Abstract
In the present study, the mixotrophic growth of the microalga Nannochloropsis sp. BR2 in sugarcane bagasse was analyzed and compared with its photoautotrophic cultivation. Nannochloropsis cultures cultivated mixotrophically in sugarcane bagasse showed significantly higher biomass productivity, fatty acid methyl ester (FAME) and protein contents of 63.28 mg L−1 d−1, 170.51 mg g−1 and 35.2% of dry weight, respectively, compared to the photoautotrophic cultivations with biomass productivity, FAME and protein contents of 51 mg L−1 d−1, 139.21 mg g−1 and 31.6% of dry weight. Whereas, total carotenoid during photoautotrophic cultivation was 5.833 mg g−1 and decreased to 4.542 mg g−1 in mixotrophic cultures. This can be explained by the additional carbon source in the form of sugars that are metabolized to the fatty acid building block acetyl-CoA, while photosynthetic pigments were less needed. Findings of this study demonstrate that acid-pretreated hydrolysate of lignocellulosic waste from sugarcane bagasse can be developed into a potential feedstock for efficient microalgal cultivation.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Novelty Statement
Nannochloropsis sp. BR2 was cultivated for the first time mixotrophically in sugarcane bagasse hydrolysate. Maximum biomass productivity and fatty acid contents were observed as compared to its autotrophic cultivations.
Introduction
Modern day civilization is dependent on energy generation by fossil fuels, but the major drawback of using fossil fuels is environmental pollution. Thus, fossil fuels are increasingly being replaced by biofuels [1, 2]. Among various biofuels, biodiesel is extensively used for transportation purposes as an seneration biofuels are extracted from various crops like sugarcane, oil palm, maize, soybean, rapeseed etc. but they raised controversy and questions on global food security and competition for biodiverse landscapes (i.e. rainforests) [3]. This led the need to explore non-food biomass, like microalgae, for biofuels that do not need to compete for arable land, biodiverse landscapes or even freshwater resources [4]. Microalgae are potential candidates for production of various products of interest, such as proteins, pigments, omega-3 fatty acids and triglycerides that can be converted into biofuels. Light reduction in cultures of high density is a major hindrance for production of lipids from microalgae. Mixotrophic cultivation of microalgae on fermentative lignocellulosic wastes helps solving this problem and may represent a cost-effective approach to achieve higher biomass while improving environmental footprints.
Microalgae consist of a group of organisms that potentially offer a number of different avenues for sustainable fuel options. Microalgae are ubiquitous and able to grow in diverse environments like freshwater habitats, marine/brackish environments and even wastewaters [5]. Microalgae cultivation is typically photoautotrophic, but they can also be grown heterotrophically and mixotrophically by utilizing organic carbon sources [6]. Microalgae can store carbon molecules like triacylglycerols (TAGs) that may provide a feedstock for biodiesel. Many microalgae also have the ability to grow mixotrophically in various organic carbon sources (acetate, glycerol, glucose, etc.), which leads to high productivities as compared to photoautotrophic growth because mixotrophic cultures display more energy utilization for biomass productivity [7]. Photomixotrophic cultivations can also lead to accumulation of higher cellular fatty acid contents [8, 9]. This ability of microalgae to grow on carbon substrates increases the likelihood to explore lignocellulosic feedstocks which in turn can increase net productivity and decreases the costs of cultivation. At the laboratory scale, the most commonly utilized carbon source is glucose but would be very expensive for use at large scale cultivation [10]. Marine microalgae are considered a potential source of biofuels due to their high lipid productions [11, 12], reducing reliance on freshwater [13, 14]. Nannochloropsis sp. BR2 is a fast-growing microalgal strain that has the ability to accumulate up to 60% more TAGs/lipids than other microalgae and is capable of growing mixotrophically in different organic carbon sources [15]. It also produces high amounts of the long chain polyunsaturated fatty acid, eicosapentanoic acid (EPA), a potentially sustainable source of omega-3 fatty acid for human and animal nutrition [16].
Lignocellulosic feedstocks are the most abundantly available raw material of plants that can serve as a promising feedstock for cultivating bacteria, fungi, yeasts and mixotrophic microalgae to produce biofuels and other value added products [17,18,19,20]. Sugarcane bagasse is a lignocellulosic by-product generated by the sugar industry during production/processing of sugars and ethanol [21]. For the effective hydrolysis of lignocellulosic waste, various methods are employed, amongst them dilute acid hydrolysis is a commonly used pretreatment to release sugars [22]. Sun et al. [23] reported that the major constituents from hydrolyzed sugarcane bagasse are glucose, xylose and arabinose, and glucose, which are promising carbon sources. Sugarcane bagasse has been used as organic carbon source for growing microalgae mixotrophically [24, 25]. Owing to the abundant availability of low cost sugarcane bagasse, it was employed as major carbon source feedstock to cultivate microalgae mixotrophically in this study. In the present study Nannochloropsis sp. BR2 was cultivated on sugarcane bagasse hydrolysate (SCBH) for lipid production.
Methodology
Substrate Collection and Pretreatment
Sugarcane bagasse was provided by School of Agriculture and Food Sciences, University of Queensland, Australia. Before pretreatment process, bagasse was washed in a glass container thrice with distilled water to remove any dirt, and then dried. It was pretreated with 0.5% H2SO4 followed by steam pressurization [20]. In the present study two concentrations of sugarcane bagasse hydrolysate were used i.e. SCBH-I (5 g L−1 of bagasse) and SCBH-II (10 g L−1 of bagasse) (Table 1).
Batch cultivation of Nannochloropsis sp. BR2
Nannochloropsis sp. BR2 had been isolated originally from the Brisbane River, Queensland, Australia [26] and was maintained at the Queensland Microalgae Collection in the Microalgae Biotechnology Laboratory, University of Queensland, Australia. Nannochloropsis sp. BR2 was cultivated in F/2 medium (silicate free) (Algaboost F/2 (2000x) silicate free, Aus Aqua) [27]. The medium was phosphate enriched (100 μmol L−1). The microalgal strain was grown with constant bubbling at 25 ppt salinity in artificial seawater. The cultures were then used as inoculum at their logarithmic phase. For the photoautotrophic cultivation mode, 5 mL of the microalgal stock culture was shifted to a 250 mL Erlenmeyer flask containing 195 mL of F/2 medium with seawater at 25 ppt salinity. Microalgal cultures were examined regularly under a microscope (Olympus CX21LED) to check whether there was any zooplankton contamination or any other microalgal growth. All the flasks were kept at 25 °C for a photoperiod cycle of 16:8 h (light:dark), with fluorescent light (120 μmol m−2 s−1) under continuous bubbling. When the medium was depleted of measureable nitrates (API, Aquarium Pharmaceutical test kit), all cultures were incubated for five additional days to attain fatty acids/oil induction phase. Absorbance (OD) at 440 nm was measured on a daily basis to check microalgal growth rates. Biomass productivity was calculated by centrifuging 20 mL of cultures at 4800×g for 5 min. Pellets obtained were washed thrice with deionized water and dried in a hot oven at 60 °C until constant weight was achieved. Biomass productivity (P) was calculated by using:
where ∆X is the difference in biomass (g L−1), ∆t is cultivation time (day) [28].
For the mixotrophic cultivations, 5 mL of the microalgal stock culture was shifted to a 250 mL Erlenmeyer flask containing 15 mL of F/2 medium with seawater at 25 ppt salinity and 180 mL of sugarcane bagasse hydrolysate (SCBH). The experiment was conducted in triplicates. When cells reached stationery growth phase, they were collected and centrifuged at 4800×s then freeze-dried for further biochemical analyses. The supernatant was also stored at 4 °C for carbohydrate/sugar analysis.
X-Ray Diffraction (XRD) Analysis
X-Ray diffraction analysis was performed in order to evaluate the crystallinity index of cellulose fibers of sugarcane bagasse by using a Shimadzu XRD, Japan. The analysis was used to determine the amorphous structures of the lignocellulosic substrate and the changes and modifications that occurred in the crystalline structures of cellulose. Th crystallinity index was determined by analyzing the ratio between maximum 002 peak intensities and minimal intensities of peak 001, 002 [29, 30].
Analytical Methods
Optical density (OD) was measured at 440 nm on a daily basis to determine the growth of the algae. pH was measured on a daily basis with a pH meter. Phosphates and nitrates were also measured daily by using colorimetric assay (API nutrient kits) and reading the absorbance at 690 nm and 545 nm by using a UV/VIS spectrophotometer (Model U-2800, Hitachi, Tokyo, Japan) until the nutrients became undetectable. Microscopic observations of cultures were carried out by using a Zeiss Axio Fluorescence microscope. For the lipids analyses, samples were collected after 1 week when nutrients were used up. They were then centrifuged at about 4800×g for at least 10 min. Pellets were preserved at − 80 °C. After that all samples were lyophilized (freeze-dried) and stored at − 20 °C for Fatty Acid Methyl Ester (FAME) analyses [26]. Total fatty acid contents were calculated by summing up of all fatty acids. Whereas Total Fatty Acids (TFA) productivity was calculated by multiplying average biomass productivity with total fatty acids. For carotenoid analyses, samples were crushed by using mortar and pestle,60 mg were kept in Falcon tubes on ice throughout the extraction procedure by following the protocol of Ahmed et al. [31]. Contents of chlorophyll-a were calculated using the method of [32].
Protein contents were analyzed by using the protocol of Lόpez et al. [33] with a few modifications. Lyophilized and crushed biomass (10 mg) was suspended in 10 mL of lysis buffer (5 ml of Triton X-100, Chem-Supply, Australia; 0.3722 g L−1 ethylenediaminetetracetic acid disodium salts, Chem-Supply; 0.0348 g L−1 of phenylmethyl sulfonyl fluoride, Sigma Aldrich) for 30 min. Then 100 µL of sodium dodecyl sulfate salt solution was added to 100 sµL of lysis buffer before the extraction of proteins with CB-X Protein Assay Kit (G Bioscience). Absorbance was read at 600 nm using a microplate photometer (Glomax Multi Detection System, Promega). Bovine serum albumin was used as a standard.
Statistical Analysis
All experiments were conducted in triplicates (n = 3). The results were analyzed and represented in mean ± S.E.M by using software Minitab 16.
Results and Discussion
Nannochloropsis is distributed in all oceans of the world. It plays an important role in the carbon and mineral cycles [34]. Nannochloropsis is rich in proteins, polyunsaturated fatty acids, and pigments [35,36,37], and it is commonly used as feed in aquaculture [38, 39]. Nannochloropsis has been proposed as an excellent candidate for high omega-3-rich oil and biofuel production [16, 40]. The addition of organic carbon substrates during mixotrophic cultivations influence lipid, biomass productivity, and carbohydrate contents but reduces protein and pigment contents. Because nitrogen is an important source for protein synthesis hence the increased carbon may alter the metabolic pathways. Many studies were carried out that utilized different types of organic and inorganic carbon sources, such as glucose and acetate, for mixotrophic cultivation of Chlorella sp. [7, 41] and Nannochloropsis sp. [42]. In the present study, the addition of sugarcane bagasse as an external organic carbon source had an impact on algal cell metabolism. We also obtained high levels of desirable products such as fatty acids and proteins by switching over to stress-inducing treatments.
X-Ray Diffraction Analysis
XRD was performed to check and analyze the crystallinity index (CI) of the lignocellulosic substrate i.e. sugarcane bagasse. The crystallinity index of the untreated bagasse was 45.38% and the pretreated biomass with sulphuric acid only had crystallinity index of 45.59%, whereas the third sample was pretreated with 0.5% sulphuric acid and autoclaving at 121 °C for 15 min and had a crystallinity index of 48.3% (Supplementary Fig. S1). It was confirmed from the XRD analysis that there was a higher delignification in bagasse samples pretreated with sulphuric acid followed by autoclaving than in the other two samples, as there had higher cellulose levels and thus an increased crystallinity index. This suggests that removal of lignin after acidic pretreatment leads to increased cellulose contents. Increased CI of pretreated biomass showed the removal of lignin and hemicellulose contents in previous studies [43] Raw sugarcane bagasse presented lowest crystallinity because it had a high content of hemicellulose and lignin, which are amorphous. XRD analysis of biomass is also important to observe how the pretreated substrates release more sugars compared to the untreated substrates and a high crystalinity index means that more sugars are released [44]. In the present study, the bagasse pretreated with sulphuric acid showed a higher crystallinity index compared to the raw substrate which is consistent with previous studies [45,46,47].
Growth/Biomass Productivity
Biomass productivity of Nannochloropsis sp. BR2 was determined for both, photoautotrophic in standard F/2 media and two modes of mixotrophic cultivation (SCBH-I and SCBH-II). Following the photoautotrophic cultivation, biomass productivity was found to be 0.051 ± 0.004 g L−1 d−1. Whereas, biomass raised mixotrophically in SCBH-I had a biomass productivity of 0.058 ± 0.002 g L−1 d−1, and the value was found to be 12.06% higher than the respective figures obtained for F/2 cultivation. For biomass raised mixotrophically in medium SCBH-II, biomass productivity was 0.063 ± 0.000 g L−1 d−1 and the value was found to be 19.04% higher than the respective values obtained for BBM cultivation (Fig. 1). Most of the reported studies are focused on low-cost carbon sources instead of using simple glucose for growing microalgae heterotrophically or mixotrophically. For example, corn powder hydrolysate (CPH), Cyperus esculentus waste hydrolysate (CEWH), cassava starch hydrolysate (CSH), and sorghum juice (SJ) have been utilized to grow Chlorella with good quality lipid production [48,49,50,51].
In a previous study, biomass production of Neochloris oleabundans increased up to 1.4 g L−1 as the concentration of sodium acetate and glucose was increased in the medium [52]. Cheirsilp and Torpee [53] also observed that Chlorella sp. as well as two marine species Nannochloropsis sp. and Chlorella sp. (marine) had the ability to accumulate more lipids when grown heterotrophically or mixotrophically. The same results were reported in another study where C. vulgaris was cultivated mixotrophically in glucose and yielded higher biomass productivity as compared to cells grown photoautotrophically [54]. Mixotrophic cultivation utilizing both organic carbon source and light has been considered as the most effective process for the production of microalgal biomass [55]. In another study, the growth rate was promoted by adding 0.1 g L−1 glucose in Nannochloropsis oculata cultures [56]. A high productivity of 0.52 g L−1 d−1 was obtained when Chlorella was cultivated in white wine lees, glycerol, and cheese whey, as compared to 0.24 g L−1 d−1 when grown autotrophically [57]. The results of the present study are also consistent with a report in which Chlorella protothecoides and Nannochloropsis salina grew faster under mixotrophic conditions than under heterotrophy or autotrophy [58]. In another study, the cell culture density of Nostoc flagelliforme was also highest under mixotrophic conditions than under heterotrophy or autotrophy, resulting in higher biomass of 4.98 g L−1 in mixotrophic cultures at the time of harvesting [59]. Rattanapoltee and Kaewkannetra [20] reported a biomass production of 3.85 g L−1 in Scenedesmus acutus PPNK1 when cultivated in sugarcane bagasse hydrolysate and Chlorella protothecoides also showed increased biomass of 10.79 g L−1 when grown in sugarcane bagasse hydrolysate [60].
Total Fatty Acid Methyl ester Contents and Productivity
Fatty acid methyl esters (FAMEs) of Nannochloropsis sp. BR2 were analyzed for the photoautotrophic and the two modes of mixotrophic cultivation (SCBH-I and SCBH-II). It was found that for the biomass cultivated in photoautotrophic conditions TFA (Total Fatty Acids), MUFA (Monounsaturated Fatty Acids), PUFA (Polyunsaturated Fatty Acids) and SFA (Saturated Fatty Acids) were 139.21, 44.05, 52.81 and 42.35 mg g−1 of dry biomass, respectively (Fig. 2). For the biomass cultivated mixotrophically i.e. SCBH-I, TFA, MUFA, PUFA, and SFA were found to be 165.42, 52.41, 47.92, and 65.09 mg g−1 of dry biomass, respectively. For the biomass cultivated mixotrophically in SCBH-II, TFA, MUFA, PUFA, and SFA were found to be 170.51, 55.10, 43.41, and 72 mg g−1 of dry biomass, respectively. It was evident from the results that MUFA, PUFA, and SFA increased in both mixotrophic cultivations as compared to the F/2 cultivation, and thus overall TFA also increased as compared to the photoautotrophic (F/2) cultivation mode. It was found that C14:0, C16:0, C18:0, and C22:0 increased in SCBH-I cultivations as compared to photoautotrophic (F/2) cultivation. Whereas, C12:0, C16:0, and C18:0 increased for the biomass grown mixotrophically in the medium SCBH-II as compared to BBM and SCBH-I cultivation modes. C16:0 was the major saturated fatty acid methyl ester and increased up to 50.22% and 73.34% in mixotrophic medium SCBH-I and SCBH-II, respectively, compared to photoautotrophic cultivation. C20:5n3 decreased in biomass raised mixotrophically when the concentration of sugarcane bagasse was increased. (Fig. 2).
During mixotrophic cultivation, the growth rate enhanced significantly due to the abundance of carbon sources in the form of hydrolysate and inorganic carbon in the form of CO2 as compared to the autotrophic mode where the only carbon source was CO2 [60]. Furthermore, CO2 produced during consumption of organic carbon can be reutilized during the process of photosynthesis, thus the growth rate increased, leading to exhaustion of nutrients more rapidly and thus shifting cell metabolism to lipid production [61]. Many studies also showed enhanced growth rates and higher lipid production of microalgae when grown mixotrophically as compared to autotrophy or heterotrophy [20, 62, 63]. A previous study also reported that with organic carbon sources added to the medium of Nannochloropsis sp., the percentage of fatty acids from C16–C18 increased, while C20:5n3 (EPA) contents decreased [64]. This may be due to the relative abundance of neutral lipids [65]. Mixotrophic growth of certain microalgae in the presence of organic carbon sources showed higher intracellular lipids to photoautrophic cultures alone e.g. Phaeodactylum tricornutum in glycerol and Chlorella sp. in glucose [66] and Nannochloropsis sp. in glycerol [7, 67]. FAME profiles depend on the organism used and its growth conditions [68]. The main fatty acids produced and analyzed in this study were C16–C18, mainly composed of palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linolenic acid (C18:2), and gamma-linolenic acid (C18:3) which are suitable for biodiesel production [69,70,71]. Biodiesel with more saturated fatty acids means higher cetane number (CN), decreased emissions of NOx, oxidative stability, and a reduced ignition delay time [72, 73].
Estimation of Chlorophyll
Chlorophyll-a was detected spectrophotometrically for both, photoautotrophic and mixotrophic cultivation modes. Following photoautotrophic cultivation, chlorophyll-a was 3.46 ± 0.24 µg mg−1 of dry biomass. For biomass raised mixotrophically in medium SCBH-I and II, chlorophyll-a was 3.1 ± 0.05 and 3.11 ± 0.01 µg mg−1 of dry biomass, respectively, and these values were found to be 10.4% and 10.1% lower than the respective figures obtained for photoautotrophic cultivation (Fig. 3). The presence of organic substrates may have inhibited the photosynthetic apparatus formation in Nannochloropsis [74], resulting in decreased levels of photosynthetic pigments when compared to those obtained in photoautotrophic cultivation. This was also confirmed by the decreased chlorophyll contents in mixotrophic cultures as compared to the photoautotrophic cultures.
Comparison of Carotenoid Pigments of Nannochloropsis sp. BR2
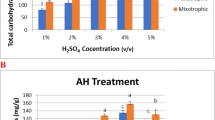
The total carotenoids were analyzed for the photoautotrophic and two modes of mixotrophic cultivation (SCBH-I and SCBH-II). Total carotenoids in F/2 were 5.834 ± 1.037 mg g−1 of dry biomass and decreased to 2.698 ± 0.636 mg g−1 of dry biomass in mixotrophic medium SCBH-I whereas it increased in case of mixotrophic medium SCBH-II down to 4.543 ± 1.042 mg g−1 of dry biomass as compared to SCBH-I cultivation (Fig. 4). The carotenoid pigments detected through HPLC for Nannochloropsis sp. BR2 were cis-betacarotene, trans-betacarotene, violaxanthin, and lutein. In F/2 cultivation, lutein, trans-betacarotene, cis-betacarotene, and violaxanthin were 0.237 ± 0.112, 2.731 ± 0.684, 0.642 ± 0.139 and 2.224 ± 0.292 mg g−1 of dry biomass, respectively. For biomass raised mixotrophically in medium SCBH-I, lutein, trans-betacarotene, cis-betacarotene and violaxanthin were 0.032 ± 0.007, 1.158 ± 0.269, 0.253 ± 0.046 and 1.254 ± 0.318 mg g−1 of dry biomass, respectively and these values were found 100%, 58%, 61% and 44% lower than the respective values obtained for F/2 cultivation. For the biomass cultivated mixotrophically in SCBH-II, lutein, trans-betacarotene, cis-betacarotene and violaxanthin were 0.073 ± 0.013, 2.011 ± 0.543, 0.477 ± 0.120 and 1.980 ± 0.369 mg g−1 of dry biomass, respectively and these values were 100%, 26%, 26% and 11% lower than respective values obtained for F/2 cultivation (Fig. 4).
Total carotenoids decreased under both SCBH-I and SCBH-II cultivations as compared to F/2 growth. The same has been previously reported by several authors for Chlorella sp. [54, 75]. A previous study has shown that the lower contents of chlorophyll in mixotrophic cultures decrease the dependence on light [76]. Therefore, reduced levels of chlorophylls in mixotrophic cultures may relieve the process of photoinhibition. Amongst the various nutritional modes analyzed, the highest carotenoid contents (5.834 mg g−1) were also found in photoautotrophic cultures. This value decreased to 2.698 mg g−1 when biomass was cultivated mixotrophically in SCBH-I but it increased to 4.543 mg g−1 with increased loading of substrates, i.e. SCBH-II. The same results were also observed by Liu et al. [77] and Abreu et al. [78] for Phaeodactylum tricornutum and Chlorella vulgaris. Moreover, growth limitations can still occur due to photo-inhibition or low illumination of cultures under mixotrophic cultivation [79].
Total Protein Content
Total protein contents (% DW) of Nannochloropsis sp. BR2 was determined for photoautotrophic and mixotrophic cultivation modes (SCBH-I and SCBH-II). For the photoautotrophically-grown cultures, the protein content was 31.6 ± 0.48% DW (Fig. 5). Whereas, for biomass cultivated mixotrophically in medium SCBH-I, the protein content was 33.9 ± 0.07% DW and the value was found 7% higher than the respective figures obtained for F/2 cultivation. For biomass grown mixotrophically in SCBH-II, the protein content was 35.2 ± 0.04% DW and the value was found 10.2% higher than the respective values obtained for F/2 cultivation. Another study reported 63.5% and 474 mg L−1 d−1 increase in protein contents and protein productivity, respectively, when C. vulgaris was grown mixotrophically in hydrolyzed cassava waste powder solution [78]. Another study conducted by Hu and Gao [42] also concluded that there were 39 and 41% increases in protein content when microalgae were grown mixotrophically in acetate with different CO2 concentrations. There was an unusually high protein content of 58.6% DW for Chlamydomonas humicola when it grew mixotrophically on acetate [80].
Carbon Source Utilized by Nannochloropsis sp. BR2 Following Mixotrophic Cultivation
For mixotrophic cultivation of Nannochloropsis sp. BR2 two initial sugar concentrations were investigated. Time-course profiles from day 0 to day 7 with added sugar in the F/2 medium are shown in Fig. 6. It can be seen from this figure that glucose was completely consumed when 5 and 10 g L−1 were the initial sugar concentrations. Furthermore, for the biomass cultivated mixotrophically in SCBH-I medium, glucose concentrations on day 0, 3 and 6 were 252 ± 1.45, 151.3 ± 3.28 and 0.00 ± 0.00 µg mL−1, respectively and the consumption of glucose for day 3 and 6 was found to be 40% and 100%, respectively. For the biomass cultivated mixotrophically in SCBH-II, glucose concentrations on day 0, 3, and 6 were 477 ± 1.2, 209 ± 6.56 µg mL−1, respectively, while it was undetectable on day 6 and the consumption of glucose for day 3 and 6 was 56.3% and 100%, respectively. It is to be noted that no sugar was added for the photoautotrophic mode of cultivation.
Whereas, xylose was not consumed by cultures completely with 5 and 10 g L−1 initial sugar concentration. Many studies reported that xylose is not being utilized/consumed as a carbon source by microalgae [70, 81,82,83]. Glucose stimulated rapid growth of the algae because it is a simple sugar and can be easily assimilated to produce acetyl-CoA, which was then utilized in multiple pathways including the synthesis of fatty acids. It has been reported that glucose enhanced high growth rates and high respiration rates in many microalgae as compared to many other carbon sources, such as like sugars alcohols, monohydric alcohols, organic acids, sugar phosphates and many other sugars [84, 85]. Glucose alone can drive only one metabolic pathway, but sugars from sugarcane bagasse (such as xylose, glucose, and many other carbohydrate components) can help in driving many metabolic pathways leading to increased biomass and oil production [60, 71]. Annual worldwide processing of 5.4 × 108 dry tons of sugarcane produces 1.51 × 108 tons of sugarcane bagasse [86] and is utilized for energy production [86, 87]. Thus sugarcane bagasse can serve as a cheap carbon source for the cultivation of microalgae at an even larger scale.
Conclusion
This study showed that sugarcane bagasse hydrolysate served as a potential and economical candidate to cultivate marine microalgae for carotenoid and fatty acid production. Fatty acid content and productivity were increased during mixotrophic cultures as compared to the photoautotrophic conditions. The main fatty acid profiles obtained were between C16–C18 indicating potential for a good quality biodiesel production.
References
Amaro, H.M., Guedes, A.C., Malcata, F.X.: Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy. 88, 3402–3410 (2011)
Manzoor, M., Tabssum, F., Javaid, H., Qazi, J.I., 2015. Lucrative future of microalgal biofuels in Pakistan: a review. Int. J. of Energy and Environ. Eng. 6, 393–403.
Moore, A.: Biofuels are dead: long live biofuels(?)-Part one. New Biotechnol. 25, 6–12 (2008)
Sukarmi, S., Hanidi, N., Yanuhar, U., Wardana, I.N.G.: Potemtial and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int. J. Energy Environ. Eng. 5(4), 279–290 (2014)
Lordan, S., Ross, R.P., Stanton, C.: Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar. Drugs 9, 1056–1100 (2011)
Chojnacka, K., Marquez-Rocha, F.J.: Kinetic and stoichiometric relationship of the energy and carbon metabolism in the culture of microalgae. Biotechnol. 3, 21–34 (2004)
Liang, Y., Sarkany, N., Cui, Y.: Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 31, 1043–1049 (2009)
Feng, F.Y., Yang, W., Jiang, G.Z., Xu, Y.N., Kuang, T.Y.: Enhancement of fatty acid production of Chlorella sp. (Chlorophyceae) by addition of glucose and sodium thiosulphate to culture medium. Process Biochem. 40, 1315–2131 (2005)
Oh, S.H., Kwon, M.C., Choi, W.Y., Seo, Y.C., Kim, G.B., Kang, D.H., Lee, S.Y., Lee, H.Y.: Long-term outdoor cultivation by perfusing spent medium for biodiesel production from Chlorella minutissima. J. Biosci. Bioeng. 110, 194–200 (2010)
Doan, Y.T.T., Obbard, J.P.: Two-stage cultivation of a Nannochloropsis mutant for biodiesel feedstock. J. Appl. Phycol. 27, 2203–2208 (2015)
Rodolfi, L., Chini Zittelli, G., Bassi, N., Padovani, G., Biondi, N., Bonini, G., et al.: Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112 (2009)
Tang, G., Suter, P.M.: Vitamin A, nutrition, and health values of algae: spirulina, chlorella, and dunaliella. J. Pharm. Nutr. Sci. 1, 111–118 (2011)
Kim, M.K., Jeune, K.-H.: Use of FT-IR to identify enhanced biomass production and biochemical pool shifts in the marine microalgae, Chlorella ovalis, cultured in media composed of different ratios of deep seawater and fermented animal wastewater. J. Microbiol. Biotechnol. 19, 1206–1212 (2009)
Craggs, R.J., McAuley, P.J., Smith, V.J.: Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res. 31, 1701–1707 (1997)
Ma, X.N., Chen, T.P., Yang, B., Liu, J., Chen, F.: Lipid production from Nannochloropsis. Mar. Drugs 14, 61 (2016)
Chua, E.T., Schenk, P.M.: A biorefinery for Nannochloropsis: induction, harvesting and extraction of EPA-rich oil and high-value protein. Biores. Technol. 244, 1416–1424 (2017)
deVrije, T., Claassen, P.A.M.: Dark hydrogen fermentations. In: Reith, J.H., Wijffels, R.H., Barten, H. (eds.) Bio-methane & Bio-hydrogen, pp. 103–123. Smiet Offset, The Hague (2003)
Villareal, M.L.M., Prata, A.M.R., Felipe, M.G.A., Almeida, E., Silva, E.: Detoxification procedure of eucalyptus hemicelluloses hydrolysate for xylitol production of Candida gulliermondii. Enzyme Microb. Technol. 40, 17–24 (2006)
Klement, T., Milker, S., Jäger, G., Grande, P.M., de-Maria, D., Büchs, J.: Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Factories. 11, 43 (2012)
Rattanapoltee, P., Kaewkannetra, P.: Utilization of agricultural residues of pineapple peels and sugarcane bagasse as cost-saving raw materials in Scenedesmus acutus for lipid accumulation and biodiesel production. Appl. Biochem. Biotechnol. 173, 1495–1510 (2014)
Huang, C., Chen, X.F., Xiong, L., Chen, X.D., Ma, L.L., Chen, Y.: Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol. Adv. 31, 129–139 (2013)
Taherzadeh, M.J., Karimi, K.: Pretreatment of lognocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9, 1621–1651 (2008)
Sun, J.X., Sun, X.F., Zhao, H., Sun, R.C.: Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 84, 331–339 (2004)
Manzoor, M., Jabeen, F., Skye, R.T.-H., Altaf, J., Younis, T., Schenk, P.M., Qazi, J.I.: Sugarcane bagasse as a novel low/no cost organic carbon source for growth of Chlorella sp. BR2. Biofuels. 10, 1–7 (2019)
Manzoor, M., Ahmad, Q.-A., Aslam, A., Jabeen, F., Rasul, A., Schenk, P.M., Qazi, J.I.: Mixotrophic cultivation of Scenedesmus dimorphus in sugarcane bagasse hydrolysate. Environ. Prog. Sustain. Energy. 39, e13334 (2020)
Lim, D.K.Y., Garg, S., Timmins, M., Zhang, E.S.B., Thomas-Hall, S.R., Schuhmann, H., et al.: Isolation and evaluation of oil producing microalgae from subtropical coastal and brackish waters. PLoS ONE 7, e40751 (2012)
Guilard, R.R.L., Ryther, J.H.: Studies of marine planktonic diatoms. I. cyclotella nana hustedt and Detonula confervacea. Cleve. Can. J. Microbiol. 8, 229–239 (1962)
Ho, S., Chen, H.C.Y., Chang, J.S.: Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 113, 224–252 (2012)
Segal, L., Creely, J., Martin, A., Conard, C.: An empirical method for estimating the degree of crystallinity of native cellulose using the X- ray diffractometer. Text Res. J. 29, 786–794 (1959)
Rodrigues, F.G., Assuncăõ, R.M.N., Vieira, J.G., Meireles, C.S., Cerqueira, D.A., Barud, H.S., Ribeiro, S.J.L., Messaddeq, Y.: Characterization of methylcellulose produced from sugarcane bagasse cellulose: Crystallinity and thermal properties. Polym. Degrad. Stab. 92, 205–210 (2007)
Ahmed, F., Fanning, K., Netzel, M., Turner, W., Li, Y., Schenk, P.M.: Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 165, 300–306 (2014)
Jeffery, S., Humphrey, G.: New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural populations. Biochem. Physiol. Pflanz. 167, 191–194 (1975)
Lopez, C.V.G., Garcia, C.C., Fernandes, F.G.A., Bustos, C.S., Chisti, Y., Sevila, J.M.F.: Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 101, 7587–7591 (2010)
Fogg, G.E.: Some comments on picoplankton and its importance in the pelagic ecosystem. Aquat. Microb. Ecol. 9, 33–39 (1995)
Volkman, J.K., Brown, M.R., Dunstan, G.A., Jeffrey, S.: The biochemical composition of marine microalgae from the class eustigmatophyceae. J. Phycol. 29, 69–78 (1993)
Lubián, L.M., Montero, O., Moreno-Garrido, I., Huertas, I.E., Sobrino, C., González-del Valle, M., et al.: Nannochloropsis (eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 12, 249–255 (2000)
Lee, M.-Y., Min, B.-S., Chang, C.-S., Jin, E.: Isolation and characterization of a xanthophyll aberrant mutant of the green alga Nannochloropsis oculata. Mar. Biotechnol. 8, 238–245 (2006)
Osinga, R., Kleijn, R., Groenendijk, E., Niesink, P., Tramper, J., Wijffels, R.H.: Development of in vivo sponge cultures: particle feeding by the tropical sponge Pseudosuberites aff. andrewsi. Mar. Biotechnol. 3, 544–554 (2001)
Ferreira, M., Coutinho, P., Seixas, P., Fábregas, J., Otero, A.: Enriching rotifers with ‘‘premium’’ microalgae Nannochloropsis gaditana. Mar. Biotechnol. 11, 585–595 (2009)
Griffiths, M.J., Harrison, S.T.L.: Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21, 493–507 (2009)
Heredia-Arroyo, T., Wei, W., Hu, B.: Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 162, 1978–1995 (2010)
Hu, H., Gao, K.: Optimization of growth and fatty acid composition of unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol. Lett. 25(5), 421–425 (2003)
Xu, N., Zhang, W., Ren, S., Liu, F., Zhao, C., Liao, H., Xu, Z., et al.: Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Biotechol. Biofuels 5, 58 (2012)
Pardo, M.F., Mwndoza, J.G.S., Galán, J.E.L.: Influence of pretreatments on crystallinity and enzymatic hydrolysis in sugarcane residues. Braz. J. Chem. Eng. 36(1), 131–141 (2019)
Rezendze, C.A., Delima, M.A., Maziero, P., Deazevedo, E.R., Garcia, W., Polikarpov, I.: Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels. 11, 4–54 (2011)
Chandel, A.K., Antunes, F.A.F., Anjos, V., Bell, M.J.V., Rodrigues, L.N., Polikarpov, I., Azevedo, E.R., Bernardinelli, O.D., Rosa, C.A., Pagnocca, F.C., Silva, S.S.: Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 7, 63 (2014)
Sindhu, R., Binod, P., Satyanagalakshmi, K., Janu, K.U., Sajna, K.V., Kurien, N., Sukumaran, R.K., Pandey, A.: Formic acid as a potential pretreatment agent for the conversion of sugarcane bagasse to bioethanol. Appl. Biochem. Biotechnol. 162, 2313–2323 (2010)
Xu, F., Cong, W., Cai, Z.-L., Ouyang, F.: Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 16, 499–503 (2004)
Gao, C.F., Zhai, Y., Ding, Y., Wu, Q.Y.: Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl. Energy 87, 756–761 (2010)
Cerón-García, M.C., Macías-Sánchez, M.D., Sánchez-Mirón, A., García-Camacho, F., Molina-Grima, E.: A process for biodiesel production involving the heterotrophic fermentation of Chlorella protothecoides with glycerol as the carbon source. Appl. Energy. 103, 341–349 (2013)
Wang, W.R., Zhou, W.W., Liu, J., Li, Y.H., Zhang, Y.K.: Biodiesel production from hydrolysate of Cyperus esculentus waste by Ch1orella vulgaris. Bioresour. Technol. 136, 24–29 (2013)
Silva, H.R., Prete, C.E.C., Zambrano, F., de-Mello, V.H., Tischer, C.A., Andrade, A.S.: Combining glucose and sodium acetate improves the growth of Neochloris oleabundans under mixotrophic conditions. AMB Express 6, 10 (2016)
Cheirisilp, B., Torpee, S.: Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 110, 510–516 (2012)
Kong, W., Song, H., Cao, Y., Yang, H., Hua, S., Xia, C.: The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotech. 10, 11620–11630 (2011)
Lee, Y.-K., Ding, S.-Y., Hoe, C.-H., Low, C.-S.: Mixotrophic growth of Chlorella sorokiniana in outdoor enclosed photobioreactor. J. Appl. Phycol. 8, 163–169 (1996)
Pagnanelli, F., Altimari, P., Trabucco, F., Toro, L.: Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: interaction between glucose and nitrate. J. Chem. Technol. Biotechnol. 89, 652–661 (2014)
Salati, S., Dimorzano, G., Menin, B., Veronesi, D., Scagila, B., Abruscato, P., Mariani, P., Adani, F.: Mixotrophic cultivation of Chlorella for local protein production using agro-food by-products. Bioresour. Technol. 230, 82–89 (2017)
Sforza, E., Cipriani, R., Morosinotto, T., Bertucco, A., Giacometti, G.M.: Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour. Technol. 104, 523–529 (2012)
Yu, H., Jia, S., Dai, Y.: Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J. Appl. Phycol. 21, 127–133 (2008)
Mu, J., Li, S., Chen, D., Xu, H., Han, F., Feng, B., Li, Y.: Enhanced biomass and oil production from sugarcane bagasse hydrolysate (SBH) by heterotrophic oleaginous microalga Chlorella protothecoides. Bioresour. Technol. 185, 99–105 (2015)
Wan, M., Liu, P., Xia, J., Rosenberg, J.N., Oyler, G.A., Betenbaugh, M.J., Qiu, G.D.: The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 91, 835–844 (2011)
Zhao, G., Yu, J., Jiang, F., Zhang, X., Tan, T.: The effect of different trophic modes on lipid accumulation of Scenedesmus quadricauda. Bioresour. Technol. 114, 466–471 (2012)
Arora, N., Patel, A., Pruthi, P.A., Pruthi, V.: Boosting TAG Accumulation with Improved Biodiesel Production from Novel Oleaginous Microalgae Scenedesmus sp. IITRIND2 Utilizing Waste Sugarcane Bagasse Aqueous Extract (SBAE). Appl. Biochem. Biotechnol. 180, 109–121 (2016)
Fang, X., Wei, C., Zhao-Ling, C., Fan, O.: Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 16, 499–503 (2004)
Suen, Y., Hubbard, J.S., Holzer, G., Tornabene, T.G.: Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J. Phycol. 23, 289–296 (1987)
Sevilla, J.M.F., García, M.C.C., Mirón, A.S., Belarbi, H., Garcia, C.F., Molina, E.G.: Pilot-plant-scale outdoor mixotrophic cultures of Phaeodactylum tricornutum using glycerol in vertical bubble column and airlift photobioreactors: studies in fed-batch mode. Biotechnol. Progress. 20, 728–736 (2004)
Wood, B.J.B., Grimson, P.H.K., German, G.B., Turner, M.: Photoheterotrophy in the production of phytoplankton organisms. J. Biotechnol. 70, 175–183 (1999)
Saraf, S., Thomas, B.: Influence of feedstock and process chemistry on biodiesel quality. Process Saf. Environ. Prot. 85, 360–364 (2007)
Lin, T.S., Wu, J.Y.: Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour. Technol. 184, 100–107 (2015)
Miazek, K., Remacle, C., Richel, A., Goffin, D.: Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 230, 122–131 (2017)
Nguyen, H.C., Liang, S.-H., Chen, S.-S., Su, C.-H., Lin, J.-H., Chien, C.-C.: Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: optimization by using response surface methodology. Energy Convers. Manage. 158, 168–175 (2018)
Knothe, G.: A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae derived biodiesel perform? Green Chem. 13, 3048–3065 (2011)
Cho, K., Lee, C.H., Ko, K., Lee, Y.-L., Kim, K.-N., Kim, M.-K., Chung, Y.-H., Kim, I.D., Yeo, K., Oda, T.: Use of phenol-induced oxidative stress acclimation to stimulate cell growth and biodiesel production by the oceanic microalga Dunaliella salina. Algal Res. 17, 61–66 (2016)
Yang, C., Hua, Q., Shimizu, K.: Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 6, 87–102 (2000)
Ip, P.F., Wong, K.H., Chen, F.: Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process. Biochem. 39, 1761–1766 (2004)
Yan, R., Zhu, D., Zhang, Z., Zeng, Q., Chu, J.: Carbon metabolism and energy conversion of Synechococcus sp. PCC7942 under mixotrophic conditions: comparison with photoautotrophic conditions. J. Appl. Phycol. 24, 657–668 (2012)
Liu, X., Duan, S., Li, A., Xu, N., Cai, Z., Hu, Z.: Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycol. 21, 239–246 (2009)
Abreu, A.P., Fernandes, B., Vicente, A.A., Teixeira, J., Dragone, G.: Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 118, 61–66 (2012)
Wang, J., Yang, H., Wang, F.: Mixotrophic cultivation of microalgae for biodiesel production: status and prospects. Appl. Biochem. Biotechnol. 172, 3307–3329 (2014)
Laliberté, G., Noüie, J.: Auto-, hetero- and mixotrophic growth of Chlamydomonas humicola (Chloroimyckak) on acetate. J. Phycol. 29, 612–620 (1993)
Hassall, K.A.: Xylose as a specific inhibitor of photosynthesis. Nature 181, 1273–1274 (1958)
Leite, G.B., Paranjape, K., Abdelaziz, A.E.M., Hallenbeck, P.C.: Utilization of biodiesel derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour. Technol. 184, 123–130 (2015)
Leite, G.B., Paranjape, K., Hallenbeck, P.C.: Breakfast of champions: fast lipid accumulation by cultures of Chlorella and Scenedesmus induced by xylose. Algal Res. 16, 338–348 (2016)
Griffiths, D.J., Thresler, C.L., Street, H.E.: The heterotrophic nutrition of Chlorella vulgaris. Ann. Bot. 2, 1–11 (1960)
Perez-Garcia, O., Escalante, F.M.E., de-Bashan, L.E., Bashan, Y.: Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 45, 11–36 (2011)
Cardona, C.A., Quintero, J.A., Paz, I.C.: Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 101, 4754–4766 (2010)
Gabov, K., Hemming, J., Fardim, P.: Sugarcane bagasse valorization by fractionation using a water-based hydrotropic process. Ind. Crops Prod. 108, 495–504 (2017)
Acknowledgements
We acknowledge the Higher Education Commission of Pakistan to provide financial assistance for the first author to conduct the present study.
Author information
Authors and Affiliations
Contributions
MM, did all experimentation and contributed to manuscript writing. FJ, QAH, TY, helped in statistical analysis and acquisition of data. EE, helped in analyzing results and with experiments. PMS, contributed to study design, critical discussions and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manzoor, M., Jabeen, F., Ahmad, QuA. et al. Sugarcane Bagasse Hydrolysate as Organic Carbon Substrate for Mixotrophic Cultivation of Nannochloropsis sp. BR2. Waste Biomass Valor 12, 2321–2331 (2021). https://doi.org/10.1007/s12649-020-01185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01185-0