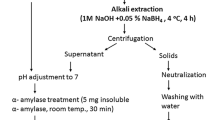
Abstract
Purpose
This paper aims to assess the potential of basidiomycete fungi to mycoremediate brewery wastewater and generate a bioactive molecule (β-glucan) for industrial applications.
Methods
Six basidiomycete fungi, Ganoderma applanatum, Ganoderma lipsiense, Pleurotus ostreatus, Pycnoporus sanguineus, Lentinula edodes, and Oudemansiela canarii, were grown in submerged fermentation using brewery wastewater (BW). β-glucan production, biomass concentration, reducing sugar content, and pH were evaluated and the fungus with the highest β-glucan production was subjected to a kinetic study of β-glucan production.
Results
Results showed that BW has important nutrients for fungi growth and all species had high biomass production. The highest production of β-glucans was for G. lipsiense (23.87%) and its kinetic study showed the highest production of β-glucans at 14 days and the greatest increase in biomass at 21 days. There was a correlation between the production of β-glucans and the consumption of BW substrate and a decrease in chemical oxygen demand (81% at 21 days), nitrate (< 3.00 mg L−1), total phosphorus (66.326 mg L−1), and total dissolved solids (634.1 mg L−1).
Conclusion
This study highlighted a sustainable use of BW for its remediation besides fungal biomass production as a source of a high-value product for the biotechnology industry, opening prospects in the circular bioeconomy.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of novelty
The novelty of this investigation was to use basidiomycete fungi for mycoremediation of brewery wastewater and concurrently study the potential of β-glucans production and its kinetics aiming for the biotechnology application.
Introduction
Beer is the fifth most consumed beverage globally, being an important contributor to the world economy [1, 2]. The beer sector has grown significantly over the last half-decade and the brewers’ impact on the global economy is estimated to have accounted for around $251 billion [3]. Brazil is the fourth main country in beer sales (around $ 23,652 million in 2019) among 70 countries, being behind the US, China, and Germany [3], and represents an important role in beer exportation (around 241 million kg in 2021) [4].
Nevertheless, with the increase and development of the brewery industry, the brewery waste streams also increase [5] and wastewater is one of the most significant waste products of brewery operations [6]. It is estimated that for each liter of beer produced, 4.5–10 L of brewery wastewater (BW) are generated from the beer production chain’s unitary operations [2], such as filtration, fermentation, malt mashing and boiling, equipment discharges, and cleaning [2, 5].
Even though BW is not toxic and does not usually contain high quantities of heavy metals, it contains biological contaminants (0.7–2.1 kg of BOD per barrel) [6] and presents high amount of organic matter, nitrogen, and phosphorus, which represent a polluting load and could cause environmental problems if do not receive the adequate destination [7]. The BW can pollute the receiving water bodies because of the organic substance’s abundance, that need to be removed before discharge into the environment. There are some effective physical, chemical, biological, and advanced treatment methods for this removal [1]. However, waste management is usually a substantial cost factor, especially for micro-breweries, creating a need for innovative solutions [7].
The BW contains residual amounts of raw materials, including suspended solids, sugars, yeasts [2], and high nutrient levels [5]. Therefore, a sustainable alternative to the reuse of brewing effluents could be through biotechnology processes using basidiomycete fungi [8], especially the white-rot fungi (WRF), once they perform an essential role in organic matter decomposition, assisting the nutrient exchange and recycling [9]. Basidiomycete fungi tend to degrade a vast number of contaminants [10,11,12,13], including the agricultural, industrial, and agrochemical [8, 14], as well as assist the biological effluents treatment through enzymatic mechanisms and biotransformation [15,16,17].
The biotransformation of pollutants and detoxification of soils and wastewater using fungi as conversion agents is known as mycoremediation [18, 19]. While this method is currently employed for investigating the detoxification of wastewater contaminated with recalcitrant substances like pharmaceuticals, endocrine disruptors [20, 21], and chlorinated compounds [8], the utilization of WRF in bioremediation processes within the field of environmental sanitation remains largely unexplored [8].
Additionally, fungi present high biotechnological interest and can act on bioconversion of molecules of commercial interest, such as the β-glucans, whose biological activities have been attracting constant interest [22,23,24], and research has been focusing on medicinal properties from these polysaccharides [25, 26]. The fungi glucans are pharmacologically classified as biological response modifiers [27] since the β-1,3-glucans stimulate the immunological system, presenting biological activities such as anti-tumor, antiviral, antibacterial [25], and anti-inflammatory [28]. These compounds also demonstrate to promote a lower elevation of the glycemic curve [23, 24] and immunomodulatory and pulmonary cytoprotective effects that may have positive relevance to SARS-CoV-2 therapeutics [29] and chemotherapy [30].
A previous study demonstrated the ability of G. lipsiense e G. applanatum to grow and produce β-glucans in solid media containing wastewater from the centrifugation process of Pilsen beer production [31]. Therefore, this study aims to assess the potential of edible and medicinal basidiomycete fungi as mycoremediator agents of wastewater from the centrifugation process of Pilsen beer and their influence on β-glucans production. This process focuses on reusing and aggregating value to the brewing wastewater and generating molecules commercially important to the biotechnology industry.
Material and Methods
The fungi cultivation was carried out by Submerged Fermentation (SF) using the wastewater from the centrifugation process of Pilsen beer (ECP) as culture media for fungi growth and β-glucans production.
Microorganisms
The fungal strain used for SF were G. applanatum (CCIBt 2978), G. lipsiense (CCIBt 2689), P. sanguineus (CCIBt 3962), O. canarii (CCIBt 2933), L. edodes (CCIBt 2920), and P. ostreatus (CCIBt 2339), purchased from fungi Culture Collection of Botanic Institute (CCIBt), São Paulo, Brazil. First, the fungi were cultivated in sterilized Petri plates using a culture media composed of 35 g L−1 of glycose, 5 g L−1 of peptone, 2.5 g L−1 of yeast extract, 0.883 g L−1 of KH2PO4, 0.5 g L−1 of MgSO4∙7H2O, 20 g L−1 of agar, and distilled water. The Petri plates were incubated at 27 ± 1 °C until complete radial growth and it was posteriorly used for the SF.
Beer Process Wastewater
The ECP used as culture media for SF was collected in an artisanal brewery of Blumenau (Santa Catarina, Brazil) at the end of the centrifugation process. The ECP was previously chemically characterized by the Laboratory of Water and Wastewater Analyses of SENAI Blumenau (Santa Catarina, Brazil) based on the Standard Methods for the Examination of Water and Wastewater [32]. The analyzed parameters were arsenic, barium, boron, cadmium, lead, dissolved copper, real color, hexavalent chromium, trivalent chromium, BOD, COD, total dissolved solids, tin, phenol, dissolved iron, fluorides, total phosphorus, dissolved manganese, mercury, nickel, nitrate, nitrite, and ammoniacal nitrogen, as well as pH and turbidity, determined according to Resolution 430 of May 13th, 2011, of the Nacional Council of Environment [33].
Submerged Fermentation
The fungi mycelial SF was performed in a static state, using cylindrical glass jars (500 ml) containing 100 ml of culture media and covered by metallic covers with an orifice containing filter paper to gas exchanges. The glass jars containing the culture media were autoclaved at 121 °C and 1 atm for 15 min, cooled and then inoculated. The inoculation of each fungus was carried out by grinding a fungi Petri plate in 120 ml of distilled water, using a sterilized laboratory grinder, and adding 20 ml of this suspension in each glass jar. The SF was performed in ECP media and also in a synthetic culture media (SM), adapted from Tang and Zhong [34], composed of glucose (35 g L−1), peptone (5 g L−1), yeast extract (2.5 g L−1), monopotassium phosphate (0.883 g L−1), heptahydrate magnesium sulfate (0.5 g L−1), and distilled water, with pH 5.5 to the SM.
The SF process was conducted in a microbiological incubator at 27 ± 1 °C for 40 days to select the fungus that presents the highest tendency to β–glucans production among the studied species. In addition, two culture systems were used for ECP media, named simple batch (ECP_SB) and feed batch (ECP_FB). The simple batch corresponds to a closed and discontinuous system where inoculation and incubation occur without supplementation of cultivation media until the end of the incubation period. The feed batch corresponds to a discontinuous system with supplementation of culture media during the incubation period due the medium adsorption by the fungi growing biomass, reducing the 15–17% of the medium from the initial volume. Both SM and the ECP for both simple and feed batches, without the inoculation of fungi, were considered blank cultivation (control).
Submerged Fermentation Analysis
pH and Fungi Biomass Dry Weight
Initial and final pH analyses were performed in triplicate for all cultivation through the potentiometric method, using a bench pHmeter (Tecnal) [35]. The fungi biomass dry weight (BDW) for each submerged culture was quantified after the incubation period. The submerged media was filtered using a paper filter Whatman n.1 and the fungal mycelia were dried in a drying oven with mechanical air circulation (Tecnal TE-324/1) at 55 °C until constant mass was obtained (2 h). The biomass dry weight was obtained with the mean of five mycelial dry weights and the results were expressed in grams (g).
Total Reducing Sugar Content
The total reducing sugar content (TRS) was determined in each submerged culture medium: SM and ECP_SB at 0 and 40 days of incubation, ECP_FB (bs) before media supplementation, and ECP_FB (as) after media supplementation (0 and 40 days). The TRS analysis was performed using the 3,5-dinitro salicylic acid (DNSA) method, according to Miller [36]. Briefly, DNSA reagent (1% of 3,5-dinitro salicylic acid and 30% of double sodium and potassium tartrate in 0.4 mol L−1 of NaOH) was added in test tubes containing 1.0 ml of sample and kept at a boiling water bath for 5 min. After cooling, 7.5 ml of distilled water was added to the solution and the absorbance was measured at 540 nm, using a UV–VIS spectrophotometer (Shimadzu UV–Vis-1650 PC). The reducing sugar content was calculated from the calibration curve of ten dilutions of standard D-glucose (Anidrol) (1.8–0.18 g L−1), and the results were expressed as g L−1 of D-glucose. Sugar consumption was also considered as substrate consumption [37,38,39,40,41].
Determination of β-glucan Content
The mycelial dried samples were ground in a laboratory mixer and then submitted to the determination of β-glucan content by an enzymatic colorimetric method, using an Enzymatic Mushroom and Yeast β-Glucan Assay Kit® (K-YBGL) (Megazyme®; IDA Business Park, Bray, Wicklow, Ireland). Total glucans and α-glucans were determined and calculated through the protocol recommended by the K-YBGL manufacturer and its difference was considered as the β-glucans fraction. The fungus that presented the highest tendency to β–glucans production in ECP media was cultivated again to study the β-glucan production kinetics.
β-glucan Production Kinetics Analysis
To evaluate the fungal behavior during the incubation period, it was established a kinetics analysis of β–glucans production, biomass growth, and substrate consumption. The fungus capacity of effluent contaminants’ reduction was also evaluated through Chemical Oxygen Demand (COD) analysis. This parameter was also compared to β–glucans production to verify the capacity of the ECP bioconversion in a product with β–glucans’ high content.
The SF was conducted as previously described, applying the simple batch and the selected fungus, and incubating at 27 ± 1 °C for 42 days [42]. The β–glucans content, fungus BDW, TRS, and COD were weekly analyzed, from the initial time until the end time of incubation (0, 7, 14, 21, 28, 35, and 42 days). Thirty-five experiments (cylindrical glass jars, fungi and substrate) were prepared and in the zero time. Every 7 days, five glass jars were selected and analyzed for β–glucans content, fungus BDW, TRS, and COD.
The specific growth rate (µx), substrate consumption (µs), and product formation (β-glucans production) (µp) were calculated according to Costa et al. [43] and modified and Bettin et al. [40]. The specific growth rate was obtained from fungi biomass dry weight in grams (g). A polynomial kinetic model was used for the experimental data fitting to obtain an equation relative to time. The µb \({\mu }_{r}\), µs, and µp were obtained by multiplying the inverse of this function by its first derivative. All experiments were running in five repetitions.
Finally, biomass substrate yield was calculated by the relationship Yx/S = (dX/dt)/(− dS/dt), where dX/dt is the first derivative of the fungi biomass dry growth; − dS/dt is the first derivative of the substrate consumption. β–glucans substrate yield was calculated by the relationship YP/S = (dP/dt)/(− dS/dt), where dP/dt is the first derivative of the β–glucans production; − dS/dt is the first derivative of the substrate consumption.
Remediation of BW
After 42 days-incubation period, a sample of ECP media was collected and analyzed based on the Standard Methods for the Examination of Water and Wastewater [32], to compare with the initial BW characteristics (item 2.2) and verify the capacity of fungi as a BW remediator.
Statistical Analysis
Statistical analysis of all data obtained was performed through Analysis of variance (ANOVA) and significance levels were set at 95% using the Tukey test (p < 0.05).
Results and Discussion
Beer Process Wastewater Characteristics
The ECP appeared to be cloudy and yellowish, and its chemical characterization is shown in Table 1.
The chemical composition of BW varies mainly according to the operational conditions, fabrication processes, production method, beer type, and system management [1, 44]. Levels of the most important water quality parameters in BW have been reported in literature to be: 1250–126366 mg L−1 for COD [2, 5, 6, 45,46,47], 1200–18000 mg L−1 O2 for BOD [2, 45], 1515.90 mg L−1 for total organic carbon (TOC) [45], 24–451 mg L−1 for total nitrogen, 5–44.33 mg L−1 for NH3–N [2, 5, 6, 46, 47], 0.50 MgNO3- mg L−1 for nitrate [45], 33.25 mg L−1 of nitrate nitrogen (NO3–N), < 0.1 mg L−1 for nitrite nitrogen (NO2–N) [47], 0.5–600 mg L−1 for total phosphorus [2, 5, 45,46,47], 10–50 mg L−1 for phosphates [5, 6], 8.6 mg L−1 orthophosphate (PO43–P) [47], 240.68 MgSO4- mg L−1 for sulfate [45], 190–3170 mg L−1 CaCO3 for Al [2], 500 mg L−1 for suspended solid (SS) [6], 3232 mg L−1 for dissolved solids [45], 63.62 mg L−1 for total solids (TS), and 0.264 mg L−1 for volatile solids (VS) [46], 160 NTU (Nephelometric Turbidity Units: 3 NTU = 1 ppm SiO2) for turbidity [45], and pH 4.5–12 [2, 5, 6, 46,47,48].
BW is considered a highly pollutant due to the high levels of organic carbon and other pollutants [1]. Organic compounds in BW are mainly from sugar, soluble starch, ethanol, and volatile fatty acids, for example, and their removal is important for the prevention of the creation of anaerobic conditions in the receiving water bodies [49]. Nevertheless, these characteristics are favorable to fungi development. A decisive factor for fungi mycelial growth is the presence and equilibrium of carbon, nitrogen, phosphorus, vitamins, and micronutrients in the culture media [50, 51]. The main carbon source in the ECP can be found as organic carbon due to the high organic charge and can be measured by the chemical oxygen demand (COD) or/and the biological oxygen demand (BOD) [6]. These parameters can vary in brewery wastewaters according to the fabrication process and the presence of ethanol, glycerol, and remaining carbohydrates, and can reach around 170,000 mg L−1 [44]. ECP presented initial COD and BOD high contents (Table 1), being a carbon source for fungi development.
Nitrogen (N) and phosphorus (P) concentrations mainly vary according to the raw materials and yeast leftover amount in the BW [1, 48] and come from malt, adjuncts, the amount of yeast discharged (nitrogen), and the used cleaning agents (nitrogen and phosphorous) [6]. Nitrogen is a critical element to cell growth and performance [52] and the nitrogen content presents favorable effects on fungi development [53, 54], being reported as 16.4–280 mg L−1 TN–N in BW [1]. Phosphorus is an essential element for life, being part of DNA and RNA molecules and participating in the reproduction and transmission processes of genetic characters. Moreover, fungi require it for nucleic acid and phospholipids synthesis [55]. ECP presented ammoniacal, nitrate, and nitrite, which can act as nitrogen sources for fungi development, and phosphorus content (Table 1), and did not exceed the concentration that could promote negative effect [56].
Other important parameters for fungi development were evaluated in the current study, such as dissolved manganese, dissolved copper, dissolved iron, boron, and nickel (Table 1). These elements are also required for fungi development, once they promote the cells’ communications (boron), cell breath (copper), nitrogenases, peroxidases, and cytochromes formation (iron), enzymes (manganese) and hydrogenases activation (nickel), for example [57]. Nickel and chromium can be from wear on machines, especially conveyors in the packaging line [6].
It is important to provide essential macro and micronutrients for the proper fungi growth [50, 51], and ECP seems to be promising to basidiomycete fungi to favor the β-glucans synthesis. The β-glucans synthesis has many influence factors, and its content is related to the substrate used in fungi cultivation [58]. For basidiomycete fungi, other factors that have great importance on β-glucans production are the mushroom species, the degree of basidiocarp maturation, the cultivation conditions [59], nitrogen and carbon sources, and their concentrations present on the cultivation substrate [60], for example.
A previous study [31] evaluated the radial mycelial growth rate (Vm) and β-glucans production by G. lipsiense and G. applanatum cultivated in Petri dishes (13 days) containing ECP as culture media, using three concentration levels (100, 75, and 50%). All treatments presented mycelial growth and β-glucans production, with the highest values of Vm in the treatment with 100% of ECP (4.48 mm day−1 to G. applanatum, and 3.90 mm day−1 to G. lipsiense). The β-glucans content was high for G. lipsiense (30.28% for treatment 50%, and 32% for treatment 100%) while G. applanatum presented the lowest values for all treatments (< 20%). These results showed that culture media and the fungus species influence the bio compounds and suggest that ECP can be recovered as a substrate for basidiomycete fungi growth and production of compounds that are of great interest to the pharmaceutical and food industry, such as the β-glucans [31].
Submerged Fermentation
The wastewater from the centrifugation process of Pilsen beer had the capability as culture media for the growth of the six studied basidiomycete fungi species. Table 2 summarizes the results of initial and final pH values of culture media and fungi biomass dry weight after 40 days of incubation of each treatment.
As can be seen from Table 2, SM cultures of G. lipsiense, G. applanatum, P. sanguineus, and L. edodes presented a media acidification behavior, with a statistical difference of initial and final pH for all species, besides P. ostreatus that did not present statistical difference comparing initial and final pH values (p ≤ 0.05). This pH decrease may indicate that fungi liberated acid compounds during metabolism and growth. When microorganisms are in the actively growing stage, the media pH is altered as long as acid or alkali compounds are produced [61]. Only the O. canarii culture showed a different pH behavior, with the pH values increasing and presenting a statistical difference (p ≤ 0.05).
ECP_SB and ECP_FB cultures showed the lowest variation pH than SM cultures. It was observed that G. lipsiense culture presented a slight pH increase for ECP_SB, statistically different from initial pH (p ≤ 0.05), while G. applanatum, P. sanguineus, and L. edodes turned the media more acid, comparing initial and final pH values, presenting significant difference (p ≤ 0.05) for ECP_SB. On the other hand, O. canarii and P. ostreatus cultures did not present significant differences between initial and final pH for ECP_SB media. ECP_FB cultures presented similar behavior for G. applanatum and L. edodes, with a pH decrease (p ≤ 0.05), although the other species presented different behavior. There was a pH increase in ECP_FB for O. canarii and P. ostreatus, while G. lipsiense and P. sanguineus presented no significant difference (p ≤ 0.05) between initial and final pH values. These pH differences among initial and final pH values occur because some basidiomycetes fungi species can autoregulate pH, making the pH media stabilized for optimum growth regardless of the initial pH value [62]. In addition, the maintenance of pH values results in better enzymatic responses, which prevent dehydration of hyphae and promote regulation of extracellular glucose concentration [63].
According to Table 2, the mycelial biomass production on SM cultures varied between 0.036 to 0.067 mg L−1, with the highest values for G. lipsiense and O. canarii, which did not present a significant difference between them (p ≤ 0.05), and the lowest values for L. edodes. Moreover, for ECP_SB and ECP_FB cultures, the highest mycelial biomass production was both for O. canarii, differing by the other species (p ≤ 0.05), while the lowest biomass values were obtained for G. applanatum, P. sanguineus, and L. edodes for ECP_SB, and L. edodes for ECP_FB. The variation in biomass production among the different fungi species studied highlights the difference in nutritional needs and adaptation of each fungus species [7].
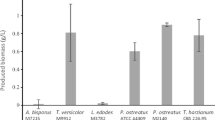
Hultberg and Bodin [7] studied fungal biomass production (T. versicolor, P. ostreatus, and T. harzianum) cultivated in synthetic brewery wastewater. The highest biomass production after 7 days of growth was for P. ostreatus (1.78 ± 0.31 g L−1 of dry weight). Also, initial pH significantly decreased for T. versicolor and T. harzianum cultures (4.2 ± 0.3 and 4.4 ± 0.5, respectively) while there were no significant effects on initial pH (6.4 ± 0.4) for P. ostreatus culture [7].
Substrate consumption is a determining factor for the increase in mycelial biomass by fungi. Gern [64] verified that substrate consumption increased proportionally to the biomass concentration increase, affected by the glucose concentration present in the culture media. Figure 1 shows the total reducing sugar content of each treatment, which is proportional to the glucose content or substrate consumption by fungi. The reducing sugar consumption in SM, ECP_SB, and ECP_FB cultures did not differ among G. lipsiense, G. applanatum, P. ostreatus, P. sanguineus, and O. canarii, and just L. edodes showed significant difference from these other species (p ≤ 0.05) but with no difference between control media. Among different culture media (SM, ECP_SB, and ECP_FB), G. lipsiense, P. ostreatus, and L. edodes did not show a significant difference in reducing sugar consumption, and P. sanguineus presented the opposite behavior. Although G. applanatum differed significantly between SM and ECP (both SB and FB), there was no significant difference between ECP_SB and ECP_FB. In addition, O. canarii reducing sugar consumption was different between SM and ECP_BA, but there was no difference comparing SM and ECP_SB and ECP_SM and ECP_FB.
Total reducing sugar content of each treatment. SM = synthetic culture media; ECP_SB = effluent media in the simple batch; ECP_FB (bs) = effluent media in feed batch before supplementation; ECP_FB (as) = effluent media in feed batch after supplementation; Control media = culture media with no fungi inoculation; The total reducing sugar content (TRS) was determined in control media and in each submerged culture: SM and ECP_SB at 40 days of incubation, ECP_FB (bs) before media supplementation, and ECP_FB (as) after media supplementation (40 days). * Lowercase letters indicate the results of comparisons between treatments. Uppercase letters indicate the results of comparisons between fungi species. Equal letters indicate means that did not differ from each other (Tukey, p ≤ 0.05).
The ECP supplementation (ECP_FB) in the feed batch system occurred when the reduction of culture media achieved 15–17% from the initial volume. Therefore, it occurred for G. lipsiense and P. sanguineus at 26 incubation days, while for the other species the supplementation occurred at 32 incubation days. The sugar content before and after the supplementation did not differ for G. lipsiense and L. edodes but differed for the other species (p ≤ 0.05).
In summary, the lowest and the highest total reducing sugar values were, respectively, 0.06 g L−1 (O. canarii) and 2 g L−1 (L. edodes) for SM; 0.04 g L−1 (G. lipsiense) and 1.47 g L−1 (L. edodes) for ECP_SB; 0.04 g L−1 (P. sanguineus); 1.62 g L−1 (L. edodes) for ECP_FB before supplementation, and 0.12 g L−1 (P. sanguineus) and 1.71 g L−1 (L. edodes) for ECP_FB after supplementation.
Total sugars are one of the most important variables to evaluate in terms of growth and nutrient removal with a microorganism system. The differences in sugar consumption among the species might rely on the media sugar profile or because of rich media use. Some microorganisms show different affinity levels to maltose, glucose, galactose, and fructose, needing different retention times to consume the sugars on the substrate [45]. This carbohydrate control is important once it can show the amount of sugar that was not fermented by the fungi, reducing the process efficiency and/or changing the product quality [65].
The grown fungi mycelium can be recovered and used as a source of income, such as an ingredient for animal feed due to the high protein content, a biomaterials component because of the high content of chitin, a source of enzymes such as laccase [5, 7], as well as a source for extraction of bioactive molecules, such as the β-glucans, as proposed in the current study. Figure 2 provides the β-glucans content of each fungi mycelium, after 40 days of culture incubation for each culture media.
β-glucans content of each fungi mycelium after 40 days of culture incubation. SM = synthetic culture media; ECP_SB = effluent media in the simple batch; ECP_FB = effluent media in the feed batch. * Lowercase letters indicate the results of comparisons between treatments. Uppercase letters indicate the results of comparisons between fungi species. Equal letters indicate means that did not differ from each other (Tukey, p ≤ 0.05).
It is apparent from Fig. 2 that SM cultures have no differences in β-glucans production between the studied species (25.60–30.62%). However, for ECP_SB, G. lipsiense and L. edodes presented the highest β-glucans content (23.87 and 23.68%, respectively), while P. ostreatus presented the lowest content (13.89%). The β-glucans production was similar for ECP_BS, which resulted in the highest value for G. lipsiense (22.00%) and the lowest value for P. ostreatus (12.98%). These results allowed us to infer that the lowest values of biomass production and sugar consumption do not necessarily result in low β-glucans production, once L. edodes and G. lipsiense presented high content of β-glucans but the lowest values for mycelial biomass production and sugar consumption. In the same way, low β-glucans production by P. ostreatus generated high biomass production.
Comparing the three studied culture media (SM, ECP_SB, and ECP_FB), the species G. lipsiense, G. applanatum, P. ostreatus, and O. canarii did not show significant differences regarding β-glucans production, while P. sanguineus and L. edodes showed significant differences among culture media. β-glucans production by P. sanguineus cultivated in SM was different from the cultures of both ECP_SB and ECP_FB media, which were similar between them. L. edodes β-glucans content was significantly different among the culture media, but not differing between SM and ECP_SB and between ECP_SB and ECP_FB.
The β-glucans content can range from 1.58 to 56.28% for Agaricus, Flammulina, Lentinula, and Pleurotus genres, for example [22, 25, 66,67,68,69,70,71]. According to Mahapatra and Banerjee [72], the β-glucans production by fungi depends mainly on the strain and the culture media composition, as well as physical conditions. Also, β-glucans content depends on the maturation degree, dietary fiber content [26], microorganism behavior, and incubation conditions [73]. Therefore, the β-glucans production resulting in the current study seems to be associated with the individual characteristics of each species during the growth and the BW degradation.
Fungal β-glucans are known as immunostimulant and immunosuppressive molecules [74], presenting an effect of inducing innate and adaptive immune responses in the human body [75]. In addition to immunomodulatory activity [25], these molecules present bioactive properties, such as antioxidant, neuroprotective [71], hepatoprotective, antimicrobial, anti-allergic, anti-inflammatory [76], anti-atherogenic, anti-diabetic, cardiovascular, radioprotective [26], anti-cancer [74] activities. Therefore, β-glucans have a great potential for application in the biotechnology industry, such as pharmaceuticals, nutraceutical products, and functional foods [77].
Although several techniques of β-glucans extraction and its analysis were already described, the majority of fungi cultivation processes are conducted by solid-state fermentation systems using conventional substrates, such as wood, or agro-industrial wastes, or by submerged fermentation system, using synthetic liquid substrates for mycelium growth [59, 78]. BW has been reported as a low-cost substrate for organisms cultivation in submerged fermentation, reducing the culture medium cost and the environmental pollution, besides allowing to obtain high content of value compounds [48, 79], as well as contributing to the circular economy [48]. However, there was no identified evidence of submerged-fermentation processes using BW for fungi β-glucans extraction and application in industrial biotechnology.
Some authors studied the use of BW for microorganism growth and molecule production. Giraldo et al. [45] studied the transformation of brewery subproducts into valuable biomass using Chlorella pyrenoidosa and associated bacteria. Depending on operation conditions, total carbohydrate depuration ranged from 50–80% [45]. Wang et al. [46] studied the cultivation of the microalga Chlorella sp. in brewery wastewater and investigate the potential value of biomass obtained as a bioproduct. For the treatment of 100% BW as cultivation media, the biomass production reached 1.96 g L−1, and the biomass presented maximum lipid, carbohydrate, and protein contents of 36.19, 36.51, and 19.77%, respectively [46]. Sheela et al. [79] studied amylase production by Aspergillus niger and Penicillium species by submerged cultivation using brewery wastewater as a substrate [79]. Dias et al. [48] reviewed different brewery wastewater treatment strategies using oleaginous yeast and microalga in pure and mixed cultures, aiming the lipids and carotenoid production [48]. Hultberg and Bodin [5] evaluated the amount and the quality of the fungal biomass produced, resulting in a total biomass amount of 13.2 g L−1 of P. ostreatus and a protein concentration of 11.6% [5].
In the same way, Morais et al. [2] reported recovered resources from some other technologies applied for BW treatment, such as Hydrogen (H2) and energy for batch anaerobic reactor and immobilized-cells continuously stirred tank reactors (immobilized-cells CSTR); methane (CH4) as biogas for up-flow anaerobic sludge blanket (UASB) reactor, anaerobic membrane bioreactor (AnMBR), and UASB reactor; algal biomass, bacterial biomass, and derived products (proteins, polysaccharides, bacteriochlorophyll, and coenzyme Q10), for photosynthetic bacterial-membrane bioreactor (PS-MBR); and carboxylic acids (CAs) for a batch anaerobic reactor.
β-glucan Production Kinetics
Ganoderma lipsiense was selected for kinetics analysis of the β-glucan production on this species showing the highest tendency to β-glucans production in ECP media and high mycelial biomass production. Additionally, studies using this fungus for wastewater remediation and β-glucans production seem to be rare. Moreover, Costa et al. [80] highlighted the great potential of G. lipsiense for the production of medicinal compounds, being promising for bioactive molecules research.
The variation of pH values ranged from 4.75 to 5.85 during the incubation period (42 days) when there was an adjustment or autoregulation of the media pH. During the first part of the period (initial time until the 21st incubation day), it can be seen an acidification behavior (4.75b ± 0.030 to 3.92c ± 0.066), and during the second part of the incubation period (from the 21st day until the 42nd day) there was a pH increasing (3.92c ± 0.066 to 5.85a ± 0.349), which might be related to the cellular autolysis [80]. Similar pH values to G. lipisiense culture (4.4–6.07) were obtained by Costa [80].
Although the kinetics of microorganisms’ growth is already studied, it is necessary to improve the models to use in reactor projects. There are some difficulties in technological applications in terms of heat and mass transfer to control microorganisms’ growth conditions, as well as reactor modeling, once most biological reactors are constructed under empirical conditions [81]. Commonly, the microorganisms’ kinetics are expressed as a first-order kinetics or a non-structured empirical model and based on experimental observations. Because of these conditions, the equations might present a complete growth curve in one equation, including the lag, exponential, and stationary phases together [82]. Therefore, it is important to study the kinetics parameters of microorganism growth to describe more efficiently the studied fungi in terms of biomass growth, substrate consumption, and specific product formation. Table 3 shows the general results obtained for kinetics parameters of G. lipsiense cultivation in ECP_SB culture media.
Figure 3 compares the relationship between the β-glucans content (%) and (A) the consumption of Chemical Oxygen Demand (COD) (mgO2 L−1), (B) the total reducing sugars content (g L−1), and (C) the fungus biomass dry weight (g), during the kinetics study of G. lipsiense in ECP_SB culture media.
From Fig. 3A, it can be seen the COD behavior during the incubation period. There was a reduction of COD from the initial day to 28 days (from 26,576.00a ± 1952.80 to 2786.67c ± 324.33 mgO2 L−1). After the 28th day, the COD values were maintained almost constant (up to 2478.67c ± 243.33 mgO2 L−1). There was a slight decrease in COD values in the first 7 days (around 13% from the initial value; up to 23,048.67a ± 1843.85 mgO2 L−1) and a significant decrease between the 7th and 14th days (around 61% from the initial value; up to 10,230.00b ± 3841.63 mgO2 L−1). On the 21st day, there was a reduction in COD values of 81% (4942.67c ± 854.70 mgO2 L−1), followed by a media color change, suggesting that G. lipsiense might have a positive response to the studied wastewater and the reduction capacity of organic charge.
Kurcz et al. [83] obtained 91% of COD reduction by Candida utilis cultivated in a supplemented potato wastewater, while Souza Filho [84] cultivated Aspergillus niger using manioc processing wastewater and obtained 91.19% of COD reduction, highlighting the fungi potential of COD reduction from wastewaters. COD is, also, an important parameter due to its influence on biomass production and concentration. High nutrient concentration and removal rates lead a high biomass production [7]. In the current study, it was also observed that G. lipsiense culture in ECP presented an inversely proportional relationship between COD reduction and β-glucans production. This behavior might be because of basidiomycetes fungi β-glucans are one of the main cellular wall compounds [70], fungal exopolysaccharides that are produced during the stationary phase of microbial growth and considered secondary metabolites [85].
The reducing sugar content profile (Fig. 3B) during the incubation period (42 days) was observed. The lowest values of reducing sugar consumption were obtained until the 7th cultivation day (1.00a ± 0.078 g L−1), when the specific sugar consumption (µs) (0.1825 g day−1) and β–glucans production (µp) (3.027% day−1) presented the maximum values (Table 3). After 7 days, it can be observed expressive sugar consumption until the 14th day (0.44b ± 0.088 g L−1), which subsequently decreased, achieving the lowest values on the 42nd cultivation day (0.05c ± 0.023 g L−1). There is a sharp decline of reducing sugars on the 14th incubation day (from 1.00a ± 0.078 to 0.44b ± 0.088 g L−1), which is associated with its consumption by the fungus, which occurs in the exponential growth phase. After that, there is a stabilization period of the reducing sugar concentration (14th until 28th days; 0.44b ± 0.088, 0.41b ± 0.316, and 0.40b ± 0.335 g L−1), associated with the stationary growth phase, when the de β-glucans are also stably synthesized. In addition, the highest yields of the substrate on biomass Yx/S (2.9612 g g−1) and yields of the substrate on product YP/S (19.410% g−1) are observed (Table 3) during this period (14 days).
In Fig. 3C there is a clear trend of the different phases of a microbial growth curve. Initially, it can be identified the lag phase (around 5 days), then, there is the exponential phase until the 17th day, with maximum specific growth rate (0.4099 day−1) (Table 3) on the 7th day and, finally, the stationary phase, until the final day (42 days). Figure 3C shows, also, the β-glucans behavior during the incubation period. The β-glucans content was 34.1% between the 0 and 14th day (from 36.89b ± 0.309 to 48.48a ± 1.245%), with significant differences (48.48%) during the exponential growth phase. Subsequently, there was a decrease of 0.5% between the 14th and 28th days (up to 48.25a ± 0.654), but also maintaining the highest values in the stationary phase. After 35 days of incubation, there was a decrease in β-glucans production (up to 32.65c ± 4.479%), which occurred, probably, because G. lipsiense needed to use its energy supply for survival when nutrients in the culture media were insufficient [86] or there was a release of enzymes that are involved in the β-glucans degradation, named β-glucanases, during the stationary phase [87]. Finally, it can be inferred that it is not necessary to maintain a long incubation period of G. lipsiense for β-glucans production, once the lowest β-glucans content was observed on the 42nd incubation day (32.65c ± 4.479%), and the maximum production occurred between the 14th and the 28th incubation days (48.48a ± 1.245 and 48.25a ± 0.654%).
Also, the β-glucans production increased while the biomass production also increased (21st day; 46.74a ± 0.146% and 0.52a ± 0.116 g, respectively) and it was stable until the 35th day (47.90a ± 1.422%) (Fig. 3C), which represents the stationary phase of the fungus growth. During the stationary phase (from 28 days on), there is also a substantial decrease in reducing sugars (from 35th to 42nd days; 0.03b ± 0.011 and 0.05b ± 0.023 g L−1) and an increase in β-glucans synthesis (42nd day; 32.65c ± 0.000%). This behavior suggests that from 28 days of incubation, there is a necessity for reducing sugars from the culture media by G. lipsiense to its maintenance on the stationary phase. It can be hypothesized that the increase of reducing sugars (35 days) induces the fungus to start to consume the synthesized β-glucans to survive until the 42nd incubation day. The production of polysaccharides such as β-glucans by fungi seems to depend on the type of carbon source and its concentration in the culture media [88]. Smits et al. [89], in a review of yeast cell wall synthesis, report that changes in cell wall metabolism and polysaccharide composition occur under stress conditions. Stress signals, such as nutrient limitation, and osmotic or temperature stress, activate a regulatory subunit of glucan synthase, increasing the production of wall glucans [89].
In this study, reducing sugar consumption during incubation was found to be 60% in the period from 0 to 14 days (from 1.09a ± 0.072 to 0.44b ± 0.088 g L−1) and just over 10% in the period from 14 to 28 days (up to 0.40b ± 0.335 g L−1). Regarding the fungal biomass, there was a formation of 12 times the initial mass in the period from 0 to 14 days (from 0.03c ± 0.010 to 0.39b ± 0.030 g) and slightly above 10% in the period from 14 to 28 days (up to 0.43ab ± 0.025 g). The highest biomass increments were observed between the 7 and 14th days and then, after this period, it was stabilized. Serbent [14] observed similar behavior when Lentinus crinitus growth kinetics was studied to promote the degradation of the 2,4D herbicide. The reducing sugar consumption was inversely proportional to the biomass increase: while sugar availability decreased, biomass remained stable. Furthermore, Hultberg and Bodin [7] studied a fungi‑based treatment of real brewery waste streams and monitored biomass production over time. No significant differences were observed on days 3 and 6, and the maximum biomass yield was obtained on day 10 (1.78 g L−1) [7]. In addition, Eliopoulos et al. [37] observed the same behavior for β-glucans and sugar consumption obtained in the current study during a solid-state fermentation (SSF) process using brewers’ spent grain (BSG) as a substrate for P. ostreatus cultivation. This behavior occurs because fungi consume fermentable sugars as an energy source for their growth, and enzymes such as cellulases and glucosidases result in reducing sugar release [37].
BW Remediation
The chemical characteristics and effluent discharge limits from the brewery process depend on local environmental legislation [49]. White-rot fungi can be promising candidates for the treatment of contaminated wastes [90]. Basidiomycetes fungi are known for degrading a wide range of materials, colonizing and using them as useful substrates, producing enzymes classified as hydrolytic (cellobiohydrolases, endoglucanases, β-glucosidases) and oxidative enzymes (laccases, lignin peroxidases, manganese peroxidases) which promote the degradation of different substances [11, 13, 37, 91].
The effectiveness of fungi in the removal of organic matter and nutrients from the studied BW was verified. The incomplete removal of COD under experimental conditions can indicate that the BW contains refractory organic matter or extracellular substances produced by microorganisms [46]. As can be seen from Table 1, BOD and COD concentrations decreased. These removals highlight the potential of fungi as a pretreatment of brewery wastewater and the use of organic matter for their growth. Morais et al. [2] summarized some technologies for BW treatment and reported a removal efficiency of 98% COD for anaerobic membrane bioreactor (AnMBR), 78.97% COD and 60.21% BOD for upflow anaerobic sludge blanket (UASB) reactor, 97% COD for photosynthetic bacterial-membrane bioreactor (PS-MBR), and 76% COD for a batch anaerobic reactor.
Moreover, nitrogen and phosphorus are important nutrient sources during fungi growth [46], and the decreasing/removal of nitrate and total phosphorus (Table 1), for example, could highlight this importance and the consumption of nitrogen sources by fungi [46]. On the other hand, the ammoniacal nitrogen increased (Table 1) after 42 days, and this might occur due to the cellular autolysis and excessive cultivation period [92] and the media mineralization [7]. Faria [92] verified that the reducing sugars exhaustion can lead to fungi mycelium concentrations decreasing and ammoniacal nitrogen increasing, which indicates possible cellular autolysis during a kinetics study.
In addition to ammoniacal nitrogen, there was an increase in copper and iron values (Table 1). Basidiomycete fungi have the potential to bioaccumulate metallic ions available in the culture medium, such as iron and copper, for example [93, 94]. When the cultivation system contains these ions, they can be bioaccumulated in the fungus hyphae. Furthermore, the addition of ions to the culture medium enhances their bioaccumulation, and the increased bioaccumulation boosts the solubility of iron and copper. The elevation of ions in the medium can be associated with the autolysis of the fungi hyphae. This typically occurs from the middle to the end of the cultivation period when the carbon reserves in the medium decrease [93, 94].
Other authors also identify low concentrations of COD, nitrogen, phosphorous, and derivative compounds after the incubation period of different microorganisms cultivated in BW. Hultberg and Bodin [5] studied a fungi‑based treatment of real brewery waste streams by five fungal species (Agaricus bisporus, L. edodes, P. ostreatus, T. harzianum, and T. versicolor) and the effects on water quality. Nitrogen was well removed and presented a maximum total nitrogen reduction of 91.5% for P. ostreatus and 77.0% for T. harzianum. Removal of total phosphorus was 30.8% for P. ostreatus and 16.6% for T. harzianum [5].
BW has been successfully described as feedstock for yeast and microalga presenting encouraging results [37]. Giraldo et al. [45] studied the transformation of brewery subproducts into valuable biomass using Chlorella pyrenoidosa and associated bacteria. The authors reported a maximal removal of nitrate and phosphate between 90 and 100%, respectively, after 6 days of cultivation. Wang et al. [46] reported the removal of ammonia nitrogen (NH4+-N), chemical oxygen demand, total nitrogen, and total phosphorus reached 92.44, 79.89, 92.46, and 78.26%, respectively, using the cultivation of the microalga Chlorella sp. in brewery wastewater [46]. Papadopoulos et al. [47] studied an integrated system of algal–bacterial brewery wastewater treatment process combined with bioethanol production using sludge. The pollutant removal was 78.7, 94.2, and 75.2% for d-COD, TKN, and PO4 3-P, respectively [47]. Dias et al. [48] reviewed different brewery wastewater treatment strategies using oleaginous yeast and microalga using pure and mixed cultures and the treatments reached more than 60% of COD removal, total N removals were higher than 70%, and until 73.86% ammoniacal N removal [48].
Some heavy metals in BW could be toxic for water body receptors and for micro-organisms [49], although the heavy metals concentration is normally very low [6]. The fungi in the current study seemed to be tolerant to these elements and other metals, such as arsenic, cadmium, lead, dissolved copper, chromium, tin, mercury, and nickel. In addition, pH is an important parameter to be monitored in wastewater treatment, since the solubility of many pollutants depends on the pH [7]. The pH of wastewater normally needs to be between 5.0 and 12.5, because when it is too acidic (pH < 5.0) or too alkaline (pH > 12.5), it can affect both the sewer system and the environment [95]. In the current study, the pH was established by the fungal growth, increasing from 4.37 (0 days) to 6.57 (42nd day), following the mentioned secure range. Finally, the total dissolved solids of BW need to be avoided and the turbidity needs to decrease [49]. These parameters presented a decreasing behavior in the current study comparing the initial and the final values (Table 1).
Treatment of wastewater from the brewing process is important for controlling water pollution and needs to be in a costly and safest way to meet the discharge regulations to protect life (both human and animal) and the environment [6]. Currently, breweries, mainly microbreweries, discharge the wastewater directly into municipal sewer systems or into water bodies, which can cause systems and management issues and environmental and health problems due to its high concentrations of organic matter and other nutrients [5]. Although some large-scale breweries have their wastewater treatment plant, using usually anaerobic reactors (UASB) and/or activated sludge systems. These treatment systems normally do not recycle nutrients and produce large quantities of low-value effluent, which also create disposal problems, such as failure to meet water quality standards set by local authorities and/or large amounts of low-value sludge, which is costly to dispose of [5, 7].
Biological processes usually used for BW treatments present some disadvantages. Aerobic processes demand high energy consumption and produce a high amount of sludge which raises the system operation cost. However, the anaerobic processes present high sensitivity to operational conditions and temperature variations, resulting in low performance. Also, both systems may not reach the discharge standards [1] and the adsorbents used in sorption technologies are high cost and raise the BW treatment costs [96]. Furthermore, biological treatment of BW is frequently used using bacteria-based treatment and the microalgae use for BW treatment has also been investigated. However, there are many limitations to their cultivation, such as the risk of low light transmittance because of the high amount of suspended particles in BW [7].
The nutritional composition and the high biodegradability of BW suggest that they could be a suitable medium for the cultivation of heterotrophic microorganisms such as fungi [7]. Therefore, it is possible to develop microbial biomasses through submerged cultivation of filamentous fungi, which use the BW’s nutrients as a source of energy for their growth [5]. Although wastewater treatment techniques using bacteria are more established, fungi-based treatment presents advantages over bacteria-based treatment, mainly because bacteria produce low-value sludge, but fungi convert organics and nutrients of BW into fungal biomass. In addition, basidiomycete fungi colonize great superficial areas, are highly adaptable to pH and temperature changes, present high tolerance to heavy metals, and have the capacity to metabolize a wide range of environmental chemicals [90, 97]. Finally, it is important to select fungal species that are considered non-harmful considering a perspective of mycelium recovery to avoid secondary health or environmental problems. Therefore, the edible basidiomycete fungi play an important role, once they have a long record of safe use, as well as their capacity to remediate the BW [7].
To the best of the authors’ knowledge, this is the first study that investigates the mycoremediation of brewery wastewater using basidiomycete fungi and the potential of β-glucans production for biotechnology application. Future research is needed to establish the techno-economic feasibility of this technology on pilot and industrial scales.
Conclusions
The high amount of brewery wastewater is recyclable or reusable through the application of appropriate techniques and procedures of the circular economy. The nutritional composition of BW (organic matter and other nutrients), allows use as a suitable and low-cost substrate for submerged fermentation for white-rot fungi cultivation, reducing the overall cost of the high-value molecules process for industrial applications, such as the fungal β-glucans. The obtained data on the current study allows us to conclude that ECP has nutritional sources adequate for the cultivation of the six basidiomycete fungi studied species, presenting significant results on β-glucans production, mainly for G. lipsiense.
The kinetics study pointed out the possibility of time reduction of the fungus incubation and, likewise, meeting the expected β-glucans production. Results also showed the G. lipsiense efficiency as an important agent to use the ECP on biotechnological processes to obtain β-glucans, opening prospects for low-cost production of bioactive molecules from mushrooms. Moreover, G. lipsiense provided COD, BOD, nitrogen, and phosphorous reductions, as well as tolerance to heavy metals, showing a promising future application for small wastewater treatment systems, such as microbreweries. Therefore, the proposed technique has potential both as mycoremediation and fungi mycelium recuperation and application in the biotechnology industry, emphasizing the use of fungi to implement technologies based on a circular bioeconomy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hosseinlou, D.: Application of an efficient, cost-effective and newly developed single-process SAO/PND technology for treating brewery industry effluent. South African J. Chem. Eng. 41, 34–42 (2022). https://doi.org/10.1016/j.sajce.2022.04.003
Morais, N.W.S., Coelho, M.M.H., de Silva, A., Pereira, S.E., E.L.: Study on Brazilian agribusiness wastewaters: composition, physical-chemical characterization, volumetric production and resource recovery. Rev. Bras. Ciências Ambient. 56, 248–265 (2021). https://doi.org/10.5327/z21769478875
Oxford Economics: Beer’s global economic footprint. 1–50 (2022)
Brazil: Anuário da Cerveja: 2021. , Brasília, DF (2022)
Hultberg, M., Bodin, H.: Fungi-based treatment of real brewery waste streams and its effects on water quality. Bioprocess Biosyst. Eng. 42, 1317–1324 (2019). https://doi.org/10.1007/s00449-019-02130-9
Olajire, A.A.: The brewing industry and environmental challenges. J. Clean. Prod. 256, 1–21 (2020). https://doi.org/10.1016/j.jclepro.2012.03.003
Hultberg, M., Bodin, H.: Fungi-based treatment of brewery wastewater—biomass production and nutrient reduction. Appl. Microbiol. Biotechnol. 101, 4791–4798 (2017). https://doi.org/10.1007/s00253-017-8185-9
Serbent, M.P., Guimarães, D.K.S., Drechsler-Santos, E.R., Helm, C.V., Giongo, A., Tavares, L.B.B.: Growth, enzymatic production and morphology of the white-rot fungi Lentinus crinitus (L.) Fr. upon 2,4-D herbicide exposition. Int. J. Environ. Sci. Technol. 17, 2995–3012 (2020). https://doi.org/10.1007/s13762-020-02693-1
Leonhardt, S., Hoppe, B., Stengel, E., Noll, L., Moll, J., Bässler, C., Dahl, A., Buscot, F., Hofrichter, M., Kellner, H.: Molecular fungal community and its decomposition activity in sapwood and heartwood of 13 temperate European tree species. PLoS ONE (2019). https://doi.org/10.1371/journal.pone.0212120
Harms, H., Schlosser, D., Wick, L.Y.: Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals, (2011)
Singh, H.: Mycoremediation: Fungal Bioremediation. (2006)
Valdez-Vazquez, I., Robledo-Rizo, J.G., Muñoz-Páez, K.M., Pérez-Rangel, M., de la Luz Ruiz-Aguilar, G.M.: Simultaneous hydrogen production and decolorization of denim textile wastewater: kinetics of decolorizing of indigo dye by bacterial and fungal strains. Brazilian J. Microbiol. 51, 701–709 (2020). https://doi.org/10.1007/s42770-019-00157-4
Zapana-Huarache, S.V., Romero-Sánchez, C.K., Gonza, A.P.D., Torres-Huaco, F.D., Rivera, A.M.L.: Chromium (VI) bioremediation potential of filamentous fungi isolated from Peruvian tannery industry effluents. Brazilian J. Microbiol. 51, 271–278 (2020). https://doi.org/10.1007/s42770-019-00209-9
Serbent, M.P., Rebelo, A.M., Pinheiro, A., Giongo, A., Tavares, L.B.B.: Biological agents for 2,4-dichlorophenoxyacetic acid herbicide degradation, (2019)
Abreu, J.A.S., Rovida, A.F.S., Pamphile, J.A.: Fungi of interest: biotechnological applications. Rev. UNINGÁ Rev. 21, 55–59 (2015)
Chicatto, J.A., Rainert, K.T., Gonçalves, M.J., Helm, C.V., Altmajer-Vaz, D., Tavares, L.B.B.: Decolorization of textile industry wastewater in solid state fermentation with peach-palm (Bactris gasipaes) residue. Brazilian J. Biol. 78, 718–727 (2018)
Hermann, K.L., Costa, T.M., Helm, C.V., Marconatto, L., Borges, L.G.D.A., Vegini, A.A., Giongo, A., Tavares, L.B.B.: Discoloration of rhodamine b dye by white-rot fungi in solid bleached sulfate paperboard coated with polyethylene terephthalate: Scale-up into non-sterile packed-bed bioreactor. J. Environ. Chem. Eng. 8, 103685 (2020). https://doi.org/10.1016/j.jece.2020.103685
Barh, A., Kumari, B., Sharma, S., Annepu, S.K., Kumar, A., Kamal, S., Sharma, V.P.: Mushroom mycoremediation: Kinetics and mechanism. In: Smart Bioremediation Technologies: Microbial Enzymes. pp. 1–22 (2019)
Bosco, F., Mollea, C.: Mycoremediation in Soil. In: Environmental Chemistry and Recent Pollution Control Approaches Fungi, p. 17. IntechOpen, London p (2019)
Taheran, M., Brar, S.K., Verma, M., Surampalli, R.Y., Zhang, T.C., Valero, J.R.: Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total. Environ. 547, 60–77 (2016). https://doi.org/10.1016/j.scitotenv.2015.12.139
Muszyńska, B., Żmudzki, P., Lazur, J., Kała, K., Sułkowska-Ziaja, K., Opoka, W.: Analysis of the biodegradation of synthetic testosterone and 17α-ethynylestradiol using the edible mushroom Lentinula edodes. 3 Biotech. 8, 1–15 (2018). https://doi.org/10.1007/s13205-018-1458-x
Timm, T.G., Pasko, R.Z., Campos, C.S., Helm, C.V., Tavares, L.B.: Drying process of lentinula edodes: Influence of temperature on β-glucan content and adjustment of mathematical models. Cienc. e Agrotecnologia. 43, 1–12 (2019). https://doi.org/10.1590/1413-7054201943025719
Timm, T., Helm, C.V., de Alves Lima, E., Henriques, G.S., Alberton, M.D., Simeone, M.L.F., Queiroz, V.A.V., Tavares, L.B.B.: Peach palm by-product bioconversion by culinary-medicinal mushroom Lentinula edodes for food products application. Int. J. Med. Mushrooms. 24, 19–36 (2022). https://doi.org/10.1615/intjmedmushrooms.2022045391
Pasko, R.Z., Timm, T.G., de Lima, G.G., Helm, C.V., de Lima, E.A., Henriques, G.S., Tavares, L.: In vivo evaluation and nutritional quality of by-product subjected to solid state-fermentation using shiitake Culinary-medicinal mushroom Lentinula edodes (Agaricomycetes). Int. J. Med. Mushrooms. 24, 53–66 (2022). https://doi.org/10.1615/intjmedmushrooms.2021041945
Sari, M., Prange, A., Lelley, J.I., Hambitzer, R.: Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 216, 45–51 (2017). https://doi.org/10.1016/j.foodchem.2016.08.010
Zhu, F., Du, B., Bian, Z., Xu, B.: β-Glucans from edible and medicinal mushrooms: characteristics, physicochemical and biological activities. J. Food Compos. Anal. 41, 165–173 (2015). https://doi.org/10.1016/j.jfca.2015.01.019
Di Bastiani, M.: β-glucans from Lasiodiplodia theobromae MMBJ: evaluation of immunomodulatory activity and as a drug carrier film (portuguese text), (2014)
Vu, V., Muthuramalingam, K., Singh, V., Hyun, C., Kim, Y.M., Unno, T., Cho, M.: Effects of β-glucan, probiotics, and synbiotics on obesity-associated colitis and hepatic manifestations in C57BL/6J mice. Eur. J. Nutr. 61, 793–807 (2022). https://doi.org/10.1007/s00394-021-02668-z
Murphy, E.J., Masterson, C., Rezoagli, E., O’Toole, D., Major, I., Stack, G.D., Lynch, M., Laffey, J.G., Rowan, N.J.: β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total. Environ. 732, 139330 (2020). https://doi.org/10.1016/j.scitotenv.2020.139330
Yehia, R.S.: Evaluation of the biological activities of β-glucan isolated from Lentinula edodes. Lett. Appl. Microbiol. 75, 317–329 (2022). https://doi.org/10.1111/lam.13727
Schipmann, D.B.D., Timm, T.G., Costa, T.M., Tavares, L.B.B.: Influence of beer production residue on growth of fungi ganoderma and obtaining β-glucans. Rev. Estud. Ambient. 22, 32 (2021). https://doi.org/10.7867/1983-1501.2020v22n2p32-42
APHA, A.P.H.A., AWWA, A.W.W.A., WEF, W.E.F.: Standard Methods for the Examination of Water and Wastewater. , Washington, D.C (2012)
Brazil: Resolução CONAMA no 430/2011, de 30 de agosto de 2011, Conselho Nacional de Meio Ambiente (CONAMA). Dispõe sobre as condições e padrões de lançamento de Diário Oficial da União, Brasília, DF, 16 maio 2011.
Tang, Y.-J., Zhong, J.-J.: Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acid. Enzyme Microb. Technol. 31, 20–28 (2002). https://doi.org/10.1016/S0141-0229(02)00066-2
AOAC: Official Methods of Analysis of AOAC International. , Gaithersburg M.D. (2006)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Eliopoulos, C., Arapoglou, D., Chorianopoulos, N., Markou, G., Haroutounian, S.A.: Conversion of brewers’ spent grain into proteinaceous animal feed using solid state fermentation. Environ. Sci. Pollut. Res. 29, 29562–29569 (2022). https://doi.org/10.1007/s11356-021-15495-w
Chan, L.G., Cohen, J.L., Ozturk, G., Hennebelle, M., Taha, A.Y., de Moura Bell, L.N.J.M.: Bioconversion of cheese whey permeate into fungal oil by Mucor circinelloides. J. Biol. Eng. 12, 25 (2018). https://doi.org/10.1186/s13036-018-0116-5
Mäkelä, M.R., Aguilar-Pontes, M.V., van Rossen-Uffink, D., Peng, M., de Vries, R.P.: The fungus Aspergillus niger consumes sugars in a sequential manner that is not mediated by the carbon catabolite repressor CreA. Sci. Rep. 8, 6655 (2018). https://doi.org/10.1038/s41598-018-25152-x
Bettin, F., da Rosa, L.O., Montanari, Q., Calloni, R., Gaio, T.A., Malvessi, E., da Silveira, M.M., Dillon, A.J.P.: Growth kinetics, production, and characterization of extracellular laccases from Pleurotus sajor-caju PS-2001. Process Biochem. 46, 758–764 (2011). https://doi.org/10.1016/j.procbio.2010.12.002
Kovárová-Kovar, K., Egli, T.: Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev 62, 646–666 (1998). https://doi.org/10.1128/MMBR.62.3.646-666.1998
Costa, T.M., Mayer, D.A., Siebert, D.A., Micke, G.A., Alberton, M.D., Tavares, L.B.B., de Oliveira, D.: Kinetics analysis of the inhibitory effects of alpha-glucosidase and identification of compounds from G. lipsiense mycelium. Appl. Biochem. Biotechnol. 191, 996–1009 (2020). https://doi.org/10.1007/s12010-020-03239-4
Costa, T.M., Costa Sperb, J.G., Roncheti, A.L., Botelho, T.K.R., Sell, T.M., Bertoli, S.L., Tavares, L.B.B.: Evaluation of radial specific growth rate fungus in residual vegetable oil. Rev. Estud. Ambient. 17, 29 (2016). https://doi.org/10.7867/1983-1501.2015v17n2p29-40
Seluy, L.G., Isla, M.A.: A process to treat high-strength brewery wastewater via ethanol recovery and vinasse fermentation. Ind. Eng. Chem. Res. 53, 17043–17050 (2014). https://doi.org/10.1021/ie500438j
Giraldo, N.D., Buchelly, R.J.R., Hincapié, D.E., Atehortua, L.: Transformation of brewery subproducts into valuable biomass using mixotrophic culture of chlorella pyrenoidosa and associated bacteria. Brazilian Arch. Biol. Technol. 63, 1–12 (2020). https://doi.org/10.1590/1678-4324-2020190229
Wang, S., Yin, C., Yang, Z., Hu, X., Liu, Z., Song, W.: Assessing the potential of Chlorella sp. for treatment and resource utilization of brewery wastewater coupled with bioproduct production. J. Clean. Prod. 367, 132939 (2022). https://doi.org/10.1016/j.jclepro.2022.132939
Papadopoulos, K.P., Economou, C.N., Stefanidou, N., Moustaka-Gouni, M., Genitsaris, S., Aggelis, G., Tekerlekopoulou, A.G., Vayenas, D.V.: A semi-continuous algal-bacterial wastewater treatment process coupled with bioethanol production. J. Environ. Manage. 326, 116717 (2023). https://doi.org/10.1016/j.jenvman.2022.116717
Dias, C., Santos, J.A.L., Reis, A., de Lopes Silva, T.: The use of oleaginous yeasts and microalgae grown in brewery wastewater for lipid production and nutrient removal: a review. Waste Biomass Valoriz. 14, 1799–1822 (2023). https://doi.org/10.1007/s12649-023-02032-8
Inyang, U.E., Bassey, E.N., Inyang, J.D.: Characterization of brewery effluent fluid. J. Eng. Appl. Sci. 4, 67–77 (2012)
Alananbeh, K.M., Bouqellah, N.A., Al Kaff, N.S.: Cultivation of oyster mushroom Pleurotus ostreatus on date-palm leaves mixed with othe agro-wastes in Saudi Arabia. Saudi J. Biol. Sci. 21, 616–625 (2014)
Costa, P.N.C., Maia, N.C., Guimarães, L.H.S., Resende, M.L.V., Cardoso, P.G.: Optimization of culture conditions for tannase production by Aspergillus sp. Gm4 in solid state fermentation. Acta Sci. Biol. Sci. 37, 23–30 (2015)
Machado, D.F.M., Parzianello, F.R., da Silva, A.C.F., Antoniolli, Z.I.: Trichoderma in Brazil: the fungus and the bioagent (portuguese text). Rev. Ciências Agrárias. 35, 1–6 (2012). https://doi.org/10.19084/rca.16182
Cutrim, F., Oliveira, S.M.A., Dantas, S.A.F., Silva, R.L.X.: Influence of culture media and the carbon-nitrogen interaction on growth and sporulation of Penicillium sclerotigenum. Summa Phytopathol. 32, 85–88 (2006). https://doi.org/10.1590/S0100-54052006000100014
Jayasinghe, C., Imtiaj, A., Hur, H., Lee, G.W., Lee, T.S., Lee, U.Y.: Favorable culture conditions for mycelial growth of Korean wild strains in ganoderma lucidum. Mycobiology. 36, 28 (2008). https://doi.org/10.4489/MYCO.2008.36.1.028
Tsai, S.M., Rossetto, R.: Microbial transformation of phosphate. In: Microbiologia do solo. p. 360. Sociedade Brasileira de Ciência do Solo, Campinas, SP (1992)
Yuan, B., Chi, X., Zhang, R.: Optimization of exopolysaccharides production from a novel strain of Ganoderma lucidum CAU5501 in submerged culture. Brazilian J. Microbiol. 43, 490–497 (2012). https://doi.org/10.1590/S1517-83822012000200009
Tortora, G.J., Funke, B.R., Case, C.L.: Microbiologia. Artmed, Porto Alegre, RS (2017)
Koutrotsios, G., Larou, E., Mountzouris, K.C., Zervakis, G.I.: Detoxification of olive mill wastewater and bioconversion of olive crop residues into high-value-added biomass by the choice edible mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. (2016). https://doi.org/10.1007/s12010-016-2093-9
Rop, O., Mlcek, J., Jurikova, T.: Beta-glucans in higher fungi and their health effects. Nutr. Rev. 67, 624–631 (2009). https://doi.org/10.1111/j.1753-4887.2009.00230.x
Fukuda, E.K., Vasconcelos, A.F., Matias, A.C., Barbosa, A.D., Dekker, R.F.H., Silva, M.D.: Fungal cell wall polysaccharides purification and characterization. Semin. Ciências Agrárias. 30, 117–134 (2009)
Pelczar, M.J., Jr., Chan, E.C.S., Krieg, N.: Microbiologia: Conceitos e Aplicações. MAKRON Books, São Paulo (1996)
Vieira, G.R.T., Liebl, M., Tavares, L.B.B., Paulert, R., Smânia, A.: Submerged culture conditions for the production of mycelial biomass and antimicrobial metabolites by Polyporus tricholoma Mont. Brazilian J. Microbiol. (2008). https://doi.org/10.1590/S1517-83822008000300029
da Corradi Silva, M.L., Martinez, P.F., Izeli, N.L., Silva, I.R., Vasconcelos, A.F.D., de Stefani Cardoso, M., Stelutti, R.M., Giese, E.C., de Melo Barbosa, A.: Chemical characterization and biotechnology applications of fungal glucans. Quim. Nova 29, 85–92 (2006). https://doi.org/10.1590/s0100-40422006000100017
Gern, R.M.M.: Cultivation study for the production of biomass and polysaccharides by Pleurotus ostreatus DM 1833 in submerged cultivation, https://repositorio.ufsc.br/handle/123456789/102431, (2005)
Negrulescu, A., Patrulea, V., Mincea, M.M., Ionascu, C., Vlad-Oros, B.A., Ostafe, V.: Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 23, 2176–2182 (2012). https://doi.org/10.1590/S0103-50532013005000003
Bak, W.C., Park, J.H., Park, Y.A., Ka, K.H.: Determination of glucan contents in the fruiting bodies and mycelia of lentinula edodes cultivars. Mycobiology. 42, 301–304 (2014). https://doi.org/10.5941/MYCO.2014.42.3.301
Kim, J., Lim, J., Bae, I.Y., Park, H.G., Gyu Lee, H., Lee, S.: Particle size effect of lentinus edodes mushroom (chamsong-i) powder on the physicochemical, rheological, and oil-resisting properties of frying batters. J. Texture Stud. (2010). https://doi.org/10.1111/j.1745-4603.2010.00231.x
Kozarski, M., Klaus, A., Nikšić, M., Vrvić, M.M., Todorović, N., Jakovljević, D., Van Griensven, L.J.L.D.: Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. (2012). https://doi.org/10.1016/j.jfca.2012.02.004
McCleary, B.V., Draga, A.: Measurement of β-Glucan in mushrooms and mycelial products. J. AOAC Int. 99, 364–373 (2016). https://doi.org/10.5740/jaoacint.15-0289
Bach, F., Helm, C.V., Bellettini, M.B., Maciel, G.M., Haminiuk, C.W.I.: Edible mushrooms: a potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 52, 2382–2392 (2017). https://doi.org/10.1111/ijfs.13522
Henriques, G.S., Helm, C.V., Busato, A.P., Simeone, M.L.F.: Lipid profile and glycemic response of rats fed on a semi-purified diet supplemented with Agaricus brasiliensis mushroom. Acta Sci. Heal. Sci. 38, 71–79 (2016). https://doi.org/10.4025/actascihealthsci.v38i1.29185
Mahapatra, S., Banerjee, D.: Fungal exopolysaccharide: production composition and applications. Microbiol. Insights. 6, MBI.S10957 (2013). https://doi.org/10.4137/MBI.S10957
Stroparo, E.C., Beitel, S.M., Resende, J.T.V., Knob, A.: Filamentous fungi and agro-industrial residues selection for enzyme production of biotechnological interest. Semin. Ciências Agrárias. 33, 2267–2278 (2012). https://doi.org/10.5433/1679-0359.2012v33n6p2267
Wasser, S.P.: Medicinal mushrooms in human clinical studies Part I anticancer, oncoimmunological, and immunomodulatory activities: a review. Int. J. Med. Mushrooms. 19, 279–317 (2017). https://doi.org/10.1615/IntJMedMushrooms.v19.i4.10
Graubaum, H.-J., Busch, R., Stier, H., Gruenwald, J.: A double-blind, randomized, placebo-controlled nutritional study using an insoluble Yeast Beta-glucan to improve the immune defense system. Food Nutr. Sci. 03, 738–746 (2012). https://doi.org/10.4236/fns.2012.36100
Chang, S.T., Wasser, S.P.: Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products (Review). Int. J. Med. Mushrooms. 20, 1121–1133 (2018). https://doi.org/10.1615/IntJMedMushrooms.2018029378
Giavasis, I.: Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 26, 162–173 (2014). https://doi.org/10.1016/j.copbio.2014.01.010
Maheshwari, G., Sowrirajan, S., Joseph, B.: Extraction and Isolation of β-Glucan from grain sources—a review. J. Food Sci. 82, 1535–1545 (2017). https://doi.org/10.1111/1750-3841.13765
Sheela, J.M., Divya, K., Premina, S.: Amylase production by aspergillus Niger and penicillium species by solid-state and submerged cultivation using two food industrial wastes. Nat. Environ. Pollut. Technol. 20, 1127–1135 (2021). https://doi.org/10.46488/NEPT.2021.V20I03.020
Costa, T.M., Kaufmann, V., Paganelli, C.J., Siebert, D.A., Micke, G.A., Alberton, M.D., Tavares, L.B.B., de Oliveira, D.: Kinetic identification of phenolic compounds and potential production of caffeic acid by Ganoderma lipsiense in solid-state fermentation. Bioprocess Biosyst. Eng. 42, 1325–1332 (2019). https://doi.org/10.1007/s00449-019-02131-8
Mitchell, D.A., Berovič, M., Krieger, N.: Solid-State Fermentation Bioreactor Fundamentals: Introduction and Overview. In: Solid-State Fermentation Bioreactors, pp. 1–12. Springer-Verlag, Berlin/Heidelberg (2006)
Fanaei, M.A., Vaziri, B.M.: Modeling of temperature gradients in packed-bed solid-state bioreactors. Chem. Eng. Process. Process Intensif. 48, 446–451 (2009). https://doi.org/10.1016/j.cep.2008.06.001
Kurcz, A., Błażejak, S., Kot, A.M., Bzducha-Wróbel, A., Kieliszek, M.: Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass. Valoriz. 9, 57–64 (2018). https://doi.org/10.1007/s12649-016-9782-z
Souza Filho, D.A. de: Reduction of the polluting potential of manipueira and citric acid production in reactors inoculated with fungus, (2016)
Fonseca, M. da S.: Obtaining and characterizing an exopolysaccharide produced by Neodeightonia phoenicum, (2019)
Costa, R., Choupina, A.: β-Glucanases enzymes—appliance of polysaccharides hydrolisis. REB-Revista eletrónica Biol. 6, 205–213 (2013)
Bauermeister, A., Rezende, M.I., Giese, E.C., Frans, R., Dekker, H., Barbosa, A.D.M.: Fungal β-1,3-Glucanases: production and biotechnological applications. Semin. Ciencias Exatas e Tecnológicas. 31, 75–86 (2010)
Barbosa, A.D.M., da Cunha, P.D.T., Pigatto, M.M., da Silva, M.D.L.C.: Production and applications of fungal exopolysaccharides. Semin. Ciências Exatas e Tecnológicas. (2004). https://doi.org/10.5433/1679-0375.2004v25n1p29
Smits, G.J., van den Ende, H., Klis, F.M.: Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology 147, 781–794 (2001). https://doi.org/10.1099/00221287-147-4-781
Akhtar, N., Mannan, M.A.: ul: Mycoremediation: expunging environmental pollutants. Biotechnol. Reports. 26, e00452 (2020). https://doi.org/10.1016/j.btre.2020.e00452
Purnomo, A.S., Mori, T., Kamei, I., Nishii, T., Kondo, R.: Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. Int. Biodeterior. Biodegrad. (2010). https://doi.org/10.1016/j.ibiod.2010.04.007
Faria, R. de A.: Study of the production of ligninolytic enzymes by Ceriporiopsis subvermispora, (2010)
Umeo, S.H., Faria, M.G.I., Vilande, S.S.S., Dragunski, D.C., do Valle, J.S., Colauto, N.B., Linde, G.A.: Iron and zinc mycelial bioaccumulation in Agaricus subrufescens strains. Semin. Ciências Agrárias. 40, 2513 (2019). https://doi.org/10.5433/1679-0359.2019v40n6p2513
Albert, Q., Baraud, F., Leleyter, L., Lemoine, M., Heutte, N., Rioult, J.P., Sage, L., Garon, D.: Use of soil fungi in the biosorption of three trace metals (Cd, Cu, Pb): promising candidates for treatment technology? Environ. Technol. 41, 3166–3177 (2020). https://doi.org/10.1080/09593330.2019.1602170
United States: Wastewater Best Management Practices for Breweries. New Hampshire (2020)
Ciont, C., Epuran, A., Kerezsi, A.D., Coldea, T.E., Mudura, E., Pasqualone, A., Zhao, H., Suharoschi, R., Vriesekoop, F., Pop, O.L.: Beer safety: new challenges and future trends within craft and large-scale production. Foods. 11(17), 2693 (2022). https://doi.org/10.3390/foods11172693
Bosco, F., Mollea, C.: Mycoremediation in soil. In: Environmental Chemistry and Recent Pollution Control Approaches (2019)
Acknowledgements
The authors are grateful to the Graduate Program of Environmental Engineering of the Regional University of Blumenau, FURB, Blumenau, Santa Catarina, Brazil; and the local company Cervejaria Blumenau for the wastewater donation.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil (Grant numbers [Finance Code 001] and [DS 88887.490173/2020-00]) and National Council for Scientific and Technological Development (CNPq), Brazil (Grant number [309903/2016-5-CNPq Nº 11/2016]); the author LBB Tavares is a fellowship holder of CNPq (Grant number [305880/2020-9-*CNPq Nº 02/2020]).
Author information
Authors and Affiliations
Contributions
Material preparation and data collection were performed by Djonice Beatriz Doege Schipmann. The analysis and preparation of data, writing, and editing of the manuscript were carried out by Thaynã Gonçalves Timm. Design experiments and the reviewing of the manuscript were made by Tania Maria Costa. Fundraising to develop this research and the refining of the manuscript were carried out by Lorena B. B. Tavares. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The sponsors and the funding sources had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Timm, T.G., Schipmann, D.B.D., Costa, T.M. et al. Remediation of Brewery Wastewater and Reuse for β-Glucans Production by Basidiomycete Fungi. Waste Biomass Valor 15, 4629–4645 (2024). https://doi.org/10.1007/s12649-024-02468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-024-02468-6