Abstract
Introduction
Oral squamous cell carcinoma (OSCC) is characterized by a high risk of cervical lymph node metastasis; however, it is still not clear whether patients with early stage OSCC with clinical N0 neck should undergo elective neck dissection (END) at the time of primary tumor removal, or they should undergo a conservative approach of observation (OBS), with therapeutic neck dissection at the time of lymph nodal recurrence. We conducted a meta-analysis of randomized controlled trials (RCTs) that compared these two approaches.
Methods
PubMed and Scopus databases were searched for RCTs published in English language related to END and OBS in patients with early stage OSCC with clinical N0 neck. A meta-analysis was performed using random effects model with hazard ratio (HR) as the effect size for survival parameters and odds ratio (OR) as the effect size for lymph nodal recurrence.
Results
A total of 7 RCTs, comprising 1250 patients were included in the meta-analysis. Results of the meta-analyses showed that as compared to OBS approach, END could significantly improve overall survival (HR 0.67; 95% CI 0.53, 0.86) and disease-free survival (HR 0.64; 95% CI 0.46, 0.89), and significantly reduce lymph nodal recurrence (OR 0.28; 95% CI 0.12, 0.66). After correcting for heterogeneity, the disease specific survival was also found to be improved by the END approach (HR 0.53; 95% CI 0.29, 0.98).
Conclusion
The results of this meta-analysis suggest that elective neck dissection at the time of resection of the primary tumor not only leads to a reduced chance of nodal recurrence, but also confers a survival benefit in patients with clinically node-negative early stage oral cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer is the sixth most common cancer globally with South Asia contributing to more than one-third of the total oral cancer burden [1, 2]. Tobacco consumption, betel-quid chewing, excessive alcohol consumption, poor oral hygiene, nutrient-deficient diet, and sustained viral infections such as human papillomavirus are some of the risks associated with the occurrence of oral cancer. Oral squamous cell carcinoma (OSCC) is the most predominant form of oral cancer, and the majority of these tumors arise from the tongue, floor of mouth and buccal mucosa.
OSCC is characterized by a high risk of cervical lymph node metastasis, and frequently these metastases are occult with non-palpable neck nodes (clinical N0) [3]. Although screening of clinical N0 neck by ultrasound, CT, MRI, or positron emission tomography (PET) can help to detect some of these non-palpable nodal metastases, the recurrence rate even in radiologic N0 neck has been up to 40% [4]. In view of the high incidence of nodal recurrence, prophylactic neck dissection at the time of primary tumor excision has been advocated as routine management protocol of N0 neck by many surgeons. However, many surgeons on the contrary prefer watchful waiting with therapeutic neck dissection for nodal relapse. Proponents of watchful waiting approach cite the potential advantage of avoiding the neck dissection procedure in up to 70% of patients who eventually remain node-negative. In addition, neck dissection is associated with increased morbidity, complications and costs. In spite of multiple retrospective observational, prospective case–control, and randomized controlled trials (RCTS), conducted over last six decades, it is still not clear whether patients with early stage OSCC with clinical N0 neck should undergo elective neck dissection (END) at the time of primary tumor removal, or they should undergo a conservative approach of observation (OBS), with therapeutic neck dissection at the time of lymph nodal recurrence. There have been many meta-analyses also published on this subject [5,6,7,8,9,10,11,12,13,14], however, most of the previous meta-analyses had combined the results of RCTs with those of either retrospective studies or matched case–control studies, thus lowering the certainty of evidence and increasing the risk of bias.
To solve any such contentious issue, the highest quality of evidence comes from RCTs and meta-analysis of RCTs. In the present paper, we performed a meta-analysis of RCTs that compared elective neck dissection (END) at the time of primary tumor removal, with watchful observation (OBS), with therapeutic neck dissection at the time of lymph nodal recurrence in patients with clinical node-negative early stage OSCC.
Materials and Methods
Protocol and Registration
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines was consulted during the stages of design, analysis, and reporting of this meta-analysis [15]. Since, this is a meta-analysis, therefore an institutional review board or an ethics committee approval was not required. The protocol of this meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) vide registration number CRD42020214911 and is available in full on the NIHR (National Institute for Health Research) website (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020214911).
Eligibility Criteria
The purpose of this meta-analysis was to compare the outcome of patients of clinically node-negative oral squamous cell carcinoma, who received surgical treatment of the primary tumor along with either elective neck dissection (END group) or a conservative surgical approach comprising of observation followed by therapeutic neck dissection in the event of nodal relapse (OBS group). The primary outcome of interest was overall survival, while the secondary outcomes of interest were disease specific survival, disease-free survival and frequency of lymph node recurrence.
The studies were included if they met the following inclusion criteria:
-
(1)
The studies were RCTs, published in English language and published between January 01, 1980 and May 01, 2021.
-
(2)
The included patients in the RCTs were diagnosed as clinically node-negative (cN0) oral squamous cell carcinoma (OSCC) without any prior treatment.
-
(3)
These patients were treated with surgical excision of the primary tumor with or without END. The patients in the END group had primary neck dissection at the time of the surgery of the primary tumor and the patients in the OBS group had surgery of the primary tumor only, while the neck was put under close observation during follow-up, and therapeutic neck dissection was performed only when neck node metastasis was detected.
-
(4)
The studies had reported the clinical outcomes for both these groups and the reported outcome measures included either overall survival (OS), disease specific survival (DSS), disease-free survival (DFS), or lymph nodal recurrence.
The exclusion criteria were as follows:
-
(1)
The primary tumor site was outside the boundaries of the oral cavity.
-
(2)
The study included less than 50 patients
Search Strategy and Study Selection
Comprehensive electronic searches were performed in PubMed and Scopus databases to identify relevant RCTs meeting the above inclusion and exclusion criteria. The PubMed database was searched with the following search strategy: ((“cancer” OR “carcinoma” OR “neoplasm” OR “tumor” OR “squamous cell carcinoma”, OR “SCC”) AND (“neck dissection” OR “cervical lymphadenectomy” OR “observation” OR “conservative” OR “surgery” OR “resection”) AND (“lingual” OR “tongue” OR “gum” OR “cheek” OR “buccal” OR “palatal” OR “palate” OR “floor of mouth” OR “retromolar” OR “lip” OR “labial” OR “mouth” OR “oral”)) AND (“English”[Language]). Scopus database was searched with the following search strategy: (TITLE-ABS-KEY ({elective neck dissection})) AND (carcinoma OR cancer) AND ( LIMIT-TO ( LANGUAGE, “English”)) AND (LIMIT-TO ( EXACTKEYWORD, “Neck Dissection”)). The last search was performed on May 01, 2021. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies and all identified articles were systematically assessed using the inclusion criteria. Other sources searched included bibliographies of previously published relevant original and review articles including meta-analyses on this topic. First, the titles and abstracts of the searched studies were screened for potential eligibility. Thereafter, the full texts of potentially eligible studies were reviewed for final inclusion. Two authors (SG and RS) independently searched, screened and selected the studies according to the search strategy, inclusion criteria and exclusion criteria.
Data Extraction
The following data were extracted from each included study: year of publication, PMID number, country of study, total number of patients in each group, their demographic data, site of cancer, tumor staging, follow-up duration, number of patients having occult LN metastasis in the END group, and clinical outcomes in terms of overall survival (OS), disease specific survival (DSS), disease-free survival (DFS), and lymph nodal recurrence. If the clinical outcome data were unavailable, these were calculated using the raw numbers provided in the studies. For discordant or unavailable data, an attempt was made to contact the corresponding authors via e-mail to provide the required data.
Risk of Bias Assessment
The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 6.2) [16]. It included seven entries: the random sequence generation (for selection bias), allocation concealment (for selection bias), blinding of participants and personnel (for performance bias), blinding of outcome assessment (for detection bias), incomplete outcome data (for attrition bias), selective reporting (for reporting bias), and other bias. Since, blinding of participants and clinicians is not feasible in surgical trials, hence, performance bias was removed from the quality assessment. For each domain the risk of bias was evaluated as high, low or unclear. If any study had two or more “high-risk” entries, it was considered to be of low quality; otherwise, it was considered to be of high quality. These results were then presented in graph form. All discrepancies were resolved by full discussions within the group of researchers.
Data Synthesis and Statistical Analysis
The meta-analysis was performed using hazard ratios (HR) with 95% confidence intervals (CIs) as the effect sizes for OS, DSS and DFS, while odds ratio (OR) as effect size for LN recurrence.
Pooling of the effect sizes were done using only a random effects model to calculate a more conservative result. P < 0.05 was considered to have a statistically significant difference in the outcomes between END and OBS groups. To assess the heterogeneity among studies I2 and P values were calculated (I2 > 50% and/or P < 0.05 were considered statistically significant). If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored. The meta-analysis was conducted with Review Manager software (version 5.3.5, The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study Selection and Data Collection
Using the described search strategy 2124 studies were retrieved from the PubMed and 593 studies were retrieved from Scopus and other sources. After removing duplicates, 2130 studies were screened for potential inclusion. The titles and abstracts of these articles were screened, and 2121 studies were excluded. The full text of remaining 9 articles were examined and a further 2 articles were excluded. The study by Mirea et al. [17] was excluded because although it was prospective case–control study, it was not randomized. The study by Otsuru et al. [18] was excluded because it was propensity score matched study and not an RCT. Thus, 7 RCTs were finally included in the meta-analysis. The flow chart of the study selection is shown in Fig. 1.
Characteristics of Included Studies
The main characteristics of the included studies are summarized in Table 1. Two RCTs were from Europe, two from India, and one each from Brazil, Hong Kong, and China. A total of 1250 patients were enrolled in these studies, and of them 1019 (81%) had tongue carcinoma, 112 (9%) had carcinoma of floor of mouth, and 92 (7%) had buccal mucosa cancer. The mean / median age ranged from 48 to 62 years, and 864 (69%) patients were males. The tumor stage was equally divided between stage T1 (51%) and stage T2 (48%). Only one study had 12 (1%) patients of stage T3. The frequency of occult lymph node metastasis in the END group was 32% (195/619) and ranged from 21 to 53%. The follow-up of patients ranged from 20 to 92 months.
Risk of Bias
Risk of bias assessment for each study is summarized in the Figs. 2 and 3. None of the studies had two or more of “high-risk” entries to be considered as low quality. At least 50% of studies were free of selection bias, 100% studies were free of detection bias, 70% studies were free of attrition bias, and 86% studies were free of reporting bias.
Clinical Outcomes
The clinical outcome of patients of both the groups in all the studies is given in Table 2. The HR for overall survival was provided by 5 studies, the HR for disease specific survival was provided by 5 studies, the HR for disease-free survival was provided by 6 studies, and the OR for the LN recurrence was provided by all the 7 studies.
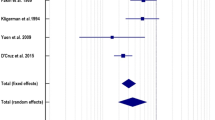
The primary aim of this meta-analysis was to investigate the effect of END on overall survival in patients of clinically node-negative oral squamous cell carcinoma. Five of seven studies compared overall survival between the two groups (Fig. 4). The pooled HR was 0.67 (95% CI 0.53, 0.86), indicating that END led to an increase in overall survival as compared to the strategy of OBS (Fig. 4). There was no heterogeneity among the studies (P = 0.56) with an I2 of 0%.
The secondary aims of this meta-analysis were disease-free survival, disease specific survival and lymph node recurrence. The pooled HR for disease-free survival was 0.64 (95% CI 0.46, 0.89), with a non-significant heterogeneity (I2 49%) (Fig. 5). The disease specific survival was found to be similar between the two groups with a pooled HR of 0.72 (95% CI 0.35, 1.49), but with significant heterogeneity (I2 68%). To look for the source of heterogeneity leave-one-out meta-analysis was performed to find out the influence of each study on the overall effect size estimate and to identify influential studies. The leave-one-out meta-analysis revealed that the study by Yang et al. [19] was an outlier. When the meta-analysis was performed after excluding the outlier study, the pooled HR for DSS was 0.53 (95% CI 0.29, 0.98) without any significant heterogeneity (I2 34%) (Fig. 6). The pooled OR for LN recurrence was 0.28 (95% CI 0.12, 0.66), but with significant heterogeneity (I2 86%). When the meta-analysis was performed after excluding the two outlier studies the pooled OR for LN recurrence was 0.17 (95% CI 0.12, 0.24) with no heterogeneity (I2 0%) (Fig. 7).
Discussion
In this meta-analysis of 7 RCTs, comprising 1250 patients, we found that patients with early stage OSCC, who are clinically node-negative, still have 32% (range 21–53%) of occult metastasis to neck lymph nodes. As compared to the conservative OBS approach, the END approach leads to significantly improved overall survival (HR 0.67; 95% CI 0.53, 0.86) and disease-free survival (HR 0.64; 95% CI 0.46, 0.89); and also, a significantly reduce lymph nodal recurrence (OR 0.28; 95% CI 0.12, 0.66). After correcting for heterogeneity, the disease specific survival was also found to be improved by the END approach (HR 0.53; 95% CI 0.29, 0.98).
One of the most important strengths of our study is that we have included only RCTs in this meta-analysis. In addition, we used the random effect model to pool the results and also explored the source of any significant heterogeneity using the leave-one-out meta-analysis. There have been many other meta-analyses on this subject [5,6,7,8,9,10,11,12,13,14], however, most of the previous meta-analyses had pooled the results of RCTs with those of either retrospective studies or matched case–control studies, thus lowering the certainty of evidence and increasing the risk of bias. The evidence generated from our meta-analysis is of high certainty, coming from input of 7 high-quality level-I trials pooled by use of rigorous statistical methods.
One of the most important adverse prognostic factors in patients with oral cancer is the presence of cervical lymph node metastasis [20, 21], as it reduces the overall survival by up to 50% [22, 23]. Patients in whom cervical lymph nodes are not palpable (clinical N0), may still harbor occult nodal metastasis [3]. Despite huge advancement in imaging technologies such as CT, MRI, and PET for preoperative noninvasive detection of cervical lymph nodal metastasis, in a significant number of patients the metastasis is missed by these imaging modalities [4, 24]. A meta-analysis comparing various modalities for the diagnosis of cervical lymph node metastasis in patient with head and neck squamous cell carcinoma with a clinically node-negative neck, found the pooled estimates for sensitivity of 47%, 57%, 48%, 63% and 56% for CT, MRI, PET, ultrasound, and ultrasound-guided fine-needle aspiration cytology, respectively [25]. Patients harboring occult cervical metastasis, in whom upfront END has not performed, present with overt lymph nodal recurrence soon after the surgery. Therefore, planning optimal management of cervical lymph nodes is a vital component of oral cancer management. Three main approaches have been advocated for clinical N0 neck management in patients with OSCC: (i) Upfront END at the time of surgery; (ii) observation (OBS), with therapeutic neck dissection if lymph nodal recurrence ensues; and more recently (iii) sentinel lymph node biopsy prior to surgery [26]. All the three approaches have their pros and cons, and despite numerous observational and interventional studies and their meta-analyses, conducted over last six decades, the best approach is still not clear. Even various society guidelines such as EHNS-ESMO-ESTRO guidelines [27], NICE guidelines [28], UK guidelines [29], NCCN guidelines [30], ASCO guidelines [31], German guidelines [32], and Indian guidelines [33], are not unanimous in their recommendations for the best approach [26]. In this direction, our meta-analysis would be a helpful addition in providing good evidence towards resolving this controversy.
We found in our meta-analysis the frequency of occult lymph node metastasis to be 32% (range 21–53%). Our results are in agreement with the meta-analysis conducted by Massey et al. who found that the rate of occult metastasis was between 21 and 49% in the 5 included RCTs, and between 7 and 39% in the 34 retrospective series [10]. Thus, it is now amply clear that one-in-three patients with clinically node-negative early stage oral cancer do harbor occult metastasis, and clinical examination of non-palpable lymph nodes cannot be relied upon when taking a decision to perform neck dissection or not. However, the question remains, is performing END in all patients the best strategy, when two-in-three patients may not need it. In our meta-analysis, we found that the primary outcome of overall survival in patients receiving END was considerably increased in patients receiving END as compared to OBS group (pooled HR 0.67; 95% CI 0.53, 0.86). In addition, the secondary outcomes such as disease-free survival, disease specific survival, LN recurrence rates were also in favor of END approach. Thus, our meta-analysis provides strong evidence in support of elective neck dissection in patients with clinically node-negative disease.
There are a few limitations in our study. The first limitation was the presence of significant heterogeneity when the results of LN recurrence and disease specific survival were being pooled (I2 of 86% and 68%, respectively). We were able to resolve the heterogeneity by identifying and excluding the outlier studies from our final analysis. The second limitation was that although we showed that END approach is superior to the OBS approach, but what about the third approach, i.e., the sentinel lymph node biopsy. To the best of our knowledge, there has been only one RCT that compared END with sentinel lymph node biopsy, which showed the oncologic equivalence of the sentinel LN biopsy approach to the END approach, with lower morbidity in the sentinel arm [34]. However, more studies will be needed comparing these two approaches, before any firm conclusion can be drawn.
While we and others have now shown that as compared to observation with therapeutic neck dissection approach, the elective neck dissection should be the preferred approach in the management of clinically node-negative early oral cancer, even this aggressive approach may still not guarantee against future recurrences. A meta-analysis of 21 studies that studied regional recurrence in the pathologically node-negative neck dissection (pN0) neck following END approach, still observed a recurrence rate of 7.5% over a median follow-up of about 3 years [35]. Thus, although the END approach should be preferred, however, this is still not the Holy Grail of best approach and other strategies should also be explored. Bree et al. have proposed to develop decision models that can serve to optimize choices depending on relevant variables such as individualized risk of lymph node recurrence, patient and institutional preference, and other factors, rather than proposing any one approach for all patients [36].
In conclusion, the results of our meta-analysis of seven randomized controlled trials suggest that elective neck dissection at the time of resection of the primary tumor not only leads to a reduced chance of nodal recurrence, but also confers a survival benefit as compared to the conservative approach of watchful waiting and therapeutic neck dissection at the time of lymph nodal recurrence in patients with clinically node-negative early stage oral cancer.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R (2013) Epidemiology of oral cancer in Asia in the past decade–an update (2000–2012). Asian Pac J Cancer Prev 14:5567–5577. https://doi.org/10.7314/apjcp.2013.14.10.5567
Chia C, Key S, Hasan Z et al (2021) Systematic review and meta-analysis of cervical metastases in oral maxillary squamous cell carcinoma. Cancer Rep. https://doi.org/10.1002/cnr2.1410
Liao L-J, Lo W-C, Hsu W-L et al (2012) Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer 12:236. https://doi.org/10.1186/1471-2407-12-236
Fasunla AJ, Greene BH, Timmesfeld N et al (2011) A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol 47:320–324. https://doi.org/10.1016/j.oraloncology.2011.03.009
Ren Z-H, Xu J-L, Li B et al (2015) Elective versus therapeutic neck dissection in node-negative oral cancer: evidence from five randomized controlled trials. Oral Oncol 51:976–981. https://doi.org/10.1016/j.oraloncology.2015.08.009
Abu-Ghanem S, Yehuda M, Carmel N-N et al (2016) Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 142:857–865. https://doi.org/10.1001/jamaoto.2016.1281
Bulsara VM, Worthington HV, Glenny A-M et al (2018) Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Datab Syst Rev. https://doi.org/10.1002/14651858.CD006205.pub4
Ding Z, Xiao T, Huang J et al (2019) Elective neck dissection versus observation in squamous cell carcinoma of oral cavity with clinically n0 neck: a systematic review and meta-analysis of prospective studies. J Oral Maxillofac Surg 77:184–194. https://doi.org/10.1016/j.joms.2018.08.007
Massey C, Dharmarajan A, Bannuru RR, Rebeiz E (2019) Management of N0 neck in early oral squamous cell carcinoma: a systematic review and meta-analysis. Laryngoscope 129:E284–E298. https://doi.org/10.1002/lary.27627
Cao Y, Wang T, Yu C et al (2019) elective neck dissection versus wait-and-watch policy for oral cavity squamous cell carcinoma in early stage: a systematic review and meta-analysis based on survival data. J Oral Maxillofac Surg 77:2154–2167. https://doi.org/10.1016/j.joms.2019.03.015
Cai H, Zhu Y, Wang C et al (2020) Neck nodal recurrence and survival of clinical T1–2 N0 oral squamous cell carcinoma in comparison of elective neck dissection versus observation: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 129:296–310. https://doi.org/10.1016/j.oooo.2019.10.012
Ibrahim SA, Ahmed ANA, Elsersy HA, Darahem IMH (2020) Elective neck dissection in T1/T2 oral squamous cell carcinoma with N0 neck: essential or not? a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 277:1741–1752. https://doi.org/10.1007/s00405-020-05866-3
Oh LJ, Phan K, Kim SW et al (2020) Elective neck dissection versus observation for early-stage oral squamous cell carcinoma: systematic review and meta-analysis. Oral Oncol 105:104661. https://doi.org/10.1016/j.oraloncology.2020.104661
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Higgins JPT, Savović J, Page MJ, et al (2021) Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane
Mirea D, Grigore R, Safta D et al (2014) Elective neck dissection in patients with stage T1–T2N0 carcinoma of the anterior tongue. Hippokratia 18:120–124
Otsuru M, Ota Y, Yanamoto S et al (2019) A multicenter retrospective study of elective neck dissection for T1–2N0M0 tongue squamous cell carcinoma: analysis using propensity score-matching. Ann Surg Oncol 26:555–563. https://doi.org/10.1245/s10434-018-07089-7
Yang X, Tian X, Wu K et al (2018) Prognostic impact of perineural invasion in early stage oral tongue squamous cell carcinoma: results from a prospective randomized trial. Surg Oncol 27:123–128. https://doi.org/10.1016/j.suronc.2018.02.005
Woolgar JA, Rogers SN, Lowe D et al (2003) Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol 39:130–137. https://doi.org/10.1016/s1368-8375(02)00030-1
Shingaki S, Takada M, Sasai K et al (2003) Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg 185:278–284. https://doi.org/10.1016/s0002-9610(02)01378-8
Amit M, Yen TC, Liao CT et al (2013) Clinical nodal stage is a significant predictor of outcome in patients with oral cavity squamous cell carcinoma and pathologically negative neck metastases: results of the international consortium for outcome research. Ann Surg Oncol 20:3575–3581. https://doi.org/10.1245/s10434-013-3044-0
Kowalski LP, Bagietto R, Lara JR et al (2000) Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck 22:207–214. https://doi.org/10.1002/(sici)1097-0347(200005)22:3%3c207::aid-hed1%3e3.0.co;2-9
Akoğlu E, Dutipek M, Bekiş R et al (2005) Assessment of cervical lymph node metastasis with different imaging methods in patients with head and neck squamous cell carcinoma. J Otolaryngol 34:384–394. https://doi.org/10.2310/7070.2005.34605
Liao L-J, Hsu W-L, Wang C-T et al (2016) Analysis of sentinel node biopsy combined with other diagnostic tools in staging cN0 head and neck cancer: a diagnostic meta-analysis. Head Neck 38:628–634. https://doi.org/10.1002/hed.23945
Vassiliou LV, Acero J, Gulati A et al (2020) Management of the clinically N0 neck in early-stage oral squamous cell carcinoma (OSCC). an EACMFS position paper. J Craniomaxillofac Surg 48:711–718. https://doi.org/10.1016/j.jcms.2020.06.004
Machiels J-P, René Leemans C, Golusinski W et al (2020) Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1462–1475. https://doi.org/10.1016/j.annonc.2020.07.011
(2017) Surveillance report (exceptional review) 2017 – Cancer of the upper aerodigestive tract: assessment and management in people aged 16 and over (2016) NICE guideline NG36. National Institute for Health and Care Excellence (UK), London
Kerawala C, Roques T, Jeannon J-P, Bisase B (2016) Oral cavity and lip cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol 130:S83–S89. https://doi.org/10.1017/S0022215116000499
Pfister DG, Spencer S, Adelstein D et al (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18:873–898. https://doi.org/10.6004/jnccn.2020.0031
Koyfman SA, Ismaila N, Crook D et al (2019) Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol 37:1753–1774. https://doi.org/10.1200/JCO.18.01921
Wolff K-D, Follmann M, Nast A (2012) The diagnosis and treatment of oral cavity cancer. Dtsch Arztebl Int 109:829–835. https://doi.org/10.3238/arztebl.2012.0829
Chaturvedi P, Prabhash K, Babu G et al (2020) Indian clinical practice consensus guidelines for the management of oral cavity cancer. Indian J Cancer 57:S6–S8. https://doi.org/10.4103/0019-509X.278975
Garrel R, Poissonnet G, Moyà Plana A et al (2020) Equivalence randomized trial to compare treatment on the basis of sentinel node biopsy versus neck node dissection in operable T1–T2N0 oral and oropharyngeal cancer. J Clin Oncol 38:4010–4018. https://doi.org/10.1200/JCO.20.01661
Chegini S, Schilling C, Walgama ES et al (2021) Neck failure following pathologically node-negative neck dissection (pN0) in oral squamous cell carcinoma: a systematic review and meta-analysis. British J Oral Maxillofac Surg S0266–4356(21):00131–00135. https://doi.org/10.1016/j.bjoms.2021.04.002
de Bree R, Takes RP, Shah JP et al (2019) Elective neck dissection in oral squamous cell carcinoma: past, present and future. Oral Oncol 90:87–93. https://doi.org/10.1016/j.oraloncology.2019.01.016
Vandenbrouck C, Sancho-Garnier H, Chassagne D et al (1980) Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer 46:386–390. https://doi.org/10.1002/1097-0142(19800715)46:2%3c386::aid-cncr2820460229%3e3.0.co;2-9
Fakih AR, Rao RS, Borges AM, Patel AR (1989) Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg 158:309–313. https://doi.org/10.1016/0002-9610(89)90122-0
Kligerman J, Lima RA, Soares JR et al (1994) Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg 168:391–394. https://doi.org/10.1016/s0002-9610(05)80082-0
Yuen AP-W, Ho CM, Chow TL et al (2009) Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck 31:765–772. https://doi.org/10.1002/hed.21033
D’Cruz AK, Vaish R, Kapre N et al (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. New England J Med 373:521–529. https://doi.org/10.1056/NEJMoa1506007
Hutchison IL, Ridout F, Cheung SMY et al (2019) Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort. British J Cancer 121:827–836. https://doi.org/10.1038/s41416-019-0587-2
Acknowledgements
Authors declare that there is no conflict of interest.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the conception and design of the work, analysis, and interpretation of data. SG drafted the manuscript. AK, SM and RS revised it critically for important intellectual content. All the authors approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Ghai, S., Mhaske, S. et al. Elective Neck Dissection Versus Therapeutic Neck Dissection in Clinically Node-Negative Early Stage Oral Cancer: A Meta-analysis of Randomized Controlled Trials. J. Maxillofac. Oral Surg. 21, 340–349 (2022). https://doi.org/10.1007/s12663-021-01677-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-021-01677-z