Wang SY, Hao SP, Yao Q, Tang LL (2010) Characteristics of sediment resuspension in Taihu Lake under simulative disturbing conditions. Adv Water Sci 21:399–404 (In Chinese)
Wang H, Holden J, Spera K, Xu XH, Wang ZD, Luan JH, Xin X, Zhang ZJ (2013a) Phosphorus fluxes at the sediment–water interface in subtropical wetlands subjected to experimental warming: a microcosm study. Chemosphere 90:1794–1804
Wang P, Shen X, Wang SY, Hao SP (2013b) Remobilization of phosphorus from sediments of Taihu Lake during periods of simulated resuspension. Water Environ Res 85:2209–2215
Wu D, Hua ZL (2014) The effect of vegetation on sediment resuspension and phosphorus release under hydrodynamic disturbance in shallow lakes. Ecol Eng 69:55–62
Zeng T, Ziegelgruber KL, Chin YP, Arnold WA (2011) Pesticide processing potential in prairie pothole porewaters. Environ Sci Technol 45:6814–6822
Zeng T, Chin YP, Arnold WA (2012) Potential for abiotic reduction of pesticides in prairie pothole porewaters. Environ Sci Technol 46:3177–3187
Zhang XY, Wu YG, Hu SH, Lu C, Yao HR (2014) Responses of kinetics and capacity of phenanthrene sorption on sediments to soil organic matter releasing. Environ Sci Pollut R 21:8271–8283
Zhao Q, Weise L, Li PJ, Yang K, Zhang YQ, Dong DB, Li P, Li XJ (2010) Ageing behavior of phenanthrene and pyrene in soils: a study using sodium dodecylbenzenesulfonate extraction. J Hazard Mater 183:881–887
Acknowledgments
This research was financially supported by the State Major Project of Water Pollution Control and Management (2014ZX07101-011), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Qing Lan Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Wang, P., Hua, Z., Cai, Y. et al. Effects of hydrodynamic conditions on the sorption behaviors of aniline on sediment with coexistence of nitrobenzene. Environ Sci Pollut Res 22, 11595–11605 (2015). https://doi.org/10.1007/s11356-015-4399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4399-5