Abstract
Background
In recent years, submucosal tunneling endoscopic resection (STER) has emerged as a novel therapeutic endoscopic technique for upper gastrointestinal submucosal tumors (SMTs). The aim of this study was to evaluate the safety and efficacy of STER for upper gastrointestinal SMTs.
Methods
A systematic search of both English and Chinese databases was performed until November 15, 2015. Complete resection and en bloc resection rates were considered the primary outcome measures. Prevalence of complications was considered the secondary outcome measure. A random-effects model was used to generate conservative estimates of the prevalence of the main outcome variables. All data analyses were performed using Meta-Analyst software (version beta 3.13).
Results
A total of 28 studies were included in the final meta-analysis. The pooled complete resection and en bloc resection rates were 97.5 % (95 % CI 96.0–98.5 %) and 94.6 % (95 % CI 91.5–96.7 %), respectively. The common complications associated with STER were air leakage symptoms and perforation. The pooled prevalence of air leakage symptoms was 14.8 % (95 % CI 10.5–20.5 %) for subcutaneous emphysema and pneumomediastinum, 6.1 % (95 % CI 4.0–9.0 %) for pneumothorax and 6.8 % (95 % CI 4.7–9.6 %) for pneumoperitoneum. Additionally, the pooled prevalence of perforation was 5.6 % (95 % CI 3.7–8.2 %). Only a few cases of bleeding were reported in two studies.
Conclusions
STER is a highly feasible and safe treatment option for upper gastrointestinal SMTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most upper gastrointestinal submucosal tumors (SMTs) are regarded as clinically benign if they are smaller than 30 mm in size. However, some of these tumors do have a malignant potential (especially gastrointestinal stromal tumors, GISTs), which cannot be detected by endoscopic ultrasonography (EUS). Management by periodic endoscopic surveillance may lead to missed or delayed diagnosis of malignancy [1]. Moreover, such periodic endoscopic follow-ups can not only be stressful and troublesome for patients but can also cause long-term financial burdens. Gastrointestinal SMTs originating from the proper muscle layer (muscularis propria, MP) are routinely resected using a surgical operation [2]. However, with the development of endoscopic techniques in recent years, endoscopic operations, including endoscopic submucosal excavation (ESE), submucosal tunneling endoscopic resection (STER) and endoscopic full-thickness resection (EFR), could provide both a definitive histologic diagnosis and a minimally invasive therapeutic approach to such tumors [3].
STER uses a submucosal tunnel as an operating space to resect targeted tumors. This technique originated from the technique of peroral endoscopic myotomy (POEM). The main advantage of STER is the maintenance of GI tract mucosal integrity while achieving an en bloc resection of SMTs [4, 5]. Compared with ESE and EFR, this method may reduce the risks of postoperative gastrointestinal tract leakage and secondary infection. Several studies have attempted to assess the efficacy and safety of STER for upper gastrointestinal SMTs in recent years, especially in China. However, most of these studies had limited samples. The aim of this systematic review was to pool the results of the STER procedures to evaluate its effectiveness and address relevant clinical and technical issues.
Methods
This study did not require ethical approval or informed consent because only published materials were included.
Search strategy
This study was conducted following the meta-analysis of observational studies in epidemiology guidelines [6]. A systematic literature search was performed in English databases, including PubMed, Embase, Web of Science and the Cochrane Library, and Chinese databases, including Chinese biomedicine literature database (CBM), Chinese technological periodical full-text database (VIP) and Chinese periodical full-text database (CNKI). Relevant published articles were searched until November 15, 2015. The medical terms “submucosal tunneling endoscopic resection,” “STER,” “endoscopic resection,” “submucosal tunnel,” “gastrointestinal tumor” and “gastrointestinal neoplasms” were used in the search. References of relevant articles were also scanned for potential missed studies.
Study selection
The inclusion criteria were as follows: (1) clinical studies focused on the efficacy and safety of STER for upper gastrointestinal SMTs; (2) clinical outcomes, such as resection rates and complications, were reported; and (3) full-text articles could be obtained. The exclusion criteria were as follows: (1) experimental studies in animal models; (2) studies including participants without upper gastrointestinal submucosal tumors; (3) studies including patients who were duplicated in similar studies; and (4) abstracts or unsuitable publication types, such as comments, reviews, guidelines or case reports. There was no language restriction in the selection. Two investigators independently evaluated each study for eligibility, and any disagreements were resolved by discussion.
Data extraction
A predefined data sheet was developed by the two reviewers who independently extracted the data. The following characteristics were collected from each study: (1) study and population characteristics, including first author’s name, year of publication, country of origin, number of patients, number of lesions, age distribution, gender distribution and study design, and (2) technical and clinical characteristics, including complete resection rate, en bloc resection rate, location of lesions, diameter of lesions (in mm), procedural time (in minutes), days of hospitalization (in days), follow-up duration (in months), result of histology, prevalence of complications and recurrence of tumors. We did not attempt to contact the corresponding authors for any missing data.
Outcome measures
The main outcome measures were complete resection rate, defined as complete tumor removal with negative margins established, and en bloc resection rate, defined as complete removal of the tumor into one non-fragmented piece. Short-term complications (most commonly air leakage symptoms, perforation and bleeding) and long-term oncologic outcomes (local recurrence rate) were carefully recorded as secondary outcome measures. We performed a subgroup analysis in the process of pooling the prevalence of complications based on lesion location because the incidence of complications was diverse in different locations in the upper gastrointestinal tract.
Quality assessment
The Downs and Black quality checklist was used to evaluate all studies. This checklist was designed to ensure the quality of both randomized and nonrandomized studies [7] and provides an overall numeric score of 30 points based on 5 domains as follows: reporting (overall quality), external validity (ability to generalize findings), bias (intervention and outcome measures), confounding (bias in sampling) and power (negative findings). Two reviewers judged the quality results independently. A final score for each study was determined using the average of the two reviewers’ scores.
Statistical analysis
The prevalence of the main outcome variables in each study was combined to yield a pooled prevalence with a 95 % CI for all studies. Data were pooled using a random-effects model to generate a more conservative estimate of the prevalence. Heterogeneity among studies was assessed by using Cochran’s Q test and an inconsistency index (I 2). Heterogeneity was present if the P value was less than 0.05 for Cochran’s Q test, while values of I 2 of 25, 50 and 75 % represented low, moderate and high heterogeneity, respectively [8]. All data analyses in this study were conducted using Meta-Analyst software (version beta 3.13) [9].
Results
Search results
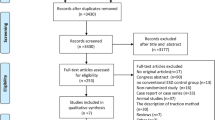
A flow diagram of the systematic review is shown in Fig. 1. After an initial search, 282 studies were identified. The studies included 191 English articles and 91 Chinese articles. Of these articles, 49 studies were selected for further full-text evaluation after excluding duplicates and a review of the remaining titles and abstracts. Of the remaining 49 records, 28 studies fulfilled the criteria for inclusion for a quantitative synthesis (meta-analysis) [10–37]. The reasons for the final exclusion of 21 studies were as follows: (1) different endoscopic techniques (ESE and EFR) were used; (2) insufficient data about the clinical outcomes were provided; and (3) possible cohort overlaps between studies were found.
Study characteristics
The included studies were published between 2011 and 2015. All but one of the studies, which were performed in Japan, were conducted in China. There were 20 retrospective and 8 prospective studies included, comprising a total of 1041 patients and 1085 lesions. More male patients were discovered in the included studies. The total M/F ratio was 611/387 out of 27 studies. The median of the mean ages across all studies was 48.0 years (range 36.7–54.2 years). The median of the mean days in the hospital (reported in 18 studies) was 4.9 days (range 2.3–12.6 days), and the median of the mean follow-up periods (reported in 16 studies) was 7.1 months (range 1.9–18.0 months). All included studies were assessed as medium to high quality according to the criteria from the Downs and Black quality checklist. The study characteristics of the included studies are given in Table 1. The clinical characteristics are given in Table 2.
Efficacy outcomes
A complete resection rate was reported in 27 studies except one [28]. The pooled prevalence was 97.5 % (95 % CI 96.0–98.5 %), which is shown in Fig. 2. No significant heterogeneity was detected in the analysis of the complete resection rate (I 2 = 0.0 %, Q = 0.894, p = 0.500). An en bloc resection rate was reported in 18 studies, and the pooled prevalence was 94.6 % (95 % CI 91.5–96.7 %), which is shown in Fig. 3, with low heterogeneity detected among the studies (I 2 = 23.2 %, Q = 0.961, p = 0.099).
Safety outcomes
As shown in Table 3, air leakage symptoms frequently occurred in the upper gastrointestinal STER procedures, which generally consisted of subcutaneous emphysema and pneumomediastinum (SEP), pneumothorax and pneumoperitoneum. SEP was the most commonly reported complication, with a pooled prevalence of 14.8 % (95 % CI 10.5–20.5 %) from 17 studies. A moderate heterogeneity was identified (I 2 = 28.5 %, Q = 0.977, p = 0.018). The esophagogastric junction was the most common predilection site (26.1 %, 95 % CI 17.9–36.4 %) for SEP rather than the esophagus or stomach (Fig. 4). The pooled prevalence of pneumothorax was 6.1 % (95 % CI 4.0–9.0 %) from 7 studies (Fig. 5). No evidence of heterogeneity (I 2 = 0.0 %, Q = 0.950, p = 0.454) was identified. The pooled prevalence of pneumoperitoneum was 6.8 % (95 % CI 4.7–9.6 %) from 9 studies (Fig. 6). Again, no evidence of heterogeneity (I 2 = 0.0 %, Q = 0.928, p = 0.496) was identified. The esophagogastric junction and stomach were the most common predilection sites for pneumothorax (10.7 %, 95 % CI 4.6–22.9 %) and pneumoperitoneum (9.6 %, 95 % CI 5.2–17.2 %), respectively.
Furthermore, the pooled prevalence of perforation was 5.6 % (95 % CI 3.7–8.2 %) from 6 studies (Fig. 7). No heterogeneity was discovered (I 2 = 0.0 %, Q = 0.915, p = 0.499). Perforation was most likely to occur in the stomach with a pooled incidence rate of 8.7 % (95 % CI 4.5–16.2 %). Only two studies reported a bleeding occurrence, and the definitions of bleeding from these studies were diverse. Wang et al. [18] reported the prevalence of bleeding was 17 %, which could be managed successfully using conservative approaches. However, Chen et al. [28] defined bleeding as major bleeding (>200 mL) with a prevalence of 1.7 %, and the bleeding could only be stanched by coagulation. No local recurrence was found in any of the included studies during the follow-up periods, and no STER-related deaths occurred.
Discussion
Our systematic review and meta-analysis showed that STER is a highly feasible and safe treatment option for upper gastrointestinal SMTs. In a pooled population of 1041 patients, both the complete resection rate (97.5 %) and en bloc resection rate (94.6 %) of STER should be viewed as reasonable. The prevalence of complications should also be considered more acceptable compared to surgical operations [38].
The STER technique was first reported by Xu et al.[39] from China. A standard procedure for STER includes the following steps: (1) injecting a mixed solution orally 3–5 cm from the proximal margin of the SMT to make a fluid cushion; (2) a 2-cm longitudinal mucosal incision is made using a hook knife at the top of the fluid cushion to provide mucosal entry to the submucosa; (3) a submucosal tunnel to the lesion is created with a hook or hybrid knife between the submucosal and muscular layers; (4) an endoscopic resection is meticulously performed with an insulated-tip, hook or hybrid knife until the tumor is completely dissected; (5) all visible blood vessels on the edge of the resection are coagulated with hot biopsy forceps or argon plasma coagulation; and (6) the mucosal incision site is then closed with several clips. Using STER offers several advantages. First, the integrity of the digestive tract mucosa and submucosa can be preserved to promote wound healing. Second, a 5-cm submucosal tunnel offers a good leak-proofing effect, which can reduce the risk of a digestive tract fistula and pleural/abdominal infection during the procedure. Third, the MP layer can be observed directly in the submucosal tunnel. Hemorrhagic spots can be immediately detected and successfully managed with hemostasis, which also reduces the risk of major or delayed bleeding.
The results of our study show that STER has both high complete resection and en bloc resection rates. Notably, several factors may influence the resection rate. First, the maximum resectable lesion size should be less than 35 mm in diameter, which is recommended by the most researchers because large tumors could cause loss of endoscopic visualization in a limited submucosal space [12, 16–18, 40]. Although some cases for employing STER in larger tumors have been reported by a few researchers, their results may not be satisfactory with failure of en bloc resection and/or complete resection [11, 12, 41]. Second, a tumor originating from the deep layer of the MP should be considered a non-applicable option for using STER. It is difficult to excise such lesions in an en bloc resection. A deep portion of the MP layer is usually associated with a high risk of perforation, chronic fistula formation and secondary infection [25, 26]. Third, to achieve high resection rates, a clear field of vision should be obtained. The tunnels should extend at least 2 cm distal to the tumor to ensure a satisfactory endoscopic view and leave enough working space for resection [30].
There was a general low complication rate for STER according to our research. Although the air leakage symptoms occur commonly, most of them can be treated with conservative approaches [16, 17, 24, 25, 28]. When SEP, pneumothorax or pneumoperitoneum occurs, gas insufflation should be used cautiously. Oxygen saturation and abdominal signs should be monitored closely. A punctuation or thoracic closed drainage should be applied timely. Furthermore, CO2 is recommended for gas insufflation because of its quick absorption by the gastrointestinal tract [14, 15, 17, 22, 24].
We realized that perforation occurred more frequently in the stomach, although the reasons for this were not definitely clear. One explanation may be that a tunnel is difficult to build in the gastric fundus and lesser curvature, which leads to the potential of harmful operations. The direction of tunneling is opposite the direction of endoscope advancement as a result of its retroflexion in this part of the stomach [29]. We also realized that, although the muscularis propria is rich in blood vessels, the incidence rate of bleeding in our review was low, which may be mainly because the operating area in the tunnel was washed and hemostasis was carefully executed during the operation [25]. During the follow-up period of all the included studies, no tumor recurrence and no STER-related deaths were found. Notably, the ideal follow-up strategies after STER are still unknown [42].
Some technical challenges also existed for STER. First, the indication for STER is limited by the lesion size and depth. EUS was still conventionally needed to identify the size, border and originating layer of the SMTs prepared for the operation [36]. Second, not all parts of the gastrointestinal tract are suitable for tunneling. Tumors in the upper esophagus leave no spare length for tunneling. As mentioned above, a tunnel is also sometimes difficult to build in the stomach. Third, the tunnel mucosa must be kept intact during the procedure, especially when excavating the lesion because leak-proofing only works effectively when the tunnel mucosa maintains integrity [25].
There are several limitations to our study. First, most of the included studies were conducted in China. Therefore, it is difficult to represent the characteristics of patients worldwide, and the results may not be generalizable to Western countries. Second, most studies included in this systematic review were retrospective studies performed in single centers. Thus, selection bias could not be excluded. Third, most of the included studies were observational studies, and a group comparison was only conducted in one study. More prospective studies are necessary to compare the efficacy and safety of STER with other endoscopic techniques and surgical operations.
Conclusions
In conclusion, our study showed that STER is a highly effective and safe procedure. Additional data from randomized controlled studies are needed to further validate these findings as well as to compare its effectiveness with other therapeutic modalities.
Abbreviations
- STER:
-
Submucosal tunneling endoscopic resection
- SMTs:
-
Submucosal tumors
- SEP:
-
Subcutaneous emphysema and pneumomediastinum
- GIST:
-
Gastrointestinal stromal tumor
- GCT:
-
Granular cell tumor
- CFT:
-
Calcifying fibrous tumor
- EUS:
-
Endoscopic ultrasonography
- MP:
-
Muscularis propria
- ESE:
-
Endoscopic submucosal excavation
- EFR:
-
Endoscopic full-thickness resection
- POEM:
-
Peroral endoscopic myotomy
References
Nicki NJ, Wackerbarth S, Gress F, Fockens P, McClave S, Chak A, Savides T, Roesch T, Odegaard S, Catalano M, Chang K, Scheiman JM (2000) Management of hypoechoic intramural tumors: a decision tree analysis of EUS-directed versus surgical management. Gastrointest Endosc 51:AB176
Ponsaing LG, Hansen MB (2007) Therapeutic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol 13:3316–3322
Lu J, Lu X, Jiao T, Zheng M (2014) Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol 48:667–673
Abe N, Takeuchi H, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M (2013) Recent developments in gastric endoscopic submucosal dissection: towards the era of endoscopic resection of layers deeper than the submucosa. Dig Endosc 25:64–70
Li QL, Zhou PH, Xu MD, Cai MY, Yao LQ (2013) Offshoots of peroral endoscopic myotomy: submucosal tunneling endoscopic resection, pyloromyotomy, and beyond. Tech Gastrointest Endosc 15:160–163
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, Moose G (2000) Meta-analysis of observational studies in epidemiology—a proposal for reporting. JAMA 283:2008–2012
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun H 52:377–384
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Wallace BC, Schmid CH, Lau J, Trikalinos TA (2009) Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol 9:157
Xu MD, Yao LQ, Zhou PH, Cai MY, Zhong YS, Chen WF, Zhang YQ, Ma LL, Qin WZ, Hu JW, Ren Z, Chen SY (2011) Submucosal endoscopic tumor resection for upper gastrointestinal submucosal tumors originating from muscularis propria layer. Chin J Dig Endosc 28:606–610
Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B (2012) Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 44:231–235
Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S (2012) Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 44:225–230
Xu J, Lu X, Xu H, Xu Z (2012) Submucosal tunneling endoscopic resection for esophageal submucosal tumors: analysis of 5 cases. Chin J Gastroenterol 17:678–680
Zhao ZF, Ma SR, Zhang N, Yang Z, Gong ZJ, Jin XL, Shi Y, Zhang L, Shi G (2012) Submucosal tunneling endoscopic resection for esophageal leiomyoma originating from muscularis propria layer. Chin J Dig Endosc 29:251–254
Ge N, Sun S, Wang S, Liu X, Wang GX, Guo JT (2013) Endoscopic ultrasound-assisted tunnel-type endoscopic submucosal dissection for the treatment of esophageal tumors arising in the muscularis propria (with video). Endosc Ultrasound 2:11–15
Jiao CH, Yang SP, Li XL, Ding J, Xu YH, Tao G, Chen L, Zhang DQ, He X, Chen WK, Shi RH (2013) Preliminary exploration on submucosal tunneling endoscopic resection for middle and lower esophagus submucosal tumors. Chin Med J 93:2388–2391
Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ (2013) Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc 27:4354–4359
Wang L, Ren W, Zhang ZM, Yu J, Li YH, Song YK (2013) Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma. Surg Endosc 27:4259–4266
Jiang HX, Qin SY, Huang JA, Li XM, Tan FY, Lei RE (2013) Application study on submucosal endoscopic tumor resection for upper gastrointestinal submucosal tumors. J Minim Invasive Med 8:660–664
Lu J, Jiao T, Zheng M, Lu X (2014) Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 28:3401–3407
Min H, Chen ZR, Gong F, Wang H, Zhou JD, Chen WF (2014) Submucosal tunneling endoscopic resection of esophageal submucosal tumors originating from the muscularis propria layer: a retrospective analysis. World Chin J Digestol 22:915–919
Wang XY, Xu MD, Yao LQ, Zhou PH, Pleskow D, Li QL, Zhang YQ, Chen WF, Zhong YS (2014) Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 28:1971–1977
Xiong Y, Hu H, Gao Y, Linghu EQ, Wang XD, Wang AM (2014) Endoscopic esophageal submucosal tunnel resection of cardiac benign tumors originating from muscularis propria. Chin Med J 94:3655–3657
Yang XZ, Dai WJ, Wang HG, Wang Q, Sun SH, Zhou JF, Ma G, Zhang J (2014) Submucosal tunneling endoscopic resection for esophageal submucosal tumors. World Chin J Digestol 22:5310–5314
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530
Li QF, Yue H, He FJ, Liu SY, Xu SH, Wang WF, Peng QQ, Chen PS, Long PQ, Yang WJ (2014) Submucosal tunneling endoscopic resection for the treatment of esophageal muscular propria layer submucosal tumors. Chin J Clin 8:1502–1506
Xu LX, Liang W, Deng WY, Wang LJ, Gao LY, Guo XB (2014) Submucosal tunneling endoscopic resection for esophageal tumors originating from muscularis propria. Chin J Exp Surg 31:2891–2893
Chen T, Zhang C, Yao LQ, Zhou PH, Zhong YS, Zhang YQ, Chen WF, Li QL, Cai MY, Chu Y, Xu MD (2015) Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy 48:149–155
Lu J, Jiao T, Li Y, Liu Y, Wang Y, Zheng M, Lu X (2015) Heading toward the right direction–solution package for endoscopic submucosal tunneling resection in the stomach. PLoS One 10:e0119870
Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou J, Duan T, Zhang J, Liu D (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroen Hepat 27:776–780
Xiong Y, Hu H, Wang A, Linghu EQ, Li YP, Zhang Z, Geng Y (2015) Preliminary experience with endoscopic gastric submucosal tumor resection through the submucosal tunnel using double tunnel and double flex endoscope. J South Med Univ 35:455–458
Zhang C, Hu JW, Chen T, Zhou PH, Zhong YS, Zhang YQ, Chen WF, Li QL, Yao LQ, Xu MD (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: a feasibility study. Indian J Cancer 51(Suppl 2):e52–e55
Zhao H, Sheng H, Huang L, Jiang L, Xie Y, Zhou P (2015) Submucosal tunneling endoscopic resection in the treatment of esophageal submucosal tumors originating from muscularis propria layer. Chin J Gastrointest Surg 18:478–482
Zhou DJ, Dai ZB, Wells MM, Yu DL, Zhang J, Zhang L (2015) Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 21:578–583
Li GH, Du GP (2015) Clinical efficacy of submucosal tunneling endoscopic resection for esophageal leiomyoma in 18 patients. Chin J Biomed Eng 21:174–176
Ruan RW, Wang S, Tao YL, Mao JS, Yu JP, Liu YJ, Zhu SW (2015) Submucosal tunneling endoscopic resection for submucosal tumors originating from the muscularis propria layer of esophagus: a report of 26 cases. Clin Focus 30(895–898):902
Wei Z, Sun ZQ, Dong W, Shang RL, Jia AQ, Sun KL, Li M, Su ML (2015) Submucosal tunneling endoscopic resection for esophageal leiomyoma originating from the muscularis propria layer: a report of 14 cases. Chin J Dig Endosc 32:190–192
Lee CM, Kim HH (2014) Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol 20:13035–13043
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199
Zhang Y, Ye LP, Mao XL (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 21:9503–9511
Kumbhari V, Saxena P, Azola A, Messallam AA, El Zein MH, Khashab MA (2015) Submucosal tunneling endoscopic resection of a giant esophageal leiomyoma. Gastrointest Endosc 81:219–220
Grotz TE, Donohue JH (2011) Surveillance strategies for gastrointestinal stromal tumors. J Surg Oncol 104:921–927
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Xiu-He Lv, Chun-Hui Wang and Yan Xie have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Lv, XH., Wang, CH. & Xie, Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 31, 49–63 (2017). https://doi.org/10.1007/s00464-016-4978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4978-7