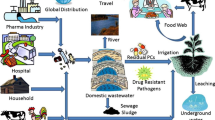
Abstract
The presence of pharmaceuticals within the environment poses serious threat to the health of humans and animals. Owing to the inability of existing wastewater treatment methods to completely remove pharmaceuticals when wastewater is treated at wastewater treatment plants, their effluent have been recognized as one of the main sources of pharmaceuticals into the environment. The negative effect of some of these pharmaceuticals in the environment has resulted in rising concern on how to improve wastewater treatment methods at wastewater treatment plants. Recently, adsorption process has been considered as an efficient method to complement the existing methods of wastewater treatment. This is because of the high affinity of suitable adsorbents for pharmaceuticals within wastewater. Nonetheless, the high price of prevalent adsorbent like activated carbon has been a major limitation. Biochar that possesses similar properties to activated carbon has recently been reported by different literature to be efficient in the removal of pharmaceuticals from wastewater and aqueous solution. Because of this, several literature were studied on pharmaceuticals adsorption with the use of biochar and a summary of our findings are presented in this review. In addition, a recent report in Estonia has shown considerable pharmaceuticals concentration above the limit of detection in the effluent streams of wastewater treatment plants. Based on the rate of human consumption data, The authors focused on three pharmaceuticals (1) Metformin, (2) Ibuprofen, and (3) Diclofenac which are part of the readily detected in wastewater treatment effluents in Estonia. In response to their inefficient removal, this paper offers the possibility of using adsorption, specifically with the use of biochar as an economical adsorbent for improving their removal. The findings in this review range from wastewater treatment methods, biochar production and characterization methods to the mechanisms involved in using biochar for the removal of pharmaceuticals. Lastly, the major challenges related with this possibility are highlighted, while recommendations for future research are also highlighted to hasten the implementation of adsorption process using biochar material as the adsorbent for improving pharmaceuticals removal from wastewater.
Graphical Abstract

Highlights
-
The presence of pharmaceuticals in the environment poses serious threat to the lives of human and animals
-
The effluents of wastewater treatment plants have been identified as a major source of pharmaceuticals within the environment
-
Concentrations of Diclofenac, Ibuprofen, and Metformin remain high in the effluents of wastewater treatment plants in Estonia
-
Adsorption has the capacity to assist in improving pharmaceuticals removal during wastewater treatment at wastewater treatment plants
-
Biochar possesses the desired features to replace high-cost adsorbents during an adsorption process
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
There have been various reports on the use of biochar for assisting in the adsorption of Phcs, however, reports on the utilization of biochar for improving Phcs removal during the treatment of wastewater at WWTP are limited. This synopsis aims to bridge that gap by specifically presenting the possibility of improving the removal of Phcs during wastewater treatment using biochar, with emphasis on diclofenac (DF), ibuprofen (IB), and metformin (MF) removal. Furthermore, Estonia is chosen as a case study owing to the limited research on biochar and its application within this region.
Introduction
Pharmaceuticals (Phcs) and Their Impact on the Environment
Pharmaceuticals (Phcs) are modified biologically active substance used to cure or prevent ailments in animals and humans [1, 2]. It includes analgesics, antibacterials, as well as antiepileptics [1, 3, 4]. Reportedly, the use of Phcs in the year 2020 was about 4.5 trillion dosages [5] and could increase in the future [6]. However, concerns about their presence in the environment and threat to aquatic lives have been raised by several authors [6,7,8,9,10,11]. The threats they pose to the environment include (1) The development of pathogens that are more resistant to treatment and threaten the health of lives within the environment [12,13,14] (2) Their bioaccumulation is poisonous to the environment [15] and (3) Their presence impacts the food web within the aquatic eco-system in an unfavorable manner [16, 17]. Reportedly, two of the major sources of Phcs within the environment is from the effluent of wastewater treatment plants (WWTP) [14, 18, 19] and untreated water [17]. Owing to this, the conventional methods of wastewater treatment have been faulted for their inability to completely eradicate Phcs, leading to their release into nearby rivers and coastal areas [20]. Other non-point sources of Phcs within the environment are connected to the inappropriate disposal of expired drugs, human feces, agriculture, and veterinary practices, leaching into surface and ground waters [10, 19, 21]. Some Phcs like paracetamol, ciprofloxacin, sulfamethoxazole, and caffeine are readily biodegradable and can be significantly removed during treatment [21], while some degrade slowly [22]. Furthermore, some could be stable in the environment and varying concentrations have been uncovered within the range of ngL−1 to μgL−1 in the effluents of WWTP, contaminating water bodies around the world [6, 19], [23,24,25].
General Wastewater Treatment Methods
In the past, different conventional methods have been considered for eliminating Phcs from wastewater, they include coagulation [26], advanced oxidative process (AOP) [27], biological method [28], ozonation and filtration [17, 27]. Coagulation is known for treating wastewater with the use of chemicals known as coagulants that bind pollutants until a huge mass that can be separated via settling is formed [29]. Common coagulants used are salts of aluminum and iron, although there are issues associated with the disposal of sludge generated from this method [29]. AOP also utilizes chemicals to remove inorganic or organic pollutants from wastewater, resulting in the formation –OH radicals [29]. AOP’s potential to breakdown complex compounds present it as an efficient technique for removing Phcs from wastewater [30]. However, its enormous operation cost [27], huge energy consumption [31], and the need for a spacious set-up increases its process cost and hinders its use globally [27]. Biological technique utilizes the activity of microbes in the treatment of wastewater, it is an eco-friendly technique, nonetheless, it takes time and there are concerns on how to manage the sludge generated when it is used [29], besides, not all Phcs are removed using this technique [14]. Ozonation is beneficial in that it produces no sludge, nonetheless, it produces other by-products in WWTP effluents [29]. Reportedly, these by-products could be more toxic when compared to the initial Phcs contaminants, however, it remains a viable wastewater treatment option [31]. Filtration utilizes a membrane (Constro [32] attached to leaky support and is useful for taking out dissolved contaminants during the treatment of wastewater [29]. Nonetheless, for membrane-filtration, there could be a need for frequent membrane replacement [29], and the problem of fouling [28]. In themselves, each of the conventional techniques may not be sufficient for fully removing Phcs from wastewater [12, 14, 33], and a summary of their demerits is presented in Fig. 1. Hence, with their shortcomings, it is expedient to complement these methods to improve the percentage of Phcs being removed during treatment [34]. Of recent, the interest in the use of adsorption for improving the removal of Phcs from wastewater [17, 35, 36] is rising [37], and this is because it is efficient [36], environmentally benign [38], and easily operated [39]. Albeit there are concerns about the huge cost of adsorbents like commercial activated carbon, paving way for more research on less-costly ones like biochar [40]. Biochar has been suggested suitable for removing Phcs in an adsorption process [17]. When compared to commercial-activated-carbon, the cost, energy-use, and greenhouse gas (GHG) emissions during the production of biochar is lesser. This is illustrated in Fig. 2.
Estonia and IB, MF& DF Phcs
Estonia is one of the countries within the North-Eastern region of Europe. It lies within the latitude and longitudinal points of 59.5° N 28° E and 57.5° N 22° E respectively. Its vegetation zone is known as hemiboreal with a relatively flat landscape, albeit its south-eastern region is more mountainous [41]. There are four seasons in Estonia, spring starts from March–April, followed by summer from June–August, then Autumn from September–November and cold winter from December to February [41]. According to Statistics, Estonia’s estimated population is 1.32 million [42], and the life expectancy of Estonians has shown a positive trend in the last decade as people are expected to live much longer; between 70–74 and 80–82 years for males and females respectively (Fig. 3). With the extended life expectancy, the consumption of IB and other medications to maintain a healthy lifestyle will increase [43]. Furthermore, according to the data available from Estonia Statistics on medicine, the consumption of different prescribed and over-the-counter drugs has risen significantly [2, 44]. Besides, three major Anatomical Therapeutic Chemical (ATC) groups of Phcs (such as alimentary tract drugs and nervous system pharmaceuticals [“non-steroidal anti-inflammatory drugs” (NSAIDs)] were frequently consumed among Estonians between 2016 and 2018, with MF, DF, and IB falling under this groups of Phcs [45, 46]. Hence, they are now frequently detected in municipal WWTP influent and effluent streams [47], resulting in their prevalence within the environment. Besides, with Estonia being one of the countries in the Baltic Area, data analysis on existing data confirmed that NSAIDs, epilepsy, diabetes and cardiovascular disease medicine were the most used, of which MF, IB and DF belongs to one of these class of drugs [18]. MF is an antidiabetic medicine [22] and is amongst the most detected Phcs in the effluent of WWTP [48]. According to statistics from the international diabetes federation, above 400 million persons worldwide live with diabetes [49], 59 million patients are in Europe and 58,700 cases in Estonia; which equals about 6.2% of the adult population in Estonia [50, 142]. MF’s prescription has increased significantly owing to its efficiency in glucose removal via acting on metabolic paths to encourage catabolism and the elimination of glucose [48]. Furthermore, in Europe (including Estonia), the consumption of MF will likely increase, because over 68 million people would reportedly have this disease by 2045 [51]. A general report on the fate of Phcs across the Baltic States shows that MF’s highest peak concentration exceeded 1 mg/l in WWTP influent while a peak optimum and average concentration of 0.92 mg/l and 0.16 mg/l respectively were detected in their effluent streams [47]. DF is a phenylacetic acid derivative, while IB is generally accessible as 2-(4-isobutylphenyl) propionic acid. Other properties of IB, DF and MF are presented in Table 1. Between 2006 and 2014, there was a significant 14.1% increase in IB consumption in Estonia [43], while the devastating side effects of DF (such as stroke and heart attack in a patient with certain cardiovascular risk factors) has prompted the European Medicine Agency to recommend that DF should be utilized at the lowest active dose [43]. Furthermore, the result of a survey in Europe found the peak absolute concentration of IB (48 μg/L), together with DF (11 μg/L) within the secondary effluent of WWTP [11]. DF has also been included as one of the emerging Phcs into the environment by the European commission, and has resulted in an effort to completely remove DF and other Phcs like MF and IB during the treatment of wastewater [14].
Life expectancy graph for Estonia between 2010 and 2019. Source. [95]
Estonia and WWTP
There are different reports on the number of WWTP in Estonia, however, from the literature that was found, we can conclude that the number varies from 664 to 730 [52,53,54]. Based on the report of [52], the capacity of each of the WWTP varies from 300 to > 100,000 PEs (person equivalents). The breakdown is presented in Table 2. Besides, the main wastewater treatment technologies utilized at Estonia’s WWTP are presented in Fig. 4, where the use of batch and plug flow reactors fall under the activated sludge technology, oxidation ponds and wetlands fall under the natural solutions while others are the biofilm reactor technology. However, these technologies utilize a combination of physical, biological, and chemical treatment methods (including those mentioned in “General wastewater treatment methods” Section) that are deemed insufficient to totally take out Phcs in WWTP. This further buttresses the need to complement these existing methods (in “General wastewater treatment methods” Section) with adsorption using lower-cost biochar, which is cheaper when compared with activated carbon for improving the removal of Phcs.
Analysis of Phcs in Wastewater
To ensure the complete removal of Phcs during the treatment of wastewater, they need to be properly analyzed to ascertain their actual concentration within the influent and effluent streams of WWTP. Some of the common techniques for the analysis of Phcs are presented in Table 3.
Methodology
For database analysis, the Scopus database was used to establish the need for a synopsis on the possibility of using biochar to augment the removal of Phcs in Estonia’s WWTP. Several searches were conducted using different keywords. The first search was steered at establishing the rising interest in the utilization of biochar for adsorption. Using biochar+adsorption as the main search word, bounded using the title of publications, abstracts, and keywords between 2005 and 2021. 5980 research documents were found. When Estonia was added to the main search words (biochar+adsorption+Estonia) under similar search conditions, no document was found. Although there might be a few documents on other databases, however, this result reflects the state of biochar research in Estonia. The second search was carried out using wastewater treatment+biochar as the main words under similar search conditions, and 1523 documents were found. This is lower than the result of the first search, and based on this, we concluded that the research into using biochar in wastewater treatment is just budding. Like the first search, when Estonia was added to the main search words (wastewater treatment+biochar+Estonia), there was no document found. Other searches were conducted to study the extent of research into using biochar for the adsorption of IB, DF, and MF, and the results are shown in Fig. 5. From the results, there was a confirmation of the need to present a summary of the potential of biochar as an adsorbent for Phcs uptake. This was later narrowed down to three of the most prevalent Phcs (IB, DF, and MF) in Estonia.
Biochar and Production
Biochar is rich in carbon and produced via the pyrolysis of various feedstocks, ranging from virgin to waste biomass. This comprises of agricultural plantings, manures, farm wastes, and municipal wastes [40, 55]. Some of biochar’s remarkable properties as shown in Fig. 6 present it as a suitable material in diverse applications, including being used as an adsorbent in treating wastewater. Furthermore, the possibility of utilizing low-cost and easily accessible biomass wastes as its precursor cements biochar as a sustainable adsorbent that can be used during wastewater treatment [56].
Biochar is produced via several thermal-chemical processes [57]. It includes torrefaction, and gasification, together with pyrolysis (Fig. 7). The choice of thermochemical process and process parameter is vital during biochar production [40]. Generally, torrefaction is used in converting biomass feedstock into a stable solid that can be used as an energy fuel [58], it could either be a dry or wet process [59]. Dry torrefaction is used to pre-treat biomass [60]. During this process, biomass is warmed-up between 200 and 300 °C, in an inert setting for about 0.5 to a few hours [61]. Although dry torrefaction produces a biochar-like substance (known as torrefied biomass) [62], however, it is best to say that torrefaction is for improving some of the properties of raw biomass before being used in energy applications [59].
Wet torrefaction, on the other hand, is also known as hydrothermal carbonization [61]. It is utilized for charring wet biomass like forestry residue and animal manure [63]. The temperature at which hydrothermal carbonization takes place is relatively low (typically between 180 and 265 °C) [59]. Furthermore, its feedstock does not require drying, thus, reducing energy consumption and production cost [61]. The process takes place within the saturation vapor pressure of water (2–10 mega-pascal) [61] and at time intervals of 5 min [59] to hours [64]. The products of hydrothermal carbonization include hydrochar (slurry-char), bio-oil (liquid), and a small amount of gas (majorly CO2) [62], [61]. Nonetheless, the problems of reactor corrosion, deposition, clogging, and high-pressure requirements limit the utilization of wet torrefaction [61].
Gasification is another thermochemical process [65]. Unlike torrefaction, gasification requires partial oxygen [65], and an elevated temperature of 500–1400 °C [66] within a short residence time, typically between 10 and 20 s [67]. The main target of biomass gasification is to produce gas mixtures of methane (CH4), alongside carbon monoxide (CO), and hydrogen (H2), and a small amount of carbon dioxide (CO2) [66]. Although it produces biochar as a byproduct at a scanty rate (typically at a yield of about 5–10%) [68]. Similarly, biochar made from biomass gasification contains various inorganic elements, together with polycyclic-aromatic-hydrocarbons (PAHs) [67, 69]. The PAHs produced are harmful, hence limiting the utilization of gasification’s biochar for environmental remediation purposes [70].
Pyrolyzing biomass is mostly known as the breakdown of biomass raw material within a zero oxygen setting using heat [68]. It is the main technique for producing biochar, such that biomass disintegrates into vapor [condensable (bio-oil, tar) and non-condensable (syngas)], and solid (biochar) [71]. The temperature interval during pyrolysis usually lies between 300 and 1000 °C [71]. It is cost-effective and possesses the ability to produce a variety of products that can be used in different applications (syngas and bio-oil can be converted into energy fuels) [72, 73]. Based on the conditions of operation, pyrolysis can be mainly as fast and slow type [71]. Fast pyrolysis takes place at a temperature interval of 400–600 °C, high heating rate (typically greater than 300 °C/min), and little vapor-residence time (typically between 0.5 and 10 s). The main target of fast pyrolysis is bio-oil, although biochar is formed as a by-product (about 15–30 wt.% yield) [67, 71]. Conversely, the slow form of pyrolysis is the choicest for producing biochar. This is because it produces the highest amount of solid product (typically between 35 and 50 wt.%) [67, 71]. It occurs between 300 and 800 °C [71], with a heating rate of 5–10 °C/min, and a few minutes to hours of residence time [67, 74].
Biochar Characterization
In understanding the features and possible application of biochar, it is subjected to various characterizations. Some of the characterization techniques include elemental, proximate, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), X-ray Diffraction (XRD), X-ray photoelectron spectrometer (XPS), Fourier transform infrared spectroscopy (FTIR), pH, and energy dispersive X-ray (EDX) analysis [75]. Elemental analysis is utilized in determining the carbon, hydrogen, nitrogen, oxygen (by difference) [76], and sometimes sulfur content of biochar samples [77]. Proximate analysis is influential in determining the volatile matter, moisture, and fixed carbon, together with ash [76]. TGA assists in understanding the thermal decomposition [78], stability [79], and kinetic pattern during the pyrolysis of biomass to form biochar [80]. SEM is an analysis that is useful in detecting biochar’s superficial morphology and structure [79]. BET is a valuable technique for determining the pore size together with the pore volume, and the surface area of biochar [79], XPS is functional in evaluating the elemental constituent (albeit at the surface alone) [79], while XRD is useful in determining the crystalline and amorphous phase present in biochar [81]. pH analysis detects the extent of acidity or alkalinity of a biochar material, and FTIR is beneficial in determining the functional groups that are on biochar’s surface [75]. EDX is another method for checking biochar’s elemental constituent [75], ThermoFisher [82]. Other characterization techniques include “inductively coupled plasma mass spectrometry (ICP-MS)” [83], electrical conductivity [84], and Raman spectroscopy analysis [85]. It is essential to note that the choice of characterization depends on the projected application and utilization of biochar. For using biochar as an adsorbent in WWTP, some of the reported analyses are mentioned in Table 4.
Phcs Adsorption Using Biochar
Globally, carbon-containing materials are used in the adsorption process, with activated carbon (AC) being the most prevalent [40]. However, the cost of its production is high and has resulted in the search for a low-cost alternative [30, 86]. Recently, biochar that possess comparable adsorbent properties to AC has been considered an economically viable option [30, 86]. The slow pyrolysis of biomass and biomass wastes under restricted oxygen condition produces biochar that has recently been described as an appropriate and low-cost adsorbent for improving Phcs removal from WWTP using adsorption [55, 87]. Furthermore, Oliveira et al., elucidated that biochar is a cheap substitute for activated carbon for removing diverse environmental contaminants like Phcs [88]. Some existing reports on the use of biochar for Phcs uptake are mentioned in Table 4 Further reports on the use of biochar for the uptake of MF, DF, and IB are presented in Tables 5, 6, and 7 respectively.
Adsorption is a surface phenomenon such that the adsorbate lies on the adsorbent either through a chemical or physical mechanism [89]. The possibilities of utilizing biochar for Phcs adsorption have been attested to at laboratory scale, with reports of several mechanisms that govern their adsorption onto biochar’s surface. Some of these mechanisms are highlighted in Tables 4, 5, 6, and 7. From these, a summary of the prevalent mechanisms controlling the adsorption of Phcs including MF, DF, and IB onto biochar’s surface is presented in Fig. 8.
Recommendations
From the summaries provided, we can conclude that it is possible to utilize biochar for improving the removal of Phcs, including MF, DF, and IB during the treatment of wastewater at WWTP. Hence, there would need to be more publicity on this possibility. Furthermore, there is a need for proper education on Phcs disposal within the environment, alongside the danger it poses if it is improperly disposed of. One of the limitations of this study is the concern on the recovery and re-utilization of spent biochar during wastewater treatment, however, there are a few reports on regenerating spent biochar during adsorption as reported in Table 8. Another concern is on augmenting biochar’s physiochemical features to ensure that they perform extremely well as an adsorbent. Most of the studies reviewed had to improve biochar’s features for better adsorption performance via activation using physical or chemical means. Hence, this could result in a rise in the overall cost of producing and utilizing biochar for Phcs adsorption. Nonetheless, a cost–benefit analysis should suffice in establishing the trade-off between the cost of biochar production and activation during Phcs’ adsorption. For future study, a feasibility study should suffice to ascertain the cost-effectiveness of using biochar for improving the removal of Phcs at WWTP. To do this, a process model that reflects the operations of a WWTP would be very useful. Lastly, most biochar-adsorption experiments have been studied at laboratory scales and there should be a further step in trying it out at pilot and industrial scales. Lastly, asides the possibility of using biochar in an adsorption process, its production from biomass wastes pave a path for a bio-circular economy universally.
Conclusions
The danger of Phcs within the environment has been expressed in this synopsis, with emphasis on MF, DF, and IB in Estonia. The effluents from WWTP have been identified as one of the point carriers of Phcs into the environment, hence, the need to improve existing treatment methods for removing Phcs during wastewater treatment at WWTP. In contrast to the idea of replacing the existing methods of wastewater treatment, it is better to complement them with adsorption. This is because adsorption alone would not be sufficient for improving Phcs removal at WWTP. Owing to this, the possibility of using adsorption with biochar being the adsorbent for improving pharmaceutical removal has been stated in this work.
Data Availability
The source of all the data utilized in the manuscript was properly cited.
Change history
02 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12649-023-02093-9
References
Ebele, A.J., Abou-Elwafa Abdallah, M., Harrad, S.: Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 3(1), 1–16 (2017). https://doi.org/10.1016/j.emcon.2016.12.004
Haiba, E., Nei, L., Ivask, M., Peda, J., Järvis, J., Lillenberg, M., Kipper, K., Herodes, K.: Sewage sludge composting and fate of pharmaceutical residues-recent studies in Estonia. Agron. Res. 14(5), 1583–1600 (2016)
Medscape. (n.d.). Diabinese (chlorpropamide) dosing, indications, interactions, adverse effects, and more. https://reference.medscape.com/drug/diabinese-chlorpropamide-342704 Accessed 28 June 2022
Nasri, H., Rafieian-Kopaei, M.: Metformin: current knowledge. J. Res. Med. Sci. 19(7), 658 (2014)
Dai, C., Li, S., Duan, Y., Leong, K.H., Tu, Y., Zhou, L.: Human health risk assessment of selected pharmaceuticals in the five major river basins China. Sci. Total Environ. 801, 149730 (2021). https://doi.org/10.1016/J.SCITOTENV.2021.149730
Alfonso-Muniozguren, P., Serna-Galvis, E.A., Bussemaker, M., Torres-Palma, R.A., Lee, J.: A review on pharmaceuticals removal from waters by single and combined biological, membrane filtration and ultrasound systems. Ultrason. Sonochem. 76, 105656 (2021). https://doi.org/10.1016/J.ULTSONCH.2021.105656
Archer, E., Petrie, B., Kasprzyk-Hordern, B., Wolfaardt, G.M.: The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere (2017). https://doi.org/10.1016/j.chemosphere.2017.01.101
González-Alonso, S., Merino, L.M., Esteban, S., López de Alda, M., Barceló, D., Durán, J.J., López-Martínez, J., Aceña, J., Pérez, S., Mastroianni, N., Silva, A., Catalá, M., Valcárcel, Y.: Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environ. Pollut. 229, 241–254 (2017). https://doi.org/10.1016/j.envpol.2017.05.060
Madikizela, L.M., Ncube, S., Tutu, H., Richards, H., Newman, B., Ndungu, K., Chimuka, L.: Pharmaceuticals and their metabolites in the marine environment: sources, analytical methods and occurrence. Trends Environ. Anal. Chem. 28, e00104 (2020). https://doi.org/10.1016/J.TEAC.2020.E00104
Undeman, E., Rasmusson, K., Kokorite, I., Leppänen, M.T., Larsen, M.M., Pazdro, K., Siedlewicz, G.: Micropollutants in urban wastewater: large-scale emission estimates and analysis of measured concentrations in the Baltic Sea catchment. Mar. Pollut. Bull. (2022). https://doi.org/10.1016/j.marpolbul.2022.113559
Verlicchi, P., Al Aukidy, M., Zambello, E.: Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 429, 123–155 (2012). https://doi.org/10.1016/j.scitotenv.2012.04.028
Ihsanullah, I., Khan, M.T., Zubair, M., Bilal, M., Sajid, M.: Removal of pharmaceuticals from water using sewage sludge-derived biochar: a review. Chemosphere 289, 133196 (2022). https://doi.org/10.1016/J.CHEMOSPHERE.2021.133196
Nawrat, A.: Pharma and the environment: why pollution remains a worrying trend. https://www.pharmaceutical-technology.com/features/pharma-and-the-environment-pollution-trend/ (2018). Accessed 10 Dec 2021
Vergeynst, L., Haeck, A., De Wispelaere, P., Van Langenhove, H., Demeestere, K.: Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography–magnetic sector mass spectrometry: method quality assessment and application in a Belgian case study. Chemosphere 119, S2–S8 (2015). https://doi.org/10.1016/J.CHEMOSPHERE.2014.03.069
Maculewicz, J., Kowalska, D., Świacka, K., Toński, M., Stepnowski, P., Białk-Bielińska, A., Dołżonek, J.: Transformation products of pharmaceuticals in the environment: their fate, (eco)toxicity and bioaccumulation potential. Sci. Total Environ. 802, 149916 (2022). https://doi.org/10.1016/J.SCITOTENV.2021.149916
Chia, M.A., Lorenzi, A.S., Ameh, I., Dauda, S., Cordeiro-Araújo, M.K., Agee, J.T., Okpanachi, I.Y., Adesalu, A.T.: Susceptibility of phytoplankton to the increasing presence of active pharmaceutical ingredients (APIs) in the aquatic environment: a review. Aquatic Toxicol. 234, 105809 (2021). https://doi.org/10.1016/J.AQUATOX.2021.105809
Yang, X., Nguyen, X.C., Tran, Q.B., Huyen Nguyen, T.T., Ge, S., Nguyen, D.D., Nguyen, V.T., Le, P.C., Rene, E.R., Singh, P., Raizada, P., Ahamad, T., Alshehri, S.M., Xia, C., Kim, S.Y., Le, Q.V.: Machine learning-assisted evaluation of potential biochars for pharmaceutical removal from water. Environ. Res. 214, 113953 (2022). https://doi.org/10.1016/J.ENVRES.2022.113953
Ek Henning, H., Putna-Nīmane, I., Kalinowski, R., Perkola, N., Bogusz, A., Kubliņa, A., Haiba, E., Bārda, I., Karkovska, I., Schütz, J., Mehtonen, J., Siimes, K., Nyhlén, K., Dzintare, L., Äystö, L., Siņics, L., Laht, M., Lehtonen, M., Stapf, M., Leisk, Ü.: Pharmaceuticals in the Baltic Sea Region – emissions, consumption and environmental risks. https://www.researchgate.net/publication/348235264_Pharmaceuticals_in_the_Baltic_Sea_Region_-_emissions_consumption_and_environmental_risks (2020)
Fedorova, G., Golovko, O., Randak, T., Grabic, R.: Storage effect on the analysis of pharmaceuticals and personal care products in wastewater. Chemosphere 111, 55–60 (2014). https://doi.org/10.1016/J.CHEMOSPHERE.2014.02.067
Vieno, N., Tuhkanen, T., Kronberg, L.: Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 41(5), 1001–1012 (2007). https://doi.org/10.1016/j.watres.2006.12.017
Al Qarni, H., Collier, P., O’Keeffe, J., Akunna, J.: Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 23(13), 13003–13014 (2016). https://doi.org/10.1007/s11356-016-6389-7
Shraim, A., Diab, A., Alsuhaimi, A., Niazy, E., Metwally, M., Amad, M., Sioud, S., Dawoud, A.: Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab. J. Chem. (2017). https://doi.org/10.1016/j.arabjc.2012.11.014
Liu, P., Wu, X., Shi, H., Wang, H., Huang, H., Shi, Y., Gao, S.: Contribution of aged polystyrene microplastics to the bioaccumulation of pharmaceuticals in marine organisms using experimental and model analysis. Chemosphere 287, 132412 (2022). https://doi.org/10.1016/J.CHEMOSPHERE.2021.132412
Milmo, S.: Pharmaceuticals in the environment. Pharm. Technol. 42(8), 1–2 (2018)
Valavanidis, A., Vlachogianni, T., Loridas, S., Fiotakis, C.: An emerging environmental problem: disposed medicinal active products pharmaceuticals, antibiotics, and disinfectants in the aquatic environment and toxicological considerations. Pharmakeftiki 26(3), 78–98 (2014)
Nicholas, N.: Pros And Cons Of Wastewater Treatment Methods Coagulation And Disinfection. Water Online. https://www.wateronline.com/doc/pros-and-cons-of-wastewater-treatment-methods-coagulation-and-disinfection-0001 (2020). Accessed 12 Dec 2021
Czech, B., Kończak, M., Rakowska, M., Oleszczuk, P.: Engineered biochars from organic wastes for the adsorption of diclofenac, naproxen and triclosan from water systems. J. Clean. Prod. 288, 125686 (2021). https://doi.org/10.1016/j.jclepro.2020.125686
Sukmana, H., Bellahsen, N., Pantoja, F., Hodur, C.: Adsorption and coagulation in wastewater treatment—Review. Prog. Agric. Eng. Sci. 17(1), 49–68 (2021). https://doi.org/10.1556/446.2021.00029
Srivatsav, P., Bhargav, B.S., Shanmugasundaram, V., Arun, J., Gopinath, K.P., Bhatnagar, A.: Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: a review. Water (Switzerland) 12(12), 1–27 (2020). https://doi.org/10.3390/w12123561
Escudero-Curiel, S., Penelas, U., Sanromán, M.Á., Pazos, M.: An approach towards Zero-Waste wastewater technology: fluoxetine adsorption on biochar and removal by the sulfate radical. Chemosphere 268, 129318 (2021). https://doi.org/10.1016/j.chemosphere.2020.129318
Grisales-Cifuentes, C.M., Serna Galvis, E.A., Porras, J., Flórez, E., Torres-Palma, R.A., Acelas, N.: Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 326, 124753 (2021). https://doi.org/10.1016/j.biortech.2021.124753
Constro Facilitator. Membrane filtration for wastewater treatment. https://www.constrofacilitator.com/membrane-filtration-for-wastewater-treatment/ (2021). Accessed 25 July 2022
Tiwari, B., Sellamuthu, B., Ouarda, Y., Drogui, P., Tyagi, R.D., Buelna, G.: Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Biores. Technol. 224, 1–12 (2017). https://doi.org/10.1016/j.biortech.2016.11.042
Deegan, A.M., Shaik, B., Nolan, K., Urell, K., Oelgemöller, M., Tobin, J., Morrissey, A.: Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 8(3), 649–666 (2011). https://doi.org/10.1007/BF03326250
Bilal, M., Ihsanullah, I., Younas, M., Ul, M., Shah, H.: Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: a critical review. Sep. Purif. Technol. 278, 1383–5866 (2022). https://doi.org/10.1016/j.seppur.2021.119510
Mansouri, F., Chouchene, K., Roche, N., Ksibi, M.: Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: state of the art and trends. Appl. Sci. 11(14), 6659 (2021). https://doi.org/10.3390/APP11146659
Kyzas, G.Z., Fu, J., Matis, K.A.: The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials 6(11), 5131–5158 (2013). https://doi.org/10.3390/ma6115131
Pigatto, R.S., Franco, D.S.P., Netto, M.S., Carissimi, É., Oliveira, L.F.S., Jahn, S.L., Dotto, G.L.: An eco-friendly and low-cost strategy for groundwater defluorination: adsorption of fluoride onto calcinated sludge. J. Environ. Chem. Eng. 8(6), 104546 (2020). https://doi.org/10.1016/J.JECE.2020.104546
Busetty, S.: Environmental Treatment Technologies: Adsorption. In: Hussain, C.M. (ed.) Handbook of Environmental Materials Management. Springer, Cham (2019). https://doi.org/10.1007/978-3-319-73645-7_37
Akintola, A.T., Akinlabi, E.T., Masebinu, S.O.: Biochar as an Adsorbent: A Short Overview. In: Daramola, M., Ayeni, A. (eds.) Valorization of Biomass to Value-Added Commodities Green Energy and Technology, pp. 399–422. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-38032-8_19
Metslaid, S., Hordo, M., Korjus, H., Kiviste, A., Kangur, A.: Spatio-temporal variability in Scots pine radial growth responses to annual climate fluctuations in hemiboreal forests of Estonia. Agric. For. Meteorol. 252, 283–295 (2018). https://doi.org/10.1016/j.agrformet.2018.01.018
Uusküla, A., Kalda, R., Solvak, M., Jürisson, M., Käärik, M., Fischer, K., Keis, A., Raudvere, U., Vilo, J., Peterson, H., Käärik, E., Metspalu, M., Jürgenson, T., Milani, L., Kolberg, L., Tiit, E.M., Vassil, K.: The 1st year of the COVID-19 epidemic in Estonia: a population-based nationwide sequential/consecutive cross-sectional study. Public Health 205, 150–156 (2022). https://doi.org/10.1016/J.PUHE.2022.02.004
Lember, E., Pachel, K., Loigu, E.: Modelling diclofenac and ibuprofen residues in major estonian seaside cities erki lember. Karin Pachel, Enn Loigu. 2, 1–7 (2016)
Republic of Estonia Agency of Medicines. (n.d.-a). Overview of Estonian medicinal products market (Ravimiamet). https://ravimiamet.ee/en/statistics/statistics-medicines. Accessed 4 July 2021
Republic of Estonia Agency of Medicines. (n.d.-b). The Estonian medicinal products market in fourth quarter 2017: Ravimiamet. https://ravimiamet.ee/en/statistics/statistics-medicines. Accessed 15 July 2021
Vilnius. Baltic Statistics on Medicines 2016–2018. https://www.zva.gov.lv/sites/default/files/2020-01/Baltic Statistics_2016-2018.pdf(2019). Accessed 15 July 2021
Helcom. Pharmaceuticals in the aquatic environment of the Baltic Sea region A status report International Initiative on Water Quality-IIWQ (Issue 149). (2017).
Niemuth, N.J., Jordan, R., Crago, J., Blanksma, C., Johnson, R., Klaper, R.D.: Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish. Environ. Toxicol. Chem. 34(2), 291–296 (2015). https://doi.org/10.1002/etc.2793
International Diabetes Federation. (n.d.). Diabetes facts & figures. https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed 4 July 2021
International Diabetes Foundation Europe Members. (n.d.). https://idf.org/our-network/regions-members/europe/members/131-estonia.html. Accessed 4 July 2021
International Diabetes Federation. IDF DIABETES ATLAS 9th Edition (Europe). www.diabetesatlas.org (2019). Accessed 27 July 2022
Kõrgmaa, V., Laht, M., Rebane, R., Lember, E., Pachel, K., Kriipsalu, M., Tenno, T., Iital, A.: Removal of hazardous substances in municipal wastewater treatment plants. Water Sci. Technol. 81(9), 2011–2022 (2020). https://doi.org/10.2166/wst.2020.264
Kõrgmaa, V., Tenno, T., Kivirüt, A., Kriipsalu, M., Gross, M., Tamm, P., Karabelnik, K., Terase, H., Värk, V., Lepik, N., Pachel, K., Iital, A.: A novel method for rapid assessment of the performance and complexity of small wastewater treatment plants. Proc. Est. Acad. Sci. 68(1), 32–42 (2019). https://doi.org/10.3176/proc.2019.1.03
Tooming, A.: Estonian experience in the water management. https://unece.org/fileadmin/DAM/env/documents/2012/wat/workshops/Nordic_Baltic_Seminar_Oslo/4a.Estonia_final_water_management_sewage.pdf (2011). Accessed 27 Dec 2022
Bimová, P., Roupcová, P., Klouda, K., Matejová, L., Stanová, A.V., Grabicová, K., Grabic, R., Majová, V., Híveš, J., Špalková, V., Gemeiner, P., Celec, P., Konecná, B., Bírošová, L., Krahulcová, M., Mackulak, T.: Biochar – An efficient sorption material for the removal of pharmaceutically active compounds, DNA and RNA fragments from wastewater. J. Environ. Chem. Eng. 9(4), 105746 (2021). https://doi.org/10.1016/J.JECE.2021.105746
Zubair, M., Ihsanullah, I., Abdul Aziz, H., Azmier Ahmad, M., Al-Harthi, M.A.: Sustainable wastewater treatment by biochar/layered double hydroxide composites: progress, challenges, and outlook. Bioresour. Technol. 319, 124128 (2021). https://doi.org/10.1016/j.biortech.2020.124128
Shackley, S., Hammond, J., Gaunt, J., Ibarrola, R.: The feasibility and costs of biochar deployment in the UK. Carbon Manag. 2(3), 335–356 (2011). https://doi.org/10.4155/cmt.11.22
Hanoğlu, A., Çay, A., Yanık, J.: Production of biochars from textile fibres through torrefaction and their characterisation. Energy 166, 664–673 (2019). https://doi.org/10.1016/j.energy.2018.10.123
Acharya, B., Dutta, A., Minaret, J.: Review on comparative study of dry and wet torrefaction. Sustain. Energy Technol. Assess. 12, 26–37 (2015). https://doi.org/10.1016/j.seta.2015.08.003
Bergman, P.C.A., Boersma, A.R., Zwart, R.W.R., Kiel, J.H.A.: “Torrefaction for biomass co-firing in existing coal-fired power stations.” [Online]. Available: https://publicaties.ecn.nl/PdfFetch.aspx?nr=ECN-C--05-013 (2005). Accessed 29 Dec 2018
Zhang, D., Wang, F., Zhang, A., Yi, W., Li, Z., Shen, X.: Effect of pretreatment on chemical characteristic and thermal degradation behavior of corn stalk digestate : comparison of dry and wet torrefaction. Bioresour. Technol. 275, 239–246 (2019). https://doi.org/10.1016/j.biortech.2018.12.044
Budai, A., Wang, L., Gronli, M., Strand, L.T., Antal, M.J., Abiven, S., Dieguez-Alonso, A., Anca-Couce, A., Rasse, D.P.: Surface properties and chemical composition of corncob and miscanthus biochars: Effects of production temperature and method. J. Agric. Food Chem. 62(17), 3791–3799 (2014). https://doi.org/10.1021/jf501139f
Xu, X., Tu, R., Sun, Y., Li, Z., Jiang, E.: Influence of biomass pretreatment on upgrading of bio-oil: comparison of dry and hydrothermal torrefaction. Biores. Technol. 262, 261–270 (2018). https://doi.org/10.1016/j.biortech.2018.04.037
Soh, M., Khaerudini, D.S., Chew, J.J., Sunarso, J.: Wet torrefaction of empty fruit bunches (EFB) and oil palm trunks (OPT): effects of process parameters on their physicochemical and structural properties. S. Afr. J. Chem. Eng. 35, 126–136 (2021). https://doi.org/10.1016/j.sajce.2020.09.004
Brewer, C.E., Schmidt-Rohr, K., Satrio, J.A., Brown, R.C.: Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 28(3), 386–396 (2009). https://doi.org/10.1002/ep.10378
Benedetti, V., Patuzzi, F., Baratieri, M.: Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications. Appl. Energy 227(2017), 92–99 (2018). https://doi.org/10.1016/j.apenergy.2017.08.076
Lee, J., Sarmah, A.K., Kwon, E.E.: Production and Formation of Biochar. In: Ok, Y.S., Daniel, C.W. (eds.) Tsang Biochar from Biomass and Waste, pp. 3–18. Elsevier, Amsterdam (2019). https://doi.org/10.1016/B978-0-12-811729-3.00001-7
Yaman, S.: Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manage. 45(5), 651–671 (2004). https://doi.org/10.1016/S0196-8904(03)00177-8
Ippolito, J.A., Laird, D.A., Busscher, W.J.: Environmental benefits of biochar. J. Environ. Qual. 41(4), 967 (2012). https://doi.org/10.2134/jeq2012.0151
Odinga, E.S., Gudda, F.O., Waigi, M.G., Wang, J., Gao, Y.: Occurrence, formation and environmental fate of polycyclic aromatic hydrocarbons in biochars. Fundam. Res. 1(3), 296–305 (2021). https://doi.org/10.1016/J.FMRE.2021.03.003
Mohan, D., Pittman, C.U., Steele, P.H.: Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20(3), 848–889 (2006). https://doi.org/10.1021/ef0502397
Laird, D.A., Brown, R.C., Amonette, J.E., Lehmann, J.: Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 3(5), 547–562 (2009). https://doi.org/10.1002/bbb.169
Leng, L., Huang, H., Li, H., Li, J., Zhou, W.: Biochar stability assessment methods: a review. Sci. Total Environ. 647, 210–222 (2019). https://doi.org/10.1016/j.scitotenv.2018.07.402
Onay, O., Kockar, O.M.: Slow, fast and flash pyrolysis of rapeseed. Renew. Energy 28(15), 2417–2433 (2003). https://doi.org/10.1016/S0960-1481(03)00137-X
Adesemuyi, F.M., Adebayo, M.A., Akinola, A.O., Olasehinde, E.F., Adewole, K.A., Lajide, L.: Preparation and characterisation of biochars from elephant grass and their utilisation for aqueous nitrate removal: effect of pyrolysis temperature. J. Environ. Chem. Eng (2020). https://doi.org/10.1016/j.jece.2020.104507
Rathnayake, D., Maziarka, P., Ghysels, S., Mašek, O., Sohi, S., Ronsse, F.: How to trace back an unknown production temperature of biochar from chemical characterization methods in a feedstock independent way. J. Anal. Appl. Pyrolysis 151, 104926 (2020). https://doi.org/10.1016/j.jaap.2020.104926
Gazulla, M.F., Rodrigo, M., Orduña, M., Gómez, C.M.: Determination of carbon, hydrogen, nitrogen and sulfur in geological materials using elemental analysers. Geostand. Geoanal. Res. 36(2), 201–217 (2012). https://doi.org/10.1111/J.1751-908X.2011.00140.X
Parsa, M., Nourani, M., Baghdadi, M., Hosseinzadeh, M., Pejman, M.: Biochars derived from marine macroalgae as a mesoporous by-product of hydrothermal liquefaction process: characterization and application in wastewater treatment. J. Water Process. Eng. 32, 100942 (2019). https://doi.org/10.1016/j.jwpe.2019.100942
Wang, P., Liu, X., Yu, B., Wu, X., Xu, J., Dong, F., Zheng, Y.: Characterization of peanut-shell biochar and the mechanisms underlying its sorption for atrazine and nicosulfuron in aqueous solution. Sci. Total Environ. 702(2), 134767 (2020). https://doi.org/10.1016/j.scitotenv.2019.134767
Sotoudehnia, F., Baba Rabiu, A., Alayat, A., McDonald, A.G.: Characterization of bio-oil and biochar from pyrolysis of waste corrugated cardboard. J. Anal. Appl. Pyrolysis 145, 104722 (2020). https://doi.org/10.1016/j.jaap.2019.104722
Tong, W., Cai, Z., Liu, Q., Ren, S., Kong, M.: Evaluation of biochar combustion reactivity under pyrolysis temperature: microstructure characterization, kinetics and thermodynamics. J. Energy Inst. 93(5), 1914–1923 (2020). https://doi.org/10.1016/j.joei.2020.04.006
ThermoFisher Sientific. EDX Analysis with SEM: How Does it Work? - Accelerating Microscopy. https://www.thermofisher.com/blog/microscopy/edx-analysis-with-sem-how-does-it-work/ (2020). Accessed 25 Nov 2022
Chen, G., Taherymoosavi, S., Cheong, S., Yin, Y., Akter, R., Marjo, C.E., Rich, A.M., Mitchell, D.R.G., Fan, X., Chew, J., Pan, G., Li, L., Bian, R., Horvat, J., Mohammed, M., Munroe, P., Joseph, S.: Advanced characterization of biomineralization at plaque layer and inside rice roots amended with iron-and silica-enhanced biochar. Sci. Rep. 11, 159 (2021). https://doi.org/10.1038/s41598-020-80377-z
Li, X., Shen, Q., Zhang, D., Mei, X., Ran, W., Xu, Y., Yu, G.: Functional groups determine biochar properties (pH and EC) as studied by two-dimensional 13C NMR correlation spectroscopy. PLoS ONE (2013). https://doi.org/10.1371/JOURNAL.PONE.0065949
De Sousa, D.V., Guimarães, L.M., Félix, J.F., Ker, J.C., Schaefer, C.E.R.G., Rodet, M.J.: Dynamic of the structural alteration of biochar in ancient Anthrosol over a long timescale by Raman spectroscopy. PLoS ONE 15(3), e0229447 (2020). https://doi.org/10.1371/JOURNAL.PONE.0229447
Escudero-Curiel, S., Acevedo-García, V., Sanromán, M.Á., Pazos, M.: Eco-approach for pharmaceutical removal: thermochemical waste valorisation, biochar adsorption and electro-assisted regeneration. Electrochim. Acta 389, 138694 (2021). https://doi.org/10.1016/J.ELECTACTA.2021.138694
Solanki, A., Boyer, T.H.: Pharmaceutical removal in synthetic human urine using biochar. Environ. Sci.: Water Res. Technol. 3(3), 553–565 (2017). https://doi.org/10.1039/C6EW00224B
Oliveira, F.R., Patel, A.K., Jaisi, D.P., Adhikari, S., Lu, H., Khanal, S.K.: Environmental application of biochar: current status and perspectives. Biores. Technol. 246, 110–122 (2017). https://doi.org/10.1016/j.biortech.2017.08.122
Oginni, O., Singh, K.: Effect of carbonization temperature on fuel and caffeine adsorption characteristics of white pine and Norway spruce needle derived biochars. Ind. Crops Prod. 162, 113261 (2021). https://doi.org/10.1016/j.indcrop.2021.113261
Borah, A.: Review of the emerging use of activated carbon or biochar media as stormwater source controls. https://sustain.ubc.ca/sites/default/files/2020-16_Review of activated charcoal and biochar_Borah.pdf (2020). Accessed 20 Nov 2021
Crini, G., Lichtfouse, E., Wilson, L.D., Morin-Crini, N.: Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 17(1), 195–213 (2019). https://doi.org/10.1007/S10311-018-0786-8/TABLES/2
Shirani, Z., Song, H., Bhatnagar, A.: Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci. Total Environ. 745, 140789 (2020). https://doi.org/10.1016/j.scitotenv.2020.140789
Thompson, K.A., Shimabuku, K.K., Kearns, J.P., Knappe, D.R.U., Summers, R.S., Cook, S.M.: Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 50(20), 11253–11262 (2016). https://doi.org/10.1021/ACS.EST.6B03239
Xiao, L., Feng, L., Yuan, G., Wei, J.: Low-cost field production of biochars and their properties. Environ. Geochem. Health 42(6), 1569–1578 (2019). https://doi.org/10.1007/S10653-019-00458-5
Statistics Estonia. Growth in life expectancy has slowed down, but Estonian people live a longer healthy life. https://www.stat.ee/en/node/183291 (2021). Accessed 21 July 2022
American Chemical Society. (n.d.). Ibuprofen. https://www.acs.org/content/acs/en/molecule-of-the-week/archive/i/ibuprofen.html. Accessed 13 Dec 2021
Bian, X., Jiang, L., Gan, Z., Guan, X., Zhang, L., Cai, L., Hu, X.: A glimepiride-metformin multidrug crystal: synthesis, crystal structure analysis, and physicochemical properties. Molecules (2019). https://doi.org/10.3390/MOLECULES24203786
Liu, Y.J., Hu, C.Y., Lo, S.L.: Comparison of the degradation of multiple amine-containing pharmaceuticals during electroindirect oxidation and electrochlorination processes in continuous system. Water Res. 203, 117517 (2021). https://doi.org/10.1016/J.WATRES.2021.117517
National Library of Medicine (PubChem). (n.d.-a). Diclofenac. https://pubchem.ncbi.nlm.nih.gov/compound/diclofenac#section=1D-NMR-Spectra. Accessed 13 Dec 2021
National Library of Medicine (PubChem). (n.d.-b). Ibuprofen. https://pubchem.ncbi.nlm.nih.gov/compound/Ibuprofen. Accessed 13 Dec 2021
National Library of Medicine (PubChem). (n.d.-c). Metformin. https://pubchem.ncbi.nlm.nih.gov/compound/metformin#section=3D-Conformer. Accessed 13 Dec 2021
Koester, V.: What is HPLC? - ChemistryViews. https://www.chemistryviews.org/details/education/9464911/What_is_HPLC/ (2016). Accessed 3 Jan 2023
Seneca: Alkaloid Chemistry. In: Aniszewski, T. (ed.) Alkaloids—Secrets of Life, pp. 61–139. Elsevier, Amsterdam (2007). https://doi.org/10.1016/B978-044452736-3/50004-0
Mccormick, J.P., Carrel, J.E.: Cantharidin Biosynthesis and Function in Meloid Beetles. In: Prestwich, G.D., Blomquist, G.J. (eds.) Pheromone Biochemistry, pp. 307–350. Academic Press, Cambridge (1987). https://doi.org/10.1016/B978-0-12-564485-3.50015-4
Tran, H.N., Tomul, F., Ha, N.T., Nguyen, D.T., Lima, E.C., Le, G.T., Chang, C.T., Masindi, V., Woo, S.H.: Innovative spherical biochar for pharmaceutical removal from water: insight into adsorption mechanism. J. Hazard. Mater. 394, 122255 (2020)
Paunovic, O., Pap, S., Maletic, S., Taggart, M.A., Boskovic, N., Turk Sekulic, M.: Ionisable emerging pharmaceutical adsorption onto microwave functionalised biochar derived from novel lignocellulosic waste biomass. J. Colloid Interface Sci. 547, 350–360 (2019). https://doi.org/10.1016/j.jcis.2019.04.011
Chakraborty, P., Show, S., Ur Rahman, W., Halder, G.: Linearity and non-linearity analysis of isotherms and kinetics for ibuprofen remotion using superheated steam and acid modified biochar. Process Saf. Environ. Prot. 126, 193–204 (2019). https://doi.org/10.1016/j.psep.2019.04.011
Mondal, S., Aikat, K., Halder, G.: Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: equilibrium, kinetics, thermodynamics and modeling. Ecol. Eng. 92, 158–172 (2016). https://doi.org/10.1016/j.ecoleng.2016.03.022
Mondal, S., Bobde, K., Aikat, K., Halder, G.: Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manage. 182, 581–594 (2016). https://doi.org/10.1016/j.jenvman.2016.08.018
Xu, D., Li, Z., Wang, P., Bai, W., Wang, H.: Aquatic plant-derived biochars produced in different pyrolytic conditions: spectroscopic studies and adsorption behavior of diclofenac sodium in water media. Sustain. Chem. Pharm. 17, 100275 (2020). https://doi.org/10.1016/j.scp.2020.100275
Rathod, R.H., Chaudhari, S.R., Patil, A.S., Shirkhedkar, A.A.: Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) in practice: analysis of drugs and pharmaceutical formulations. Futur. J. Pharm. Sci. 5(1), 1–26 (2019). https://doi.org/10.1186/S43094-019-0007-8
EAG Laboratories. Ultra-High-Performance Liquid Chromatography (UHPLC). https://www.eag.com/techniques/chromatography/ultra-high-performance-liquid-chromatography-uhplc/ (2023). Accessed 3 Jan 2023
Taleuzzaman, M., Ali, S., Gilani, S., Imam, S., Hafeez, A.: Ultra performance liquid chromatography (UPLC)—A review. J. Anal. Pharm. Chem. 2(6), 1056 (2015)
Creative Proteomics. Ultra Performance Liquid Chromatography (UPLC) Based Analysis Services - Creative Proteomics. https://www.creative-proteomics.com/technology/ultra-performance-liquid-chromatography-uplc-based-analysis-service.htm (2023). Accessed 3 Jan 2023
Dyad Labs. (n.d.). HPLC vs. UPLC. In D. https://dyadlabs.com/wp-content/uploads/2018/10/hplc_uplc_onesheet.pdf. Accessed 3 Jan 2023
Wu, Y., Engen, J.R., Hobbins, W.B.: Ultra performance liquid chromatography (UPLC) further improves hydrogen/deuterium exchange mass spectrometry. J. Am. Soc. Mass Spectrom. 17(2), 163–167 (2006). https://doi.org/10.1016/J.JASMS.2005.10.009
Wu, Q., Zhang, Y., Cui, M., Liu, H., Liu, H., Zheng, Z., Zheng, W., Zhang, C., Wen, D.: Pyrolyzing pharmaceutical sludge to biochar as an efficient adsorbent for deep removal of fluoroquinolone antibiotics from pharmaceutical wastewater: performance and mechanism. J. Hazard. Mater. (2021). https://doi.org/10.1016/J.JHAZMAT.2021.127798
Pratiwi, R.A., Nandiyanto, A.B.D.: How to read and interpret UV-VIS spectrophotometric results in determining the structure of chemical compounds. Indonesian J. Educ. Res. Technol. 2(1), 1–20 (2022). https://doi.org/10.17509/ijert.v2i1.35171
Tom, J.: UV-Vis Spectroscopy: Principle, Strengths and Limitations and Applications | Technology Networks. https://www.technologynetworks.com/analysis/articles/uv-vis-spectroscopy-principle-strengths-and-limitations-and-applications-349865 (2021). Accessed 3 Jan 2023
Singh, V., Srivastava, V.C.: Self-engineered iron oxide nanoparticle incorporated on mesoporous biochar derived from textile mill sludge for the removal of an emerging pharmaceutical pollutant. Environ. Pollut. 259, 113822 (2020). https://doi.org/10.1016/j.envpol.2019.113822
Maged, A., Dissanayake, P.D., Yang, X., Pathirannahalage, C., Bhatnagar, A., Ok, Y.S.: New mechanistic insight into rapid adsorption of pharmaceuticals from water utilizing activated biochar. Environ. Res. 202, 111693 (2021). https://doi.org/10.1016/J.ENVRES.2021.111693
Chakraborty, P., Singh, S.D., Gorai, I., Singh, D., Rahman, W.U., Halder, G.: Explication of physically and chemically treated date stone biochar for sorptive remotion of ibuprofen from aqueous solution. J. Water Process. Eng. 33, 101022 (2020). https://doi.org/10.1016/j.jwpe.2019.101022
Moreno-Pérez, J., Pauletto, P.S., Cunha, A.M., Bonilla-Petriciolet, Á., Salau, N.P.G., Dotto, G.L.: Three-dimensional mass transport modeling of pharmaceuticals adsorption inside ZnAl/biochar composite. Colloids Surf. A Physicochem. Eng. Asp. 614, 126170 (2021). https://doi.org/10.1016/j.colsurfa.2021.126170
Quesada, H.B., De Araújo, T.P., Cusioli, L.F., De Barros, M.A.S.D., Gomes, R.G., Bergamasco, R.: Evaluation of novel activated carbons from chichá-do-cerrado (Sterculia striata St. Hil. et Naud) fruit shells on metformin adsorption and treatment of a synthetic mixture. J. Environ. Chem. Eng. 9(1), 104914 (2021). https://doi.org/10.1016/j.jece.2020.104914
Shi, J., Guo, C., Lei, C., Liu, Y., Hou, X., Zheng, X., Hu, Q.: High-performance biochar derived from the residue of Chaga mushroom (Inonotus obliquus) for pollutants removal. Bioresour. Technol. (2021). https://doi.org/10.1016/J.BIORTECH.2021.126268
Liyanage, A.S., Canaday, S., Pittman, C.U., Jr., Mlsna, T.: Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas for biochar adsorbents. Chemosphere 258, 127336 (2020)
Keerthanan, S., Bhatnagar, A., Mahatantila, K., Jayasinghe, C., Ok, Y.S., Vithanage, M.: Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media. Environ. Technol. Innov. 19, 100847 (2020). https://doi.org/10.1016/j.eti.2020.100847
Mahmoud, M.E., El-Ghanam, A.M., Saad, S.R., Mohamed, R.H.A.: Promoted removal of metformin hydrochloride anti-diabetic drug from water by fabricated and modified nanobiochar from artichoke leaves. Sustain. Chem. Pharm. 18, 100336 (2020). https://doi.org/10.1016/j.scp.2020.100336
Huang, X., Liu, Y., Liu, S., Li, Z., Tan, X., Ding, Y., Zeng, G., Xu, Y., Zeng, W., Zheng, B.: Removal of metformin hydrochloride by: Alternanthera philoxeroides biomass derived porous carbon materials treated with hydrogen peroxide. RSC Adv. 6(83), 79275–79284 (2016). https://doi.org/10.1039/c6ra08365j
De Bhowmick, G., Briones, R.M., Thiele-Bruhn, S., Sen, R., Sarmah, A.K.: Adsorptive removal of metformin on specially designed algae-lignocellulosic biochar mix and techno-economic feasibility assessment. Environ. Pollut. 292, 118256 (2022). https://doi.org/10.1016/J.ENVPOL.2021.118256
dos Santos, G.E.D.S., Ide, A.H., Duarte, J.L.S., McKay, G., Silva, A.O.S., Meili, L.: Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol. 364, 229–240 (2020). https://doi.org/10.1016/j.powtec.2020.01.083
Lonappan, L., Rouissi, T., Liu, Y., Brar, S.K., Surampalli, R.Y.: Removal of diclofenac using microbiochar fixed-bed column bioreactor. J. Environ. Chem. Eng. 7(1), 102894 (2019). https://doi.org/10.1016/j.jece.2019.102894
Correa-Navarro, Y.M., Giraldo, L., Moreno-Piraján, J.C.: Dataset for effect of pH on caffeine and diclofenac adsorption from aqueous solution onto fique bagasse biochars. Data Brief 25, 104111 (2019). https://doi.org/10.1016/j.dib.2019.104111
He, L., Lv, L., Pillai, S.C., Wang, H., Xue, J., Ma, Y., Liu, Y., Chen, Y., Wu, L., Zhang, Z., Yang, L.: Efficient degradation of diclofenac sodium by periodate activation using Fe/Cu bimetallic modified sewage sludge biochar/UV system. Sci. Total Environ. 783, 146974 (2021). https://doi.org/10.1016/j.scitotenv.2021.146974
Zhang, H., Tu, Y.J., Duan, Y.P., Liu, J., Zhi, W., Tang, Y., Xiao, L.S., Meng, L.: Production of biochar from waste sludge/leaf for fast and efficient removal of diclofenac. J. Mol. Liq. 299, 112193 (2020). https://doi.org/10.1016/j.molliq.2019.112193
Chakraborty, P., Banerjee, S., Kumar, S., Sadhukhan, S., Halder, G.: Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: isotherm, kinetics, thermodynamics and cost estimation. Process Saf. Environ. Prot. 118, 10–23 (2018). https://doi.org/10.1016/j.psep.2018.06.015
Chakraborty, P., Show, S., Banerjee, S., Halder, G.: Mechanistic insight into sorptive elimination of ibuprofen employing bi-directional activated biochar from sugarcane bagasse: Performance evaluation and cost estimation. J. Environ. Chem. Eng. 6(4), 5287–5300 (2018). https://doi.org/10.1016/j.jece.2018.08.017
Show, S., Mukherjee, S., Devi, M.S., Karmakar, B., Halder, G.: Linear and non-linear analysis of Ibuprofen riddance efficacy by Terminalia catappa active biochar: equilibrium, kinetics, safe disposal, reusability and cost estimation. Process Saf. Environ. Prot. 147, 942–964 (2021). https://doi.org/10.1016/j.psep.2021.01.024
Ocampo-Perez, R., Padilla-Ortega, E., Medellin-Castillo, N.A., Coronado-Oyarvide, P., Aguilar-Madera, C.G., Segovia-Sandoval, S.J., Flores-Ramírez, R., Parra-Marfil, A.: Synthesis of biochar from chili seeds and its application to remove ibuprofen from water. Equilibrium and 3D modeling. Sci. Total Environ. 655, 1397–1408 (2019). https://doi.org/10.1016/j.scitotenv.2018.11.283
Essandoh, M., Kunwar, B., Pittman, C.U., Mohan, D., Mlsna, T.: Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 265, 219–227 (2015). https://doi.org/10.1016/j.cej.2014.12.006
Puga, A., Moreira, M.M., Figueiredo, S.A., Delerue-Matos, C., Pazos, M., Rosales, E., Sanromán, M.Á.: Electro-Fenton degradation of a ternary pharmaceutical mixture and its application in the regeneration of spent biochar. J. Electroanal. Chem. 886, 115135 (2021). https://doi.org/10.1016/J.JELECHEM.2021.115135
World Health Organization (WHO). (n.d.). Data and statistics. https://www.euro.who.int/en/health-topics/noncommunicable-diseases/diabetes/data-and-statistics. Accessed 4 July 2021
Acknowledgements
The authors would love to acknowledge Oyepeju Abioye for assisting in the proofreading of this manuscript.
Funding
This research did not obtain any donation from any organization in the community, business, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ATA, YAA: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Original draft; Writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The Table 1 has been revised.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akintola, A.T., Ayankunle, A.Y. Improving Pharmaceuticals Removal at Wastewater Treatment Plants Using Biochar: A Review. Waste Biomass Valor 14, 2433–2458 (2023). https://doi.org/10.1007/s12649-023-02070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02070-2