Abstract
Concentrations of major and trace elements (Al, Fe, Mn, B, Cd, Co, Cr, Cu, Mo, Ni, Pb, Sr, V, and Zn) were determined in water and sediments from irrigation canals and the Nile River in an agricultural area of 120 km2 to evaluate the impact of agricultural practices and the spatial distribution and mobility of these elements. The enrichment factors of cadmium indicate contamination in this area. Metal pollution indices are higher at locations downstream of the irrigation canals, possibly a consequence of waste discharges and phosphate fertilizing. Comparisons with consensus-based sediment quality guidelines revealed that ∼92 % (Cr), ∼85 % (Cu), ∼46 % (Ni), and ∼23 % (Zn) of the samples exceeded the threshold effect concentrations, with 7.7 % for Cr and Ni being above the probable effect concentration. Contamination with many metals in water was found in the secondary irrigation canals. The partition coefficients of all determined metals were evaluated. The major elements Al, Fe, and Mn were found to be very mobile while V was the least mobile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Nile flows north of Cairo across the delta in its two branches, the Rosetta and Damietta. The Nile delta has an area of ∼22,000 km2 and accounts for two thirds of Egypt’s agricultural surface. Agriculture is fundamental to Egypt’s economy. Land is entirely dependent on the Nile water. The cultivated area has a dense irrigation and drainage canal network. The agricultural drains from which the water is lifted to the irrigation canals receive effluents from sewage systems and nearby industries. Low level of sanitation services, especially in rural areas, makes nearby streams the places for inhabitants to dispose of their sewage. Many industrial establishments do not comply with the law, dumping their wastewater and partially treated water into surface water bodies. The excessive use of fertilizers and pesticides is another major source of water pollution. Continuous application of fertilizers may lead to metal accumulation in the agricultural areas.
There is therefore an urgent need for monitoring and evaluating water quality. Sediments can be sensitive indicators of contamination of aquatic environments (Harikumar et al. 2009), which might result in potentially irreversible adverse effects to ecosystems. Metal pollution not only affects the quantity and quality of crops, but may also influence the quality of the atmosphere and water bodies, and threatens the health and life of animals and human beings (Kumar 2008).
Water and sediment quality may vary spatially according to the distribution of the human activities and the use of fertilizers over the past decades. The focus of this study, therefore, was to determine the sediment and water quality in the Nile River and irrigation canals in a representative example of agricultural area of the delta. The aims are to provide information that helps to identify main pollutants currently present, to characterize their spatial patterns of distribution, and to understand the practices that lead to the current environmental status. It is of general interest to provide useful information on the mobility of different elements in this complicated environment.
Materials and methods
Study area
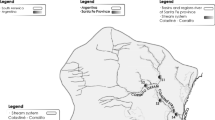
The study area is located on the western side of the Rosetta branch in Al-Beheira governorate, one of the largest governorates of Egypt (9122 km2). The study area is about 120 km2 and lies between 30° 40′ and 30° 47′ E longitude and 30° 52′ to 31° 0′ N latitude, with a total population of 450,000 in 17 main villages. The irrigation system is charged from the main canal (El-Henawy canal) streaming from south (at S01) to north (at S10) and feeds a secondary canal in east–west direction as shown in Fig. 1. The study area has two unconnected drainage canals, one in the north–south direction parallel to the main irrigation canal, and another starting at the southern location S03 and extending west. When a canal is close to a village, it receives the refuse and sewage. For example, locations S11 and S12 on the secondary irrigation canal are normally subject to such disposal. On the other hand, locations C01 and S06 on the main irrigation canal are not subject to this contamination because of the closeness of the drainage canal which is used for this purpose.
Sampling and sample preparation
Twenty water of 20 L each and surface sediment samples of about 4 kg each of the upper 15 cm were collected from the study area (S01–S20 in Fig. 1). Each sediment sample comprises material taken from 3 to 5 points over a distance of 5–10 m. In addition, 14 core samples to a depth of 75 cm (5 layers of 15 cm each) were also taken (C01–C14 in Fig. 1), but no water samples were collected from these locations. Only 13 surface sediments and 12 water samples were selected for analysis of the contents for major and trace elements. Among the analyzed water and sediment samples, only 7 were taken from the same location. Sediment samples were placed in polyethylene bags; water samples were collected in clean plastic containers. Air-dried sediments were further dried at 90 °C until constant weight. Samples were then sieved to collect 100 g of grain size of <2 mm for the analysis. Aliquots of 1 g for each sediment sample were sequentially extracted as described (USEPA 1996), to determine the concentrations of Al, Fe, Mn, B, Cd, Co, Cr, Cu, Mo, Ni, Pb, Sr, V, and Zn by inductively coupled plasma–emission spectrometry (ICAP 6500, Thermo Scientific, UK). The concentrations of the same elements in the water samples were determined without any pretreatment.
Multielement standards at different concentrations typical in water and sediment were prepared to obtain the elemental concentrations of the samples. The certified reference materials RM-8704 (river sediment) and SRM 1640a (natural water) were used at the beginning and after analyzing each series of samples for sediments and water analysis, respectively, in order to verify the sensibility of the device and the reliability of the results. Precision was estimated by replicate analyses of standard reference materials and actual samples. Standard deviations of replicate measurements were below 10 % and of chemical blanks were less than 2 %.
Physical and chemical characteristics of sediment and water samples were determined. The pH value, total organic matter (TOM), electrical conductivity (EC), and calcium carbonate (CaCO3) were therefore determined. TOM was determined as the loss on ignition at 550 °C for 2.5 h (Rowell 1994), and the measurement of CaCO3 was based on the volumetric analysis of the carbon dioxide which is liberated during the application of hydrochloric acid solution in the sediment’s carbonates in a sealed reaction cell (Allen 1989).
Results and discussion
Characteristics of sediments and water
The mean pH of sediments was slightly alkaline (8.2 ± 0.1) with a range from neutral to strongly alkaline (7.1–9.1). TOM was below 17 % (6.0 ± 0.7 %) in all samples. The mean EC value was 527 ± 47 μS cm−1 (168–1475 μS cm−1). The sediments are CaCO3-poor with contents of less than 1.6 % (0.6 ± 0.1). Figure 2 shows these measured values as a function of locations. Comparison of the mean values of main canal, secondary canals, and Nile locations indicate that both pH and EC are relatively homogeneous. On the other hand, higher means of TOM and CaCO3 found in the secondary irrigation canals compared to those in the main canal and the Nile. This is related to the significant refuse and sewage input along the secondary canals in particular, as they are shallower and their water moves more slowly. The pH of water samples had a range of 7.1–7.9 and a mean of 7.7 ± 0.2. The mean value of the measured electrical conductivity was 508 ± 221 μS cm−1 (310–1000 μS cm−1). The mean pH in irrigation canals (7.7) was slightly higher than that in the Nile River (7.5). The mean EC in the secondary canals (684 μS cm−1) was close to that of the Nile water (597 μS cm−1), and the water in the main canal had EC (375 μS cm−1) lower than both.
Elemental concentrations in sediments
Table 1 presents the descriptive statistics of the concentrations of major and trace elements in the sediments collected from 13 locations. Generally, the distributions were quite narrow and restricted to ±2σ with the exception of few cases. The cases in which the concentrations were statistically outside the confidence limits of the normal distribution (>|2σ|) are as follows: the concentrations of Al, Fe, Cr, Cu, Mo, Ni, and Sr in location C06 corresponded to 2.43σ (p = 0.74 %), 2.96σ (p = 0.15 %), 2.86σ (p = 0.21 %), 2.68σ (p = 0.37 %), 2.53σ (p = 0.57 %), 2.26σ (p = 1.18 %), and 2.31σ (p = 1.054 %), respectively, and the concentration of Pb in location S12 was 2.73σ (p = 0.31 %). These apparently higher concentrations do not necessary distinguish these locations from the rest because the observed concentrations may be associated with the variation in grain size and/or mineralogy of the sediments. Therefore, no conclusions should be reached at this point before addressing this issue (The relative contents).
The normality of Mn, Co, V, and Zn distributions was reflected by the small values of kurtosis and skewness. The rest of the distributions, and in particular Fe, Cr, Cu, Mo, and Pb, had positive values of kurtosis coefficient indicating peaked distributions. These distributions had tails toward higher values. For the three major elements Al, Fe, and Mn, the mean concentrations in the secondary irrigation canal were higher than those in the main canal, which in turn had slightly higher concentrations than the two locations on the Nile. Similarly, the mean concentrations of B, Cr, Cu, Pb, Sr, V, and Zn in the secondary irrigation canal were higher than the corresponding means in the main irrigation canal and the Nile, while the means of Cd, Co, Mo, and Ni were comparable.
The relative contents
Assessing the input of many elements resulting from human activities is difficult to deconvolute. Metal variability may be caused by grain size and/or mineralogy of the sediments. To reduce this variability and to identify anomalous metal contributions, geochemical normalization has been used with various degrees of success by employing conservative elements. The most popular methods include normalization techniques such as grain size, particulate organic carbon, iron, and aluminum. Among these, Al is the most widely used because it is one of the most abundant elements on the earth, is strongly correlated to the fine fraction, and does not depend on anthropogenic inputs (Dai et al. 2007). Al-normalization of the elemental concentrations was performed to check whether or not the observed excesses were related to variability of Al in these locations. After Al-normalization (C r e = C e /C Al , where e refers to element), the previously observed excesses of elements Fe, Cr, Cu, Mo, Ni, and Sr in location C06 no longer exist. This indicates that the observed higher concentrations of these elements is mainly due to the higher concentration of Al in this particular location. In contrary, the Al-normalized concentrations of Pb still show a slightly significant concentration in location S12 (2.77σ and P = 0.29 %). The Al-normalized concentrations of Fe in location S01 (2.56σ and P = 0.53 %) and Cd (3.18σ and P = 0.07 %), Mo (3.19σ and P = 0.07 %), and Ni (2.46σ and P = 0.70 %) in location C08 have the highest values among the studied locations. Fe is not likely susceptible to enrichment from anthropogenic sources, and its variations could be explained by particle grain size differences. The statistically most significant excess is that of Al-normalized concentrations of Cd and Mo in C08. While Cd was detected in only locations S12, C06, and C08, Mo was above the minimum detectable level in three locations S01, C06, and C08. Location S12 is characterized by the highest values of TOM (17 %) and Pb (29.8 mg kg−1). Location C06 has the highest measured values of pH, CaCO3, Al, Fe, Mn, Cd, K, Cr, Cu, Mo, Ni, Sr, and V. Location C08 had relatively high TOM content (∼11 %) and EC (692 μS cm−1) indicating high salinity. Several studies pointed out that Cd in sediment shows strong binding to organic matter. Cadmium entering waterways is adsorbed to sediments and associated with ferric or manganese oxy-hydroxides, organic matter, and sulfides (DiToro et al. 1990; Lion et al. 1982). Molybdenum is enriched in sediments forming under anoxic conditions. In oxic sediments, Mo is preferentially absorbed onto iron-oxyhydroxides, but as sediments become suboxic, Mo is leached out of sediments (Anbar and Rouxel 2007). The processes associated with redox change in sediments result in Mo accumulation include scavenging by manganese oxides, coprecipitation with iron sulfides and adsorption by organic matter (Malcolm 1985). Molybdenum can be derived from a number of agricultural activities and is present in fertilizers (NRC 2004). The abundance of Mo in sediments should reflect the redox state of the environment. The Cd and Mo enrichments in location C08 can be ascribed to diffusion of the dissolved metals from overlying water into the organic-rich, anoxic sediments, and their fixation in the solid phase.
Correlations among characteristics and metal concentrations in the sediments
To determine the controlling factors for the distributions in sediment, Pearson correlation matrix analyses were carried out for 16 items, including absolute and Al-normalized concentrations (excluding Cd and Mo) and characteristics of sediments assuming linear relation. Throughout this work, the correlations will be described as strong (r ≥ 0.95), moderate (0.85 ≤ r < 0.95), or weak (0.75 ≤ r < 0.85). No obvious correlation between the concentrations and the characteristics of the sediment was found except that a possible correlation may exist between Pb concentration and TOM (r = 0.71). Table 2 lists the Pearson correlation coefficients for the absolute concentration. Cr accounts for most of the variability of many metals. It has moderate correlations with Al, Fe, Mn, and Cu (r = 0.85–0.88) and weak correlations with Co, Ni, and V (r = 0.75–0.77). Sr is also correlated with most of the other elements (r = 0.74–0.87) with the exception of B and Pb. Strong correlations exist between different pairs of elements: Al and Cu (r = 0.94), Co and Ni (r = 0.94), and Cr and Sr (r = 0.95). The results suggest that oxides of Al, Fe, and Mn have insignificant influence on scavenging of metal ions and that they may have different anthropogenic and natural sources in sediments of the investigated area and its vicinity. Table 3 lists the Pearson correlation coefficients for Al-normalized concentrations. The concentration of Cr/Al continues to show correlations with different significances with the normalized concentrations of Co, Ni, and Sr (r = 0.90–0.97) but not with Fe and Cu and possibly correlates with Mn (r = 0.79) and V (r = 0.72). The Al-normalized concentration of Sr has more or less the same behavior with other elements. The two previously observed strong correlations between Co and Ni and between Cr and Sr are also present when the Al-normalized concentrations of these elements are used but with somewhat lower significance.
Assessment of sediment pollution
A number of approaches have been used to assess the health risks from conventional pollutants. The metal enrichment factor (EF) is used as an index to assess anthropogenic influences of heavy metals in sediments. EF is commonly defined as the observed metal to Al ratio in the sample of interest divided by the background metal to Al ratio (Martin and Meybeck 1979; Sinex and Wright 1988). EFs are calculated according to
where C e and C Al are the element and Al content in the sample, respectively, and B e and B Al refer to the content of the same element and Al in uncontaminated (preindustrial reference level) crust minerals, respectively. Due to the lack of metal background values for our study area, we used the reported background concentration of uncontaminated shale (Martin and Meybeck 1979; Turekian and Wedepohl 1961). The suggested scale of enrichment factor includes five groups (Kumar and Edward 2009). The means and ranges of EFs of all measured metals are listed in Table 4. Strontium is minimal enriched in all locations. Most of the locations have minimal enrichment in Fe, B, Pb, and Zn and have moderate enrichment in 4, 3, 2, and 3 locations, respectively. Significant enrichment exists only in locations C12 (EF = 6.1) and C14 (EF = 6.3) on the Nile River for Cu. On the other hand, the mean EF of Cd and Mo indicate moderate enrichment and minimal enrichment, respectively. The EF values of Cd in the sediment may be consistent with the use of phosphate fertilizer particularly in the main irrigation canal. Similar findings have also been found in other studies (Lambert et al. 2007). The mean EF values suggest that only Sr and possibility B, Ni, Pb, and Zn could be considered of natural origin. On the other hand, Mn, Co, Cr, and V showed higher mean EF values suggesting moderately to strongly polluted sediments particularly at locations C06, C07, and C11. However, the mean and range of EF for Cu strongly indicate its anthropogenic origin.
The geoaccumulation index (I geo) was proposed by Müller (1969) (see e.g., Farkas et al. 2007) as a criterion to evaluate the intensity of heavy metal pollution and is defined as
The factor 1.5 is introduced to take into consideration possible differences in the background values due to lithological variation. I geo assesses the degree of metal pollution defining seven classes of sediment quality (Zheng et al. 2008). The mean values of I geo are given in Table 4. The quality of sediment is between unpolluted to moderately polluted with Cu depending on the background concentration used for uncontaminated sediment. On the other hand, the concentrations of all other elements in sediments can be considered natural.
A convenient device for discussing geochemical trends and making comparisons between total concentrations of metals at different sampling locations is the metal pollution index (MPI) (Usero et al. 1996). The MPI is calculated as the geometric mean of the concentration of various major and trace elements measured in the location according to the equation
where C 1, C 2, C 3, …C n are the content of elements (μg g−1) 1, 2, 3, … n, respectively. Although MPI gathers all of the metals into one value and provides an easy way to compare one location from another, it has the drawback of not having any threshold value to denote the pollution level. The concentrations of Cd and Mo were not included in the calculation of MPI. MPI values varied between 87.2 and 249.1 mg kg−1. Generally, MPI varies slightly depending on the location of the sampling points. Sediments at the water’s entry in the South of the study area have comparable MPI to that in the nearby Nile’s sediments. As one moves to the north or from the main to the secondary irrigation canals, the MPI increases presumably because of the increasing load of contaminations. The highest level of MPI is especially marked in location C06 in the secondary canal followed by locations S14 and S10.
The degree of contamination was developed and tested for rivers (Hakanson 1980) and has already been successfully used for coastal areas (Kwon and Lee 1998). To determine an anthropogenic influence on the ecosystems, the elemental contamination factor (CF e) for each element was calculated as the ratio of the mean concentrations from upper most sediment layer to the average preindustrial background values.
where \( {\overline{C}}_e \) is the mean element content in the study area. The following terminology is suggested for describing the contamination factor; CF < 1: low contamination factor; 1 ≤ CF < 3: moderate contamination factor; 3 ≤ CF < 6: considerable contamination factor; CF ≥ 6: very high contamination factor. The calculated CFs for all data are listed in Table 4. For many elements, CFs of the secondary irrigation canal are the highest while those of the Nile River are the lowest. Obvious differences between the Nile and at least one of the irrigation canals exist for B, Cd, Cr, Mo, and Pb. The elemental degree of contamination (DC) for the study area is defined as the sum of all contamination factors for the same element divided by the number of locations.
where n is the number of contaminants, and the adopted terminology to describe the degree of contamination are DC < n: low level of contamination; n < DC < 2n: moderate degree of contamination; 2n < DC < 3n: considerable degree of contamination; and DC > 3n: very high degree of contamination. To allow the comparison between the Nile River and main and secondary irrigation canals, the DC for all locations and those associated with only the Nile River, main canal, and secondary canals were found to be 11.7, 8.3, 10.8, and 13.3, respectively. Clearly, all values are less than the number of elements (14), which categorizes the investigated area with low level of contamination.
The pollution load index (PLI) (Tomlinson et al. 1980) of each location is calculated by obtaining the nth root of the product of n-CFs according to the equation.
PLI provides a simple mean that exhibits the number of times by which the heavy metal concentrations in sediment exceed the background concentrations and gives a summative suggestion of the overall level of metal in a particular location. When PLI > 1, it means that pollution exists; otherwise, if it is <1, there is no metal pollution. PLI was calculated using the concentrations of 12 elements not including Cd and Mo. The lowest concentration of metals was in location S01 (0.28) and the highest was in location C06 (0.79). The calculated PLI values in increasing order were the same as MPI: S01, C12, C14, C08, S15, S04, C07, S13, C11, S12, S10, S14, and C06.
Sediment quality guidelines
Data on the concentrations of contaminants in sediments alone do not provide an effective basis for estimating the potential for adverse effects to living resources. Ecological risk indices compare the results for the contaminants with sediment quality guidelines (SQG) (MacDonald et al. 1996; Long and MacDonald 1998; MacDonald et al. 2000; Long et al. 2006). SQGs are very useful to screen sediment contamination by comparing sediment contaminant concentration with the corresponding quality guideline. These guidelines evaluate the degree to which the sediment-associated chemical status might adversely affect aquatic organisms. The mean sediment quality guideline quotient (SQG-Q) mixes all contaminants in the same SQG. It evaluates toxicity since it takes into account SQG comparison. It is defined as
where PEL-Q e is the probable effect level quotient for element e; PEL e the probable effect level for each contaminant e (concentration above which adverse effects frequently occur) (MacDonald et al. 1996). A value of SQG-Q ≤ 0.1 means lowest potential for observing adverse biological effects; 0.1 < SQG-Q < 1: means moderate impact potential for observing adverse biological effects; and SQG-Q ≥ 1 means highly impacted potential for observing adverse biological effects. The range of the calculated values of PEL-Q for Cr, Cu, Ni, Pb, and Zn as well as the mean and range of SQG-Q are listed in Table 5. All locations have moderate impact potential (SQG-Q = 0.10–0.68). Among the various SQGs developed during the past decade, increasing importance is given to the consensus-based threshold effect concentration (TEC) and the probable effect concentration (PEC) guidelines (MacDonald et al. 2000) for freshwater sediment assessment. The TECs were intended to identify contaminant concentrations below which harmful effects on sediment-dwelling organisms were not expected to occur. The PECs were intended to identify contaminant concentrations above which harmful effects on sediment-dwelling organisms were expected to occur frequently. For marine sediments, TEC and PEC for eight trace metals were identified (MacDonald et al. 2000). Based on the results of this assessment, the incidence of sediment toxicities due to Cu, Pb, and Zn was generally low at contaminant concentrations below the PECs, while about 15, 100, and 77 % of the samples had very low contaminant concentrations below the TEC for Cu, Pb, and Zn, respectively. Among the five trace metals, 7.7 % of samples exceeding an individual consensus-based PEC for both Cr and Ni resulted to be toxic to sediment-dwelling organisms.
Elemental concentrations in water
Table 6 gives the descriptive statistics of the concentration of dissolved metals in water. The highest concentrations of many metals were measured in location S14 close to the western end of the secondary irrigation canal, where agriculture is the predominant activity. The nearby location S15 has the highest concentrations of Al and Cd and is the second highest in Fe, Mn, B, Pb, and V. All distributions have positive values of skewness (0.83–2.99) indicating distributions with tails extending toward higher values. Similarly, all distributions with the exception of B have high positive value of kurtosis coefficient indicating peaked distribution. While the mean concentrations of all dissolved elements in the main irrigation canal is similar to that in the Nile River, the mean concentrations of all elements are higher in the secondary irrigation canals except three elements, namely, Cu, Ni, and Zn. This is in general agreement with the results obtained previously from the concentrations of these elements in sediments.
The distribution of major and trace elements between sediment and water can be quantified using the sediment partition coefficients (K d , also called distribution coefficients), which is defined as the ratio of the concentration of an element in the solid phase (C sediment) to its concentration in the liquid phase (C water) in equilibrium with this sediment and has units of L/kg. It is used to understand and determine the eventual fate of elements released into the aquatic environment. Figures 3 and 4 show the distributions of sediment partition coefficients of major and trace elements, respectively, using the data of seven locations for which both water and sediment samples were analyzed. The mean K d for the major elements Al, Fe, and Mn are 107, 63, and 51 L kg−1, respectively. The solubility of Al decreases gradually from south to north in the main irrigation canal and increases from east to west in the secondary irrigation canal. Similarly, the solubility of Fe and Mn seems to change in the secondary irrigation canal from low value in the two locations S12 and S13 to high value in the two locations S14 and S15. Among the trace elements, vanadium is the least mobile with a mean K d of 6.0 × 103 L kg−1, and zinc and lead are the most mobile with a mean K d of 146 and 118 L kg−1, respectively. The overall trace metal mobility sequence, derived for the study area, in increasing order, is V < Co < B < Cr < Cu < Sr < Ni < Zn < Pb. The calculated sediment partition coefficients are high and suggest that there is a large tendency for the metals to precipitate out from the water or that the sediments have a large specific capacity to adsorb the metals.
To determine the controlling factors for the distributions in water, the Pearson correlation matrix analyses were carried out for 16 items, including metal concentrations and characteristics of water. No dependence of the concentrations of the measured elements on the water’s pH or EC was found. Al has obvious correlations with only few elements, namely, Fe, Cd, and V (r = 0.86–0.95). Mn is correlated strongly with Fe and Pb (r = 0.96–0.97), moderately with Cr, Sr, and V (r = 0.90–0.94), and weekly with Co and Mo (r = 0.84–0.86). It seems that the previous seven elements excluding Mo are more or less related. The anthropogenic pollution in the studied area does not only derive from point sources, but also from diffuse input from human, industrial, and agricultural activities. The solubility and mobility of metals in water depend not only on their concentrations, but also on their associations, chemical properties, and environmental conditions. The results may indicate the existence of two soluble Fe compounds. This first one contains Al and Cd, and V is in strong association to this compound. Moreover, another Fe and Mn compound seems to exist to which Pb, Cr, Sr, and V and possibly Co and Mo are associated. The positive correlation among these elements may also reflect similarity in their occurrences or geochemical processes that contribute or control their behavior in water. Another possibly related group of elements is Ni, Cu, and Zn (r = 0.92–0.97). The correlations among these metals are probably indicating common influential factors on their concentrations and could also be attributed to anthropogenic inputs of these metals. Since these metals did not have significant correlations with Al, Fe, or Mn, their existence in water may be associated with carbonates. B is not correlated with any other element. The highly soluble character of boron favors its release into the aqueous environment (Chetelat and Gaillardet 2005; Marschall and Jiang 2011). B is a ubiquitous trace element generally geogenic, but it can be due to anthropogenic pollution sources from industries such as ceramics, detergents, and fertilizers (Pennisi et al. 2006; Petelet-Giraud et al. 2009). It seems to be conservative in rivers (Chetelat and Gaillardet 2005). B(OH)3 and B(OH) −4 are the two dominant aqueous species of boron which seem to explain its lack of correlation with other elements.
Pearson correlation matrices for the concentrations of major and trace elements in both sediment and water samples indicate the absence of strong or medium correlation between the parameters. Weak correlations were found between the concentration of Fe in sediment and the concentration of Co, Cu, Ni, and Zn in water indicating that the adsorption, and coprecipitation of these metals on the iron oxides is not a major mechanism for their accumulation in sediment. Possible correlations exist between the concentrations of Mn in sediments and the concentrations of Mn, Co, Cr, Ni, Pb, and Sr in water. This may suggest that the deposition of these metals is not related to the forms of Mn minerals in sediment such as MnO2, MnCO3, and MnO(OH).
Conclusion
The trash and sewage inputs in the secondary canals are responsible for the high TOM and CaCO3 levels in the sediments. The distributions of all measured elements in sediments are uniform in the irrigation canals and the Nile River. Slightly higher concentration of Fe, Cr, Cu, Mo, Ni, and Sr in location C06 was observed which is due to the higher concentration of the natural Al in this locations. Pb enrichment was found in location S12 when both absolute and Al-normalized concentrations were considered. The EF values of Cd in the sediment suggest moderate enrichment. The geoaccumulation indices indicate that the sediments are unpolluted to moderately polluted with respect to Cu. MPI values argue for increasing load of contaminations in sediment as water follows to the north and east of the study area. The contamination factors are highest in the secondary irrigation canals and lowest in the Nile River. All locations have moderate impact of ecological risk due to Cu, Pb, and Zn. Among five trace metals, 7.7 % of samples exceeding an individual consensus-based PEC for both Cr and Ni resulted to be toxic to sediment-dwelling organisms. The mean concentrations of many metals in water are higher in the secondary irrigation canals. The partition coefficients for all measured major and trace metals are high, which leads to their accumulation in the wetland. While zinc and lead and the three major elements Al, Fe, and Mn are the most mobile elements in the study area, vanadium is the least mobile.
References
Allen, S. E. (1989). Chemical analysis of ecological materials (2nd ed.). Oxford: Blackwell Scientific Publication.
Anbar, A. D., & Rouxel, O. (2007). Metal Stable isotopes in paleoceanography. Annual Review of Earth and Planetary Sciences, 34, 717–746.
Chetelat, B., & Gaillardet, J. (2005). Boron isotopes in the seine river, France: a probe of anthropogenic contamination. Environmental Science & Technology, 39, 2486–2493.
Dai, J., Song, J., Li, X., Yuan, H., Li, N., & Zheng, G. (2007). “Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China”. Environmental Pollution, 145, 656–667.
DiToro, D. M., Mahony, J. D., Hansen, D. J., Scott, K. J., Hicks, M. E., Mays, S. M., & Redmont, M. S. (1990). Toxicity of cadmium in sediments: the role of acid volatile sulphides. Environmental Toxicology and Chemistry, 9, 1487–1502.
Farkas, A., Erratico, C., & Vigan, L. (2007). “Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po”. Chemosphere, 68, 761–768.
Hakanson, L. (1980). “An ecological risk index for aquatic pollution control. A sedimentological approach”. Water Research, 14, 975–1001.
Harikumar, P. S., Nasir, U. P., & Mujeebu, M. P. (2009). “Distribution of heavy metals in the core sediments of a tropical wetland system”. International Journal of Environmental Science and Technology, 6, 225–232.
Kumar, G. P. (2008). “Growth of Jatrophacurcas on heavy metal contaminated soil amended with industrial wastes and Azotobacter – a greenhouse study”. Bioresource Technolology, 99, 2078–2082.
Kumar, S. P., & Edward, J. K. P. (2009). “Assessment of metal concentration in the sediment core of Manakudy estuary of south west coast of India”. Indian Journal of Marine Sciences, 38, 235–48.
Kwon, Y. T., & Lee, C. W. (1998). “Application of multiple ecological risk indices for the evaluation of heavy metal contamination in a coastal dredging area”. Science of the Total Environment, 214, 203–210.
Lambert, R., Grant, C., & Sauvé, S. (2007). “Cadmium and zinc in soil solution extracts following the application of phosphate fertilizers”. Science of the Total Environment, 378, 293–305.
Lion, L. W., Altmann, R. S., & Leckie, J. O. (1982). Trace metal adsorption characteristics of estuarine particulate matter: evaluation of contributions of Fe/Mn oxide and organic surface coatings. Environmental Science and Technology, 16, 660–666.
Long, E. R., & MacDonald, D. D. (1998). “Recommended uses of empirically derived sediment quality guidelines for marine and estuarine ecosystems”. Human and Ecological Risk Assessment, 5, 1019–1039.
Long, E. R., Ingersoll, C. G., & MacDonald, D. D. (2006). “Calculation and uses of mean sediment quality guideline quotients: a critical review”. Environmental Science & Technology, 40, 1726–36.
MacDonald, D. D., Carr, S., Clader, F. D., Long, E. D., & Ingersoll, C. G. (1996). “Development and evaluation of sediment quality guidelines for Florida coastal waters”. Ecotoxicology, 5, 253–78.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). “Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems”. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Malcolm, S. J. (1985). Early diagenesis of molybdenum in estuarine sediments. Marine Chemistry, 16, 213–225.
Marschall, H. R., & Jiang, S. Y. (2011). “Tourmaline isotopes: no element left behind”. Elements, 7, 313–319.
Martin, J. M., & Meybeck, M. (1979). “Elemental mass-balance of material carried by major world rivers”. Marine Chemistry, 7, 173–206.
NRC. (2004). “Canadian Minerals Yearbook: Molybdenum. National Resources Canada” Available online: http://www.nrcan-rncan.gc.ca/mms-smm/busi-indu/cmy-amc/content/2004/40.pdf
Pennisi, M., Gonfiantini, R., Grassi, S., & Squarci, P. (2006). The utilization of boron and strontium isotopes for the assessment of boron contamination of the Cecina River alluvial aquifer (central-western Tuscany, Italy). Applied Geochemistry, 21, 643–655.
Petelet-Giraud, E., Klaver, G., & Negrel, P. J. (2009). Natural versus anthropogenic sources in the surface- and groundwater dissolved load of the Dommel River (Meuse basin): constraints by boron and strontium isotopes and gadolinium anomality. Journal of Hydrology, 369, 336–349.
Rowell, D. L. (1994). Soil science: methods and applications. England: Longman Scientific and Technical.
Sinex, S. A., & Wright, D. A. (1988). “Distribution of trace metals in the sediments and biota of chesapeake Bay”. Marine Pollution Bulletin, 19, 425–431.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). “Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index”. Helgol Meeresunters, 33, 566–575.
Turekian, K. K., & Wedepohl, K. H. (1961). “Distribution of the elements in some major units of the Earth’s crust”. Geological Society of America Bulletin, 72, 175–192.
USEPA. (1996). “Acid Digestion of Sludges, Solids and Soils, USEPA 3050B” In SW-846 Pt 1. Office of Solid and Hazardous Wastes. Cincinnati: USEPA.
Usero, J., Gonzales-Regalado, E., & Gracia, I. (1996). “Trace metals in bivalve mollusks Chamelea gallina from the Atlantic Coast of southern Spain”. Marine Pollution Bulletin, 32, 305–10.
Zheng, N., Wang, Q., Liang, Z., & Zheng, D. (2008). “Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China”. Environmental Pollution, 154, 135–142.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Mashali, H.A., Badran, H.M. & Elnimr, T. Metal concentrations in irrigation canals and the Nile River in an intensively exploited agricultural area. Environ Monit Assess 187, 136 (2015). https://doi.org/10.1007/s10661-015-4357-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4357-1