Abstract
Textile wastewater (TW) from the Ban Phraek Weaving Group, Phatthalung, Thailand, showed high carbon (6405 mg/L) and nitrogen (54 mg/L) contents and a pH of 6.92, which, if not treated, will affect the environment. Newly isolated Enterobacter strain TS3 isolated from TW was used for TW treatment and PHA production under simultaneous batch conditions. The optimal conditions for the production of PHA by strain TS3 were as follows: 100% TW as the sole substrate, pH 6.92, 150 rpm, 35 °C, and 48 h of incubation at 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L). The characterization of the extracted biopolymer proves the presence of short˗chain˗length˗co˗medium˗chain˗ length PHA (scl˗co˗mcl PHA) by Fourier transform infrared spectroscopy (FTIR) and gas chromatography-mass spectrometry (GC˗MS). This is the first report on Enterobacter TS3, which can produce scl˗co˗mcl PHA using TW as a substrate. Moreover, the treated wastewater after PHA cultivation met the criteria of the Water Quality Standard (Thailand).
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
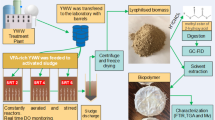
The integration of textile wastewater (TW) treatment with polyhydroxyalkanoate (PHA) production using newly isolated Enterobacter strain TS3 is a novel system. Under optimal conditions, it can be produced short chain length-co-medium chain length (scl˗co˗mcl) PHA high about 83.94 ± 2.72% cell dry mass (0.34 ± 0.02 g/L) using TW as substrate. Moreover, the TW after treatment met the criteria of the Water Quality Standard (Thailand) using the integration process. This is an interesting process as it can simultaneously treat TW and produce an environmentally friendly alternative product.
Introduction
The textile industry is an ever-growing market, which is and increases currently increasing, and is increasing at present. The textile industry encompasses the production and sale of materials such as cotton, yarn, fiber and finished products or apparels. Southern Thailand is the location of the industry sector, has the industry of where dyeing of woven bulrush mats are dyed, causing problems in the matter of wastewater. After dye processing, dye-contaminated wastewater is normally disposed of in open ponds and treated via physical methods. However, this conventional approach requires a long treatment time and results in the release of residues into natural water bodies, with the potential accumulation of hazardous compounds. The best method for treating wastewater arising from the textile industry is biological methods. Biological treatment options are currently receiving increased interest as because they are highly effective, can completely convert synthetic dye into CO2 and water, are environmentally friendly and have low operation costs [1, 2]. From other previous researches studies, it was found that has bacteria can decolourization and degradation of decolorize and degrade textile wastewater, such as Pseudomonas sp., Enterobacter EC3, Aeromonas hydrophila, Sphingomonas sp. BN6 and Bacillus sp. [3,4,5,6,7], etc. Based on our previous research, we isolated five new strains of Enterobacter from TW (Ban Phraek Weaving Group, Phatthalung, Thailand); strains TW1L, TS1P, TS1L, TS3 and TW1P can decolorcolourize and degrade dye TW [8]. Strains TS3 and TS1L showed high decolorization rates of 79.45 ± 0.24 and 81.15 ± 0.08%, respectively, under optimal conditions. In addition, strain TS3 can produce PHA at 75.34% of cell dry mass (CDM) [9]. However, the TW treatment system has not been reported in conjunction with PHA production by TS3. PHA is a family of polyesters that synthesized and accumulates in cells of various microorganisms to store carbon and energy sources. The properties of PHA are similar to those of petrochemical plastics, such as polyethylene and polypropylene [10]. The integration of TW treatment with PHA production has the advantage of utilizing the waste generated from the environment to produce a new product that is environmentally friendly.
Therefore, this research aims to use TW as a substrate to produce PHA using the newly isolated Enterobacter strain TS3. The optimization of various parameters to achieve maximum PHA production using a newly isolated of TS3 was determined. After that, PHA production was confirmed using FTIR and GC˗MS techniques. Thereafter, TW before and after TS3 cultivation was analyzed and compared with the Water Quality Standard (Thailand).
Materials and Methods
Bacterial Stored and Inoculum Preparation
Newly isolated Enterobacter strain TS3 was isolated from TW from the Ban Phraek Weaving Group (Phatthalung, Thailand) using a pretreatment method (Bushnell Haas medium-Basic Red 9 specific culture and PHA-detection agar) by Rakkan et al. [8]. The result found that five isolated strains can degrade Basic Red 9 and produce PHA. These strains were identified through 16S rDNA sequencing analysis and phylogenetic evaluation and were found to belong to Enterobacter with 100% identification. All sequences of isolates (TS1L, TS1P, TS3, TW1L, and TW1P) were submitted to GenBank with the accession numbers MN508471, MN508472, MN508473, MN508474, and MN508475, respectively. However, strain TS3 has shown a high PHA accumulation. Therefore, strain TS3 has used throughout this study. The isolate was kept in a PHA-producing agar plate containing 2 g/L (NH4)2SO4, 13.3 g/L KH2PO4, 1.2 g/L MgSO4·7H2O, 1.7 g/L citric acid, 10 mL/L trace element and 15 g/L agar at pH 7 [11]. The inoculum was prepared in a 250 mL Erlenmeyer flask containing 100 mL of PHA-producing medium; 10% of each isolate was added to the medium and incubated at 35 °C and 150 rpm for 24 h.
Sample Collection
TW was collected from the Ban Phraek Weaving Group (Phatthalung, Thailand). Samples were kept on ice and transferred to the laboratory. After that, the preparation of TW was centrifuged at 13,000×g for 10 min and then autoclaved at 121 °C for 10 min. After preparation, TW was used to optimize the parameters for PHA production and to determine the parameters pH, total dissolved solids (TDS), settleable solids, total suspended solids (TSS), oil and grease, sulfide, temperature, total Kjeldahl nitrogen (TKN), chemical oxygen demand (COD) and color.
TW Characterization
TW was characterized after preparation using the standard method. TW was analyzed for pH, total dissolved solids (TDS), settleable solids, total suspended solids (TSS), temperature, oil and grease, sulfide, total Kjeldahl nitrogen (TKN), chemical oxygen demand (COD) and color. The pH was measured using a pH meter. The parameters TDS, settleable solid, TSS, TKN and oil and grease were determined following APHA [12, 13]. Sulfide coloring reagent and hydrochloric acid were used to determine the sulfide content. The sulfide concentration was measured with a spectrophotometer at a wavelength of 667 nm [14]. Wastewater color was also determined using a spectrophotometric method (OD 200˗800 nm) [15]. The COD was evaluated using a Spectroquant® COD Cell Test (Germany) following the manufacturer´s recommendations. Textile wastewater was added to standard and miniaturized COD ampules and diluted if necessary. Subsequently, the samples were incubated at 150 °C for 2 h in a dry incubator and kept at room temperature. The COD concentration was measured using a UV–Visible spectrophotometer at a wavelength of 600 nm. A standard or semi˗micro cuvette was used to maintain a 1 cm path length of the sample [16, 17].
Effects of Cultivation Parameters on PHA Production
The effects of cultivation parameters on PHA production were determined in Erlenmeyer flasks containing 100% TW (textile wastewater medium; TWM) as the sole substrate. Afterward, we added 10% inoculum (TS3) containing 1 × 106 cells/mL. These flasks were incubated at 35 °C for 48 h at an agitation speed of 150 rpm. The effect of additional carbon sources [glycerol, molasses and used cooking oil (UCO)] at 5% (w/v) was determined while the other parameters were fixed. Subsequently, the concentration of NH4Cl (1–5 g/L), temperature (25–45 °C) and agitation speed (0–300 rpm) were evaluated. After 48 h, the CDM and PHA production of the samples was determined. Then, the effect of cultivation time was cultivated at 96 h and CDM and PHA production was determined every 12 h. The characterization of PHA was analyzed by FTIR and GC˗MS.
Analytical Methods
Cell Growth Measurement
The bacterial culture broth 10 mL was centrifuged at 13,000×g for 10 min at 4 °C. The pellet was washed twice with distilled water and then suspended in 5–10 mL distilled water. After mixing, growth was monitored by measuring absorbance at 600 nm [18].
Total Cell Mass Analysis
Total cell mass was determined by weighing the CDM. Briefly, 10 mL samples were collected and centrifuged at 13,000×g at 4 °C for 15 min. The pellet was resuspended in 10 mL of distilled water and centrifuged again for washing. Washed cells were dried at 105 °C for 24 h in a hot air oven and then cooled down in a desiccator. The samples were weighed, and the total cell mass was reported in g/L.
PHA Concentration
The extraction and quantification of PHA was determined by the method of Kemavongse et al. [19]. After the determination of dry cell weight, 10 mL of sodium hypochlorite (0.4% w/v) was added to the dried biomass pellet and mixed well. The mixtures were incubated at 37 °C for 1 h. The solids were recovered by centrifugation at 13,000×g for 15 min and washed with 10 mL of acetone. The contents were then centrifuged, washed with 10 mL distilled water and centrifuged again to obtain the pellet. Ten milliliters of chloroform was then added to the pellet and dried at 50 °C for 12 h followed by with 105 °C for 24 h. The experiments were performed in three replicates. PHA yield was calculated as follows:
Characterization of PHA
FTIR Analysis
The chemical structure of the extracted PHA was analyzed by Fourier transform infrared spectroscopy. The biopolymer was dissolved in chloroform and added to KBr pellets and then the solvent was evaporated. The infrared spectra of the samples were recorded in the wavenumber range from 4000 to 650 cm−1 using a Perkin Elmer FTIR spectrophotometer (Jasco FTIR˗6100, Japan) using KBr disc [20].
GC˗MS Analysis
The PHA composition was determined by 10 mg of PHA for methanolysis which was performed in a mixture of 2 mL of chloroform and 2 mL of methanol containing 3% (v/v) sulfuric acid and 10 mg/mL of the benzoic internal standard. Afterward, the solution was heated for 140 min at 100 °C in a closed screw-cap tube to convert HA units to their corresponding methyl esters. The sample was cooled, 2 mL of deionization water was added, vortexed for 5 min and then left out overnight. The chloroform phase was analyzed by the GC˗MS method to compare the authentic standard [21]. A GC˗MS chromatograph equipped with a capillary column (30 m × 0.25 mm) was used for PHA-esterified monomer separation. The column temperature was programmed to remain at 120 °C for 2 min and then to increase at a rate of 10 °C/min to 250 °C. It remained for 10 min at 250 °C and then increased at a rate of 10 °C/min to 280 °C. The injection temperature was 280 °C. Mass spectra and gas chromatograms were acquired and processed with an Agilent 7890B/5977 Series mass spectrometer. Spectra were obtained as electron impact (EI) spectra or as chemical ionization (CI) spectra.
Statistical Analysis
The experiments were run in triplicate. A completely randomized design was used throughout this study. Data were subjected to analysis of variance (ANOVA), and mean comparisons were carried out using Duncan’s multiple range test. All analyses were performed using the software package SPSS (SPSS 24 for Windows, SPSS Inc., Chicago, IL, USA) [22].
Results and Discussion
Characterization of Textile Wastewater
TW from the Ban Phraek Weaving Group (Phatthalung, Thailand) was collected and characterizsed. The characteristics of the wastewater before cell cultivation are shown in Table 1. Before cell cultivation, the characteristics of TW were as follows: pH 6.92, a red–black color, high TDS 2,435 mg/L, settleable solids 0.8 mg/L, TSS 325 mg/L, oil and grease 17 mg/L, sulfide (not found), temperature 30 °C, TKN 54 mg/L, COD 6405 mg/L and carbon to nitrogen (C/N) ratio 9:1. The optimum pH for bacterial growth is 6.5–7.5 [23]. The biosynthesis of PHA from bacteria is usually caused by certain deficiency conditions (e.g. lack of macroelements, such as phosphorus, trace elements, or lack of oxygen) and the excess supply of carbon sources [24]. Moreover, the optimal nitrogen content is necessary for the biosynthesis of PHA, due to the suitable pH, carbon and nitrogen content in TW. Therefore, TW was suitable for bacterial growth and could be used as a substrate for the biosynthesis of PHA by newly isolated Enterobacter TS3.
Effects of Cultivation Parameters on PHA Production
In our previous research, we isolated five new strains of Enterobacter sp. (TW1L, TS1P, TS1L, TS3 and TW1P) from TW containing BR9. All strains were capable of growing in synthetic medium containing BR9, with the ability to produce PHA [8]. Among them, TS3 showed the highest PHA production; therefore, this strain was selected and used throughout this study. Normally Enterobacter strains can found in an environment such as water, soil and waste, etc. TW from the Ban Phraek Weaving Group (Phatthalung, Thailand) contained high carbon sources (COD and oil and greases) and mineral salts (Basic Red 9 and TKN). These carbon sources and mineral salts have important for cell growth and cell viability of Enterobacter strains. Moreover, Enterobacter sp. can be utilized TW and converted into PHA, effectively. Our previous study, the result shows newly isolated Enterobacter can be biodegraded Basic Red 9 (C19H17N3) in TW through oxidative deamination and carboxylation lead to produce phthalic acid, di(2-propylpentyl) ester, which can be transformed into different fatty acids and aldehydes (carbonic acid, allyl nonyl ester, tetradecanoic acid, hexadecanoic acid methyl ester, hexadecanoic acid, bis(2-ethylhexyl) ester, 9-decanoic acid, squalene, n-hexadecanoic acid, octadecanoic acid and hexadecanoic acid ethyl ester) [9]. These intermediate compounds can use as a substrate [carbon sources, carbon 10 to 30 atoms] to produce scl˗co˗mcl PHA by newly isolated Enterobacter strain TS3. Cultivation parameters affecting PHA production using TW as a substrate, including additional carbon sources, ammonium chloride as a nitrogen source, initial pH value, temperature, agitation speed and incubation time to achieve maximum PHA production using newly isolated Enterobacter TS3, were studied.
Effect of Additional Carbon Sources
The biosynthesis of PHA and the growth of Enterobacter require carbon sources. Table 1 shows the high carbon content but to enhance PHA production we added carbon sources from molasses, glycerol and UCO. These carbon sources were selected because they are by˗products or waste from industry. These carbon sources, if released into nature, affect the environment; however, these carbon sources are cheap. Therefore, this research studied different carbon sources including molasses, glycerol and UCO (fixed concentration of carbon source at 5%). The effects of cultivation parameters on PHA production were determined in Erlenmeyer flasks containing 100% TWM as the sole substrate and 10% TS3. The other cultivation parameters were kept constant at 150 rpm and 35 °C for 48 h. Figure 1 shows that effect of carbon sources was evaluated using TWM supplemented with 5% molasses, glycerol and UCO. The results showed that TWM supplemented with 5% of molasses showed the highest CDM of PHA production (0.37 ± 0.01 g/L, 84.01 ± 3.45% CDM). Corresponding to the research of Naheed and Jamil [25], PHA production was observed at the highest 80.95 ± 0.01% CDM by Enterobacter SEL2 supplemented with 5% of molasses as a carbon source. However, TWM without carbon sources (83.94 ± 2.72% CDM) showed no significant difference in PHA production when compared to TWM supplemented with 5% molasses. The results of the characteristics of TWM were found to have a high carbon content. Therefore, it is not necessary to add a carbon source to TWM for PHA production. Consequently, the TWM was suitable for bacterial growth and can be used as the substrate for Enterobacter to produce PHA. However, PHA production from TWM supplemented with 5% glycerol and UCO yielded PHA contents of 39.27 ± 2.09% CDM (0.58 ± 0.02 g/L) and 62.00 ± 2.70% CDM (0.81 ± 0.03 g/L), respectively. In this study, TWM without a supplemental carbon source was selected to study the NH4Cl concentration as a nitrogen source.
Effect of NH4Cl Concentration as a Nitrogen Source
In this study, NH4Cl was selected and used as a nitrogen source for PHA production [26]. NH4Cl has mostly been used as a nitrogen source for producing PHA. To cause PHA accumulation, nutrient limitation is required, and generally, ammonia is used as the essential control factor for uncoupling cell growth and PHA development [26]. Therefore, this research has studied the effect of NH4Cl at 0–5 g/L as a nitrogen source for PHA production. Figure 2 shows that the TWM without NH4Cl showed the highest 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L) of PHA production. On the other hand, the increases in NH4Cl concentration from 1 to 5 g/L showed decreased PHA content (60.84 ± 4.18 to 70.54 ± 3.85% CDW) and PHA concentration (0.15 ± 0.01 to 0.21 ± 0.01 g/L). The results of the characteristics of TWM showed a high nitrogen content. Consequently, it is not necessary to add a nitrogen source to TWM for PHA production. When NH4Cl added as a nitrogen source into TWM, the nitrogen content exceeded that in the culture. Moreover, the CDM decreased when NH4Cl was added as a nitrogen source into the TWM (0.24 ± 0.03 to 0.32 ± 0.01 g/L). Therefore, TW has also been an attractive material for use in the production of PHA, because it was shown to have a high nitrogen content. In this study, TWM and without NH4Cl concentration as a nitrogen source were selected for further study.
Effect of Initial pH Value
The pH level is one of the important factors affecting cell growth, enzymatic activities and PHA accumulation because of its effect on the bioavailability of the trace elements [27] and the regulatory enzymes responsible for the synthesis of PHA, β-ketothiolase, acetoacetyl-CoA reductase, and PHA polymerase [28]. Figure 3 shows that TS3 grown on TWM had the highest PHA production at 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L) at pH 7. Although there was no significant difference in the PHA production obtained at pH 5, 6, 8 and 9, 63.27 ± 1.24 to 74.21 ± 1.19% CDM (0.16 ± 0.01 to 0.27 ± 0.03 g/L) was observed. In the same way, Palleroni et al. [29] and Wei et al. [30], showed that the optimal pH range for the growth of bacteria involved in the production of PHA is 6.0 to 7.5. The pH did not affect the production of PHA. Therefore, pH 6.29 (pH of raw material) was selected to study the incubation temperature.
Effect of Incubation Temperature
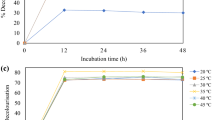
The cultures were incubated at 25–45 °C. We achieved maximum PHA production at 35 °C at 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L), as shown in Fig. 4. The maximum PHA production and bacterial biomass occurred at 35 °C, which was in accordance with the research of Mostafa et al. [31], who that the optimization of PHB accumulation by Erythrobacter aquimaris was achieved at 120 h, pH 8.0, 35 °C, and 2% sodium chloride, using glucose and peptone as the best carbon and nitrogen sources at a C:N ratio of 9.2:1. The cultivation temperatures lower (25–30 °C) and higher (40˗45 °C) had significantly lower PHA content at 43.20 ± 6.82 to 58.09 ± 4.66% CDW in the cells and PHA concentration at 0.17 ± 0.02 to 0.24 ± 0.02 g/L. The temperature of the incubation plays a significant role in a microorganism's metabolic process. A microorganism's growth temperature correlates to the optimal temperature for PHA accumulation. TS3 can grow at an optimum temperature of 35 °C and survive. The effect of temperature on the accumulation of PHA production may be because temperatures other than the optimal temperature reduced the activity of the enzymes responsible for PHA synthesis [32]. In this study, an incubation temperature of 35 °C was selected to study the agitation speed.
Effect of Agitation Speed
Biosynthesis of PHA production by aerobic bacteria requires oxygen for cell growth and maintenance of cell viability. Therefore, agitation is necessary to provide oxygen and to increase the oxygen gas˗liquid mass transfer [33]. The influence of agitation speed at 100–300 rpm on PHA production by TS3 was investigated as shown in Fig. 5. The highest PHA production was obtained at an agitation speed of 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L) at 150 rpm after 48 h. Consistent with the research of Musa et al. [34] the agitation speed at 150 rpm stimulated maximum (6.08 mg/mL) polyhydroxybutyrate (PHB) production by Bacillus sp.1. Choonut et al. [35], and the optimal agitation speed at 150 rpm showed the maximum mcl-PHA accumulation of 63.27% CDM by Bacillus thermoamylovorans. The agitation speeds lower and higher than 150 rpm showed PHA contents lower at 45.79 ± 1.56 to 59.21 ± 4.22% DCW in the cells and PHA concentrations of 0.09 ± 0.01 to 0.26 ± 0.01 g/L. Therefore, an agitation speed of 150 rpm was considered the optimal agitation speed for PHA fermentation by TS3. In other research, however, the PHA accumulation has used optimal agitation speeds differently at 150 rpm. The strains Halomonas campisalis, Cupriavidus necator (CCUG52238T), Bacillus endophyticus and Vibrio harveyi MCCB 284 have achieved maximum PHA production at 100, 200, 220 and 250 rpm [36,37,38,39], respectively. Therefore, the oxygen demand for each species of bacteria is different. In this study, an agitation speed of 150 rpm was selected to study the incubation time.
Effect Incubation Time
Incubation time is important for producing PHA. Therefore, after achieving the optimal conditions, we studied the incubation time for PHA production at 0–96 h. PHA fermentation was collected every 12 h for analyzing PHA production. PHA production increased gradually from 0 to 96 h (0.00 ± 0.00 to 84.37 ± 1.47% CDM), as shown in Fig. 6. The results showed that after 60 h of incubation, the highest 84.37 ± 1.47% CDM (0.36 ± 0.01 g/L) PHA production was observed. However, there was no significant difference in the 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L) of PHA at 48 h. The PHA biosynthesis of bacteria takes different incubation times for each strain. The strains Cupriavidus taiwanensis, Cupriavidus necator (CCUG52238T), Vibrio harveyi MCCB 284, and Bacillus thermoamylovorans PHA005 have achieved maximum PHA production at 28, 36, 72 and 120 h of incubation time [40, 41], respectively. In this study, the optimal conditions (TWM without carbon and nitrogen sources, pH 6.92, 150 rpm, 35 °C and 48 h of incubation) were selected to study the characterization of PHA production.
Characterization of PHA Production
Functional Group of PHA Polymer Analysis
FTIR spectroscopy was used to analyze PHA production from TS3. To confirm the functional groups of the extracted polymer, it was used in the 4000–650 cm−1 spectral range (Fig. 7). The absorption band at 3438.00 cm−1 was assigned to the hydroxyl group of PHA. The typical spectra of methyl (CH3) and methylene (CH2) peaks in the extracted PHA can be detected in the 3000–2600 cm−1 region. An absorption peak at 2978.73 cm−1 corresponds to the stretching of the asymmetrical methyl (CH3) group. It has been reported that the presence of asymmetric CH3 can possibly produce a union of C–H–O, that is, hydrogen bridges. Moreover, stretching in the 2978.73 cm−1 region is also involved in the formation of interactions between CH3 and carbonyl (C=O) groups [35]. The absorption peak at 2924.27 cm−1 corresponds to the stretching of asymmetrical CH2, and these functional groups are mainly responsible for the formation of the lateral chains of the monomeric units. The absorption peak at 2852.67 cm−1 corresponds to the stretching of symmetrical CH3 and the strength of this peak is correlated to the conformational change acquired in the process of crystallization [40]. A strong signal at 1719.52 cm−1 was assigned to carbonyl (C=O) ester bond stretching vibrations [41], which are also associated with the amorphous region of the polymer and can be considered a PHA marker peak. A number of solid peaks at wavenumber values between 1450 and 1000 cm−1 were due to CH3, CH2 twist, and C–O stretches. A typical terminal CH3 group signal was recorded at 1378.69 cm−1, which is quite a common feature of PHA. In the region 1300 to 1000 cm−1, stretching of the ethers features C–O–C linkages [42]. More specifically, strong vibrations at 1274.72 and 1179.15 cm−1 were observed due to asymmetric C–O–C and C–O stretching, respectively. Multiple absorption peaks at 1054.31 to 677.46 cm−1 were assigned to C–O and C–C extending pulsation in the amorphous phase. Therefore the polymer extracted from Enterobacter TS3 was confirmed to be PHA.
PHA Polymer Monomer Composition
The newly isolated Enterobacter TS3 was analyzed by GC˗MS to confirm and to characterize the PHA monomers they synthesized. Table 2, shows that TS3 accumulated scl˗co˗mcl PHA, consisting of 3˗hydroxybutyrate (3HB, C4), 3˗hydro˗ xyvalerate (3HV, C5), 3˗hydroxyhexanoate (3HHx, C6), 3˗hydroxyoctanoate (3HO, C8), 3˗hydroxydecanoate (3HD, C10), 3˗hydroxy˗5˗dodecenoate (3H5DD, C12:1, Δ5) and 3˗hydroxydodecanoate (3HDD, C12) is 3(HB˗co˗HV˗co˗HHx˗co˗HO˗co˗HD˗co˗ H5DD˗co˗HDD). The 3(HB˗co˗HV˗co˗HHx˗co˗HO˗co˗HD˗co˗H5DD˗co˗ HDD) was also observed to be 6.38, 8.32, 36.00, 18.05, 18.26, 6.45 and 6.55 mol%, respectively. In the same way, Samrot et al. [43] and Wecker et al. [44] found that the Enterobacter strain FAK 1384 and cloacae SU˗1 can produce mcl˗PHA. Valentin and Dennis [45] reported that recombinant bacteria can accumulate scl˗co˗mcl PHA at 3(HB˗co˗HHx). Doi et al. [46] showed that Aeromonas caviae produced 3(HB˗co˗HHx) under aerobic conditions. Chen and Wu [47] demonstrated the potential of scl˗co˗mcl PHA for applications such as bone tissue engineering. Hence, PHA produced from TWM by a new strain of Enterobacter TS3 has the potential to be an important biodegradable material for biomedical applications. This is the first report of scl˗co˗mcl PHA; 3(HB˗co˗HV˗co˗HHx˗co˗HO˗co˗HD˗co˗H5DD˗co˗HDD) by Enterobacter TS3 using TW with a TWM under optimal conditions. In addition, TS3 can produce more than 36.00 mol% of the 3HHx monomer. Therefore, TW was found to be an attractive material for utilization as a substrate for producing PHA.
The most industrial production PHA is of type PHB (C4, scl-PHA), but the limitations of PHB homopolymer are rigid and brittle in nature, limited chemical resistance as it is attacked by acids and alkalis and dissolves in chlorinated solvents and PHB’s degradation rate at melt-processing temperature is high; thereby its copolymers and blends are ideal for general applications [48]. In this current study, PHA containing high 3HHx at 36% and other types of mcl-PHA are 3HV, 3HO, 3HD, 3H5DD and 3HDD was obtained. The composition of mcl-PHA can improving the mechanical properties and lowering the melting point (thermal stability and stabilization of the melt flow index), slowing down the degradation during processing and widening the application range of PHA processing methods [49]. In addition, blends with copolymers are convenient as material application especially for biomedical industry.
After Cell Cultivation
The TW after strain TS3 cultivation was collected and characterized. The characteristics of TW after cell cultivation are shown in Table 1. The initial pH of 6.92 was changed to 5.03 after cell cultivation. This result is in agreement with the findings of Rakkan and Sangkharak [9], who found that pH decreased TW treatment by newly isolated Enterobacter TS1L. TDS (mg/L), settleable solids (mg/L), TSS (mg/L), sulfide and temperature (°C) after cell cultivation were 495, < 0.1, 28, not found and 30, respectively. Notably, all parameters related to water quality were significantly decreased. The COD decreased from 6405 to 2670 mg/L, indicating that TS3 can decompose solid and organic substrates in the TW. In addition, the initial TKN of 54 mg/L was lowered to 34 mg/L after cell cultivation. The quality of the wastewater after treatment was determined using the guidelines of the Quality Standard (Thailand). Regarding parameters such as pH, TDS, settleable solids, TSS, oil and grease, sulfide and COD, these guidelines were met (Table 1). The color of the wastewater changed from red–black to colorless. According to our results, the quality of the TW was improved dramatically after biological degradation using TS3. The treated wastewater after cell cultivation met the criteria of the Water Quality Standard (Thailand).
Conclusions
In this study, TW treatment was integrated with PHA production by strain TS3, and it showed a high PHA content of 83.94 ± 2.72% CDM (0.34 ± 0.02 g/L) under optimal conditions. The characterization of the extracted biopolymer proves the presence of scl˗co˗mcl PHA. Moreover, the treated wastewater after PHA cultivation passed the Water Quality Standard (Thailand). Based on the successful laboratory results, future work should then be scaled up. Moreover, in genomics and proteomics studies, there is a possibility of increasing PHA production. In addition, we will analyze the properties and use PHA for biomedical applications.
References
dos Santos, A.B., Cervantes, F.J., van Lier, J.B.: Review paper on current technologies for decolorization of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour. Technol. 98, 2369–2385 (2007)
Sarayu, K., Sandhya, S.: Current technologies for biological treatment of textile wastewater a review. Appl. Biochem. Biotechnol. 167, 645–661 (2012)
Lin, J.X., Zhang, Z., Li, L.: Biodegradation of reactive blue 13 in a two˗stage anaerobic/aerobic fluidized beds system with a Pseudomonas sp. isolate. Bioresour. Technol. 101, 34–40 (2010)
Wang, H., Zheng, X.W., Su, J.Q., Tian, Y.X., Xiong, J., Zheng, T.L.: Biological decolorization of the reactive dyes reactive black 5 by a novel isolated bacterial strain Enterobacter sp. EC3. J. Hazard. Mater. 171, 654–659 (2009)
Chen, B.Y., Lin, K.W., Wang, Y.M., Yen, C.Y.: Revealing interactive toxicity of aromatic amines to azo dye decolorizer Aeromonas hydrophila. J. Hazard. Mater. 166, 187–194 (2009)
Russ, R., Rau, J., Stolz, A.: The function of cytoplasmic flavin reductases in the reduction of azo dyes by bacteria. Appl Environ. Microbiol. 66(1429), 1434 (2020)
Dawkar, V.V., Jadhav, U.U., Ghodake, G.S., Govindwar, S.P.: Effect of inducers on the decolorization and biodegradation of textile azo dye navy blue 2GL by Bacillus sp. VUS. Biodegradation. 20, 777–787 (2009)
Rakkan, T., Chana, N., Chirapongsatonkul, N., U˗taynapun, K., Sangkharak, K.: Screening and identification of Basic Red 9˗degrading bacteria from textile wastewater and their ability to produce medium˗ and long chain length polyhydroxyalkanoate. J. Polym. Environ (2021). https://doi.org/10.1007/s10924-021-02206-2
Rakkan, T., Sangkharak, K.: Enhanced decolourisation and biodegradation of textile wastewater using single and mixed cultures of a newly isolated Enterobacter strain. Curr. Microbiol. 77, 4085–4094 (2020)
Reddy, C.S.K., Ghai, R., Rashmi, Kalia, V.C.: Polyhydroxyalkanoates: an overview. Bioresour. Technol. 87, 137–146 (2002)
Sangkharak, K., Prasertsan, P.: The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotechnol. Bioprocess Eng. 18, 272–279 (2012)
APHA.: Standard methods for the examination of water and wastewater. American Public Health Association. Washington, DC (1999)
Sugahara, S., Suzuki, M., Kamiya, H., Yamamuro, M., Semura, H., Senga, Y., Egawa, M., Seike, Y.: Colorimetric determination of sulfide in microsamples. Anal Sci. 32, 1129–1131 (2016)
Anouzla, A., Abrouki, Y., Souabi, S., Safi, M., Rhbal, H.: Colour and COD removal of disperse dye solution by a novel coagulant: application of statistical design for the optimization and regression analysis. J. Hazard. Mater. 166, 1302–1306 (2009)
Lapara, T.M., Alleman, J.E., Pope, P.G.: Miniaturized closed reflux, colorimetric method for the determination of chemical oxygen demand. Waste Manage. 20, 295–298 (2000)
Shimizu, H., Shioya, S., Suga, K.I.: Cultivation condition for production of biodegradable poly˗β˗hydroxybutyric acid (PHB) production in Alcaligenes eutrophus H16 and Myoplans ruba R14 with respect to the dissolved oxygen tension in ammonium limited batch cultures. Eur. J. Appl. Microbiol. Biotechnol. 7, 1–10 (1990)
APHA.: Standard Methods for the examination of water and wastewater. American Public Health Association, Washington, DC (1992)
Steinbüchel, A., Wiese, S.: A Pseudomonas strain accumulating polyesters of 3˗hydroxybutyric acid and medium˗chain˗length 3˗hydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 37, 691–697 (1992)
Kemavongse, K., Prasertsan, P., Upaichit, A., Methacanon, P.: Poly˗β˗hydroxyalkanoate production by halotolerant Rhodobacter sphaeroides U7. J. Microbiol. Biotechnol. 24, 2073–2085 (2008)
Shamala, T.R., Chandrashekar, A., Vijayendra, S.V., Kshama, L.: Identification of polyhydroxyalkanoate PHA producing Bacillus spp. using the polymerase chain reaction. J. Appl. Microbiol. 94, 369–374 (2003)
Abe, H., Doi, Y., Fukushima, T., Eya, H.: Biosynthesis from gluconate of a random copolyester consisting of 3˗hydroxybutyrate and medium˗chain˗length 3˗hydroxyalkanoates by Pseudomonas sp. 61˗3. Int. J. Biol. Macromol. 16(3), 115–119 (1994)
Steel, R.G.D., Torrie, J.H.: Principles and procedures of Statistics. McGraw˗Hill, New York (1980)
Horikoshi, K.: Alkaliphiles: some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev. 63(4), 735–750 (1999)
Kim, Y.B., Lenz, R.W.: Polyesters from microorganisms. Adv. Biochem. Engin/Biotechnol. 71, 51–79 (2001)
Naheed, N., Jamil, N.: Optimization of biodegradable plastic production on sugar cane molasses in Enterobacter sp. SEL2. Braz. J. Microbiol. 45(2), 417–426 (2014)
Wang, F., Lee, S.Y.: Poly3˗hydroxybutyrate production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 63, 3703–3706 (1997)
Gomaa, E.Z.: Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Braz Arch Boil Technol. 57, 145–154 (2014)
Bhagowati, P., Pradhan, S., Dash, H.R., Das, S.: Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. Biosci. Biotechnol. Biochem. 79, 1454–1463 (2015)
Palleroni, N.J., Palleroni, A.V.: Alcaligenes latus, a new species of hydrogen utilizing bacteria. Int. J. Syst. Bacteriol. 28, 416–424 (1978)
Wei, Y.H., Chen, W.C., Huang, C.K., Wu, H.S., Sun, Y.M., Lo, C.W., Janarthanan, O.M.: Screening and evaluation of polyhydroxybutyrate˗producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int. J. Mol. Sci. 12(1), 252–265 (2011)
Mostafa, Y.S., Alrumman, S.A., Otaif, K.A., Alamri, S.A., Mostafa, M.S., Sahlabji, T.: Production and characterization of bioplastic by polyhydroxybutyrate accumulating Erythrobacter aquimaris isolated from mangrove rhizosphere. Molecules 25(1), 179–199 (2020)
Getachew, A., Woldesenbet, F.: Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes. 9, 509–518 (2016)
Swamy, J., Ramsay, J.A.: The evaluation of white rot fungi in the decoloration of textile dyes. Enzyme Microb. Technol. 24, 130–137 (1999)
Musa, H., Bolanle, B.B., Kasim, F., Arbain, D.: Screening and production of polyhydroxybutyrate (PHB) by bacterial strains isolated from rhizosphere soil of groundnut plants. Sains Malays. 45(10), 1469–1476 (2016)
Choonut, A., Prasertsan, P., Klomklao, S., Sangkharak, K.: Bacillus thermoamylovorans˗related strain isolated from high temperature sites as potential producers of medium˗chain˗length polyhydroxyalkanoate (mcl˗PHA). Curr. Microbiol. 77, 3044–3056 (2020)
Kshirsagar, P., Suttar, R., Nilegaonkar, S., Kulkarni, S., Kanekar, P.: Scale up production of polyhydroxyalkanoate (PHA) at different aeration, agitation and controlled dissolved oxygen levels in fermenter using Halomonas campisalis MCM B-1027. J. Biochem. Technol. 4(1), 512–517 (2014)
Zahari, M.A.K.M., Ariffin, H., Mokhtar, M.N., Salihon, J., Shirai, Y., Hassan, M.A.: Factors affecting poly(3˗hydroxybutyrate) production from oil palm frond juice by Cupriavidus necator (CCUG52238T). J Biomed Biotechnol (2012). https://doi.org/10.1155/2012/125865
Geethu, M., Vrundha, R., Raja, S., Chandrashekar, H.R., Divyashree, M.S.: Improvement of the production and characterisation of polyhydroxyalkanoate by Bacillus endophyticus using inexpensive carbon feedstock. J. Polym. Environ. 27, 917–928 (2019)
Mohandas, S.P., Balan, L., Lekshmi, N., Cubelio, S.S., Philip, R., Singh, I.S.B.: Production and characterization of polyhydroxybutyrate from Vibrio harveyi MCCB 284 utilizing glycerol as carbon source. J. Appl. Microbiol. 122(3), 698–707 (2016)
López˗Cuellar, M.R., Alba˗Flores, J., Rodríguez, J.N.G., Pérez˗Guevara, F.: Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 48, 74–80 (2011)
Gumel, A.M., Annuar, M.S.M., Heidelberg, T.: Biosynthesis and characterization of polyhydroxyalkanoates copolymers produced by Pseudomonas putida bet 001 isolated from palm oil mill effluent. PLoS One 7(9), 1–8 (2012)
Sathiyanarayanan, G., Bhatia, S.K., Song, H.S., Jeon, J.M., Kim, J., Lee, Y.K., Yang, Y.H.: Production and characterization of medium˗chain˗ength polyhydroxyalkanoate copolymer from Arctic psychrotrophic bacterium Pseudomonas sp. PAMC 28620. Int. J. Biol. Macromol. 97, 710–720 (2017)
Samrot, A.V., Avinesh, R.B., Sukeetha, S.D., Senthilkumar, P.: Accumulation of poly[(R)-3-hydroxyalkanoates] in Enterobacter cloacae SU˗1 during growth with two different carbon sources in batch culture. Appl. Biochem. Biotechnol. 163, 195–203 (2011)
Wecker, P., Moppert, X., Simon˗Colin, C., Berteaux˗Lecellier, V.C. : Discovery of a mcl˗PHA with unexpected biotechnical properties: the marine environment of French Polynesia as a source for PHA˗producing bacteria. AMB Express 5(74), 1–9 (2015)
Valentin, H.E., Dennis, D.: Production of poly(3˗hydroxybutyrate˗co˗4˗hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J. Biotechnol. 58, 33–38 (1997)
Doi, Y., Kitamura, S., Abe, H.: Microbial synthesis and characterization of poly(3˗hydroxybutyrate˗co˗3˗hydroxyhexanoate). Macromolecules 28, 4822–4828 (1995)
Chen, G.Q., Wu, Q.: The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26, 6565–6578 (2005)
Rajan, K.P., Thomas, S.P., Gopanna, A., Chavali, M.: Polyhydroxybutyrate (PHB): a standout biopolymer for environmental sustainability. Springer, Cham (2018)
Poltronieri, P., Kumar, P.: Polyhydroxyalkanoates (PHAs) in industrial applications. Springer, Cham (2018)
Acknowledgements
The authors would like to acknowledge the support of Thailand Science Research and Innovation (TSRI) through the Royal Golden Jubilee Ph.D. (RGJ˗PHD) Program through Grant Number PHD/00073/2559 for RGJ˗PHD. Acknowledgment is also made to the Department of Chemistry, Faculty of Science, Thaksin University, Phatthalung Campus, Thailand. Finally, acknowledgement is made to Professor Dr. Ken’ichiro MATSUMOTO.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakkan, T., Chana, N. & Sangkharak, K. The Integration of Textile Wastewater Treatment with Polyhydroxyalkanoate Production Using Newly Isolated Enterobacter Strain TS3. Waste Biomass Valor 13, 571–582 (2022). https://doi.org/10.1007/s12649-021-01504-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01504-z