Abstract
In order to improve the economic feasibility and environmental sustainability of microalgal bioethanol production, a nontoxic, copious agricultural waste, sugarcane bagasse aqueous extract (SBAE) was used for cultivating Nannochloropsis oculata microalga (NNO-1 UTEX Culture LB 2164) as potential sources of substitutes for traditional nutrition to reduce the costs in cultivation through acid digestion and enzymatic treatment before being fermented by Saccharomyces cerevisiae (NRRLY-2034). The primary target of this research was to find out the ethanol from hydrolysate of the defatted biomass of N. oculata grown mixotrophically on SBAE and CO2 as carbon sources. For acid hydrolysis (AH), the highest carbohydrate yield 252.84 mg/g DW has been obtained with 5.0% (v/v) H2SO4 at 121 °C for 15 min for defatted biomass cultivated mixotrophically on sugarcane bagasse aqueous extract (SBAE) regarding 207.41 mg/g DW for defatted biomass cultivated autotrophically (control treatment). Whereas, the highest levels of reducing sugars has been obtained with 4.0% (v/v) H2SO4 157.47±1.60 mg/g DW for defatted biomass cultivated mixotrophically compared with 135.30 mg/g DW for the defatted control treatment. The combination of acid hydrolysis 2.0% (v/v) H2SO4 followed by enzymatic treatment (AEH) increased the carbohydrate yields to 268.53 mg/g DW for defatted biomass cultivated mixotrophically on SBAE regarding 177.73 mg/g DW for the defatted control treatment. However, the highest levels of reducing sugars have been obtained with 3.0% (v/v) H2SO4 followed by enzyme treatment that gave 232.39±1.77 for defatted biomass cultivated mixotrophically on SBAE and 150.75 mg/g DW for the defatted control treatment. The sugar composition of the polysaccharides showed that glucose was the principal polysaccharide sugar (60.7–62.49%) of N. oculata defatted biomass. Fermentation of the hydrolysates by Saccharomyces cerevisiae for the acid pretreated defatted biomass samples gave ethanol yield of 0.86 g/L (0.062 g/g sugar consumed) for control and 1.17 g/L (0.069 g/g sugar consumed) for SBAE mixotrophic. Whereas, the maximum ethanol yield of 6.17±0.47 g/L (0.26±0.11 g/g sugar consumed) has been obtained with samples from defatted biomass grown mixotrophically (SBAE mixotrophic) pretreated with acid coupled enzyme hydrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioenergy has gained more attention in recent decades because of the depletion of fossil fuel forms and increasing requirements for eco-friendly energy to alleviate global warming (Koyande et al. 2019). Sustainable processing of biomass to produce biofuels, biochemicals, and bioenergy is referred to as bio-refining (Taylor et al. 2011; Knoshaug et al. 2018; Osman et al. 2020; Demiray et al. 2019; El-Sheekh et al. 2019; Aristizábal-Marulanda et al. 2021; Dong et al. 2016; Yao et al. 2020; Malhotra and Suman 2021; Rodríguez-Valderrama et al. 2020; Attia et al. 2021; Abdelsalam et al. 2019; Hijazi et al. 2020a, b). We can consider bio-refineries as environment-friendly analogous to oil refineries. Biofuels have the advantage of being renewable and emitting less environmental contaminants, resulting in a reduction of global warming. Because of its high oxygen content, ethanol is an excellent example of a biofuel that could be combined with gasoline to increase octane number, preventing cylinder knocking in engines and lowering greenhouse gas emissions (Wyman 1996; Sanchez and Cardona 2008; Walker 2011; Swana et al. 2011). In the perspective of bio-refining, biomass is a generic term for all organic materials which can be converted to fuels and chemicals. Biomass is a natural or anthropogenic organic, renewable and not fossil, composite biogenic material. It is obtained through the conversion of solar energy into lipids and carbohydrates in plants, algae, and certain bacteria. It is essential to categorize the variety of biomass sources in order to better understand the produced biofuels. The first-generation biomass comprises edible plant material and plants such as maize, wheat, sugarcane, and food grain, which, because of the disputes between food and fuel, appears unreasonable to commercial use. The second-generation biomass includes nonedible plant residues such as straw, wood, and sugarcane bagasse (SBA) that are rich in cellulose, which can be hydrolyzed into glucose. However, lignin and hemicellulose, materials which limit its conversion, are associated with this polysaccharide (Siqueira et al. 2013); therefore, the delignification process must be carried out before hydrolysis. During the delignification process, toxic compounds are formed (Mesa et al. 2011). Conversion of lignocellulosic residues into cellulosic ethanol comprises three phases: (i) pretreatment of raw materials to minimize the content of lignin and enhance polysaccharide exposure; (ii) transformation of polysaccharides into glucose and xylose monomers; (iii) sugar fermentation to ethanol. Thus, ethanol production from the lignocellulosic material is non-economic. The third-generation biomass includes microalgae and macroalgae. Marine biomasses such as seaweed, hyacinth, caltrop diatoms, duckweed, kelp, and Salvinia are candidates for the production of biofuels (Demiray et al. 2019; Nanda et al. 2018; Vassilev et al. 2012). The development of biofuel from microalgae biomass is regarded as one of the most promising third-generation technologies due to the effective photosynthetic process combined with a higher growth rate results in a greater ability to sequester carbon dioxide than other biomasses (Matsumoto et al. 1997). However, there are still significant complications to commercial microalgae development, such as economic barriers, a lack of technical readiness, and a shortage of common products developed to make overall energy production from algae biomass desirable. Selection of microalgae cultivation mode is of vital importance to achieve the highest possible cost-effectiveness of microalgae production.
In general, microalgae are usually cultivated by fixing dissolved, inorganic carbon (CO2) and absorbing solar energy; thus, they perform photosynthesis and are photo-autotrophs, while in heterotrophic cultivation organic carbon sources are directly used instead of light as carbon and energy source (Wen et al. 2019; Ceron-Garcia et al. 2005; Dragone et al. 2010; Cheah et al. 2018). Mixotrophic cultivations provide a viable method for maximizing algal biomass production and valuable metabolites in addition to lowering the expense of continuous light energy usage (Sipauba et al. 2020; Ceron-Garcia et al. 2005). Many microalgae species can be transit from the photoautotrophic to the mixotrophic pathway. Furthermore, it can only depend on autotrophic or heterotrophic metabolic pathways for growth (Choi and Lee 2015). The use of low-cost carbon sources is more cost-effective than using refined glucose. As a result, sugars extracted from sugarcane residues have been established as a promising energy source for mixotrophic microalgae cultivation (Nguyen et al. 2018). Defatted microalgal biomass can be hydrolyzed to produce a considerable amount of reducing sugars which can be fermented to ethanol (Sivasankar et al. 2017). In order to overcome economic problems combined with microalga production, the biomass remaining after extracting oil and other valuable compounds can be used in the production of ethanol after proper pretreatment (mechanically, chemically, or enzymatically) to liberate fermentable sugars (Sivasankar et al. 2017; Chaudhary et al. 2014; Nobre et al. 2013). In the present study, a nontoxic, copious agricultural waste, sugarcane bagasse aqueous extract has been used as a possible source of traditional nutritional substitutes to minimize the costs of cultivation and biodiesel production. In addition, the zero-value defatted N. oculata microalga was used as a feedstock source for bioethanol production after hydrolysis under variable acid concentration and/or constant enzyme dosage.
Materials and methods
Algae and growth conditions
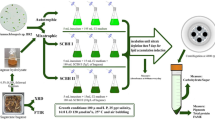
Marine microalga Nannochloropsis oculata (NNO-1 UTEX Culture LB 2164) belonging to Chrysophyta were obtained from Algal Biotechnology Unit, National Research Centre. We grew cultures under conditions of F2 medium (Guillard 1975) containing the following composition (g L−1): NaNO3 0.075, NaH2PO4 H2O 0.005, and Na2SiO3.9H2O 0.030. Sea water was artificially formulated from 22.0 g/L of sea salt dissolved in pre-sterilized tap water. We performed mixotrophic carbon nutrition based on the examined volume of bagasse extract (El-Sayed et al. 2020). We locally collected sugarcane bagasse from Giza Governorate Egypt. The bagasse was dried at 70 °C for 24 h in a hot air oven and homogenized using a blender. We then subjected dried bagasse to acid hydrolysis at 30 °C for 24 h with 1.0N HNO3, and we removed suspended solids using a sieve (0.2 mm); then, filtrate was sugarcane bagasse vacuum-filtered using a 0.45-μm membrane filter (El-Sayed et al. 2020). Biochemical profile sugarcane bagasse extract was presented in our previous work (El-Sayed et al. 2020).
Light intensity was adjusted at 120μ.e from one side white light bank, and aeration was done by free oil compressed air. According to the previously described structure (El-Sayed et al. 2020), Nannochloropsis oculata was cultivated for mass production in 1000-l capacity tubular photobioreactor. Prior to cultivation, the unit was sterilized by circulating hypochlorite solution for 10 min, followed by water to remove the hypochlorite residues, then filled with 1000 l tap water containing 25% F2 artificial growth medium, 10% sugarcane bagasse aqueous extract (SBAE) filtrate, and enriched by commercial fertilizer compounds as described by El-Sayed et al. (2020). Night illumination was provided by the upper surface fluorescent lamp (6 lamps × 40 watts) along with the growth surface. When the alga reached its maximum growth dry weight (g/L), stress was induced with 20 kg NaCl (2%), and we collected continuously samples from the bioreactor until the end of the experiments. Control treatment of N. oculata was grown by the autotrophic way of nutrition on the F2 medium.
Fat extraction from algal biomass
We switched off the aeration of the culture at the end of the incubation cycle to allow algal cells to settle. To concentrate the algal slurry, we used thermo laboratory centrifuge (4000 rpm/1800g) after removal of the transparent supernatant. After drying the biomass, we extracted the lipids by using a hexane/isopropanol mixture (3:2). Using the Soxhlet method, the extracted biomass had been removed, and the residual hexane had evaporated by bubbling with either nitrogen or carbon dioxide gas in a downflow mode. The procedure was repeated until no odor of hexane was detected. We collected the LEAB as dry biomass and held at 4 °C until it has been used.
Acid hydrolysis and preparation of defatted biomass hydrolysates
We accomplished acid hydrolysis in 250-mL conical flasks with 0%, 1%, 2%, 3%, 4%, and 5% of 98% H2SO4 for 15 min at 121 °C. The solid/liquid ratio of defatted biomass content was set at 10% (w/v). (Miranda et al. 2012). The liquid fraction was isolated after hydrolysis by centrifugation at 14,000 rpm (26342 RCF with radius of rotor (120 mm)) for 15 min). We neutralized the supernatant to pH 7 with 5.0 M NaOH and then used to determine total carbohydrates, reducing sugars, monosaccharides, and hydroxymethyl furfural (HMF) before being stored at 5–7 °C until used to produce ethanol. We conducted all experiments in triplicates.
Enzyme hydrolysis and preparation of defatted biomass hydrolysates
Defatted dry biomass was mixed in 1:10 (w/v) ratios with 0.0, 1.0, 2.0, and 3.0% H2SO4 solutions and autoclaved for 15 min at 121 °C. The autoclaved solutions were cooled, neutralized with 5 M NaOH, and the saccharification process was carried out with a mixture of commercially available enzyme mixtures from Trichoderma reesei ATCC 26921 (3.2.1.4 (BRENDA, IUBMB)) and Trichoderma longibrachiatum C 9748 (3.2.1.4 BRENDA, IUBMB) containing multiple enzyme activities, mainly exoglycanase (1.6 FPU/mL), endoglucanase (33.3 U/mL), cellobiohydrolase (30.02 U/mL), xylanase (21.0 U/mL), and β-glucosidase content (12.4 U/mL). The hydrolysis was carried out by incubating the mixture in a water bath at 60 °C, pH 5.5, for 24 h (pH was maintained using 0.05M citrate buffer). The residual materials were separated by centrifugation at 14,000 rpm for 15 min. For complete free sugar analysis, the resulting hydrolysate has been used to determine total carbohydrates, reducing sugars, monosaccharides, and hydroxymethyl furfural (HMF) and then cooled up to 5–7 °C until used for ethanol production (Mirsiaghi and Reardon 2015). We conducted all tests three times. We bought the commercial enzymes used in this study from Sigma-Aldrich.
Ethanol production from defatted algal biomass
Inoculum preparation
The inoculum of Saccharomyces cerevisiae (NRRLY-2034) obtained from the Department of Microbiology, Soils, Water, and Environment Research Institute, Agriculture Research Center—Giza, Egypt was cultured on yeast extract peptone dextrose agar (YPD) and incubated at 30 °C and 150 rpm for 48 h. The cell density at the end of incubation was 5 × 107 CFU/mL.
Ethanol production
The medium used for ethanol fermentation was composed of (%) 0.25 yeast extract, 0.25 (NH4)2SO4, 0.1 KH2PO4, and 0.05 MgSO4.7H2O, which were added to 100 mL of the filtrate from saccharified biomasses and sterilized at 121 °C for 15 min. The medium was cooled at room temperature and inoculated with Saccharomyces cerevisiae at 2.0% v/v then incubated at 30 °C for 4 days under static conditions. We monitored glucose and ethanol concentrations in a fermentation medium at 12-h intervals. Ethanol yield was calculated according to the equation derived by Yoswathana et al. (2010).
Ethanol yield = measured ethanol in the sample (g) / theoretical ethanol (g)
where theoretical ethanol (g) = amount of initial sugar content (g) in fermentation solution × 0.5.
Analytical procedures
The method described by Dubois et al. (1956) performed the measurement of the total carbohydrate. Estimation for the amount of reducing sugars was carried out by the dinitro salicylic acid (DNS) method using glucose as standard (Miller 1959). We analyzed the monosaccharides and hydroxymethyl furfural (HMF) in the hydrolysates using high-performance liquid chromatography (HPLC, Agilent, USA). The chromatographic conditions were waters amino chromatographic column with a refractive index detector, elution influent. The mobile phase was deionized water that had been degassed in an ultrasonic bath under vacuum. At a temperature of 40 °C and an injection volume of 10 L, the flow rate was 0.7 mL/min. The repeatability relative standard deviation of this method is below 5%, and the recovery is over 97%. A satisfactory result had been obtained under these optimum conditions, and we finished the complete separation within 25 min. Comparison with analytical curves achieved the quantification using glucose, fructose, sucrose, fucose, ribose, arabinose, xylose, and mannose standards. HMF was determined using a diode-array detector and an ODS analytical column with detection of 280 nm. We prepared a filtered and degassed mobile phase at a flow rate of 1 mL/min with a sodium acetate buffer (0.08 M) and adjusted to pH 3.6 with glacial acetic acid. Gas Chromatography was used to determine the concentration of bioethanol. Purification by distillation is very low when the retention time of impurities considered (below the boiling point of ethanol). The fermentation efficiency (FE%) was determined by dividing the average generated ethanol by the theoretically produced ethanol in the biochemical conversion of the sugars consumed.
Statistical analysis
Statistical analysis was done by analysis of variance (ANOVA) test using the Microsoft Excel program. The difference in values was indicated in the form of probability (p < 0.05) values.
Results
To increase the economic viability and environmental sustainability of microalgal bioethanol production, sugarcane bagasse aqueous extract (SBAE) as a nontoxic and abundant agricultural waste was used for cultivating Nannochloropsis oculata microalga. The content of carbohydrates in microalgae is mainly present within the cell wall; its disruption and collapses are required to release monosaccharides and make them accessible to fermentative microorganisms as carbon sources. The efficiency of each pretreatment method was determined by measuring reducing sugars in hydrolysates. The sugar composition of the polysaccharides and hydroxymethyl furfural (HMF) in hydrolysates was determined by HPLC after each pretreatment. Effective pretreatment processes take into consideration the cost of the production process, degradation capacity of fermentable sugars, energy efficiency, and ease of application (Sritrakul et al. 2017; Phwan et al. 2019).
Effect of acid hydrolysis on total carbohydrates and total reduced sugar content
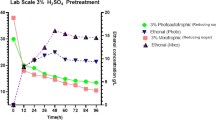
Giang et al. (2019) showed that the acid concentration is a limiting factor that can impair microalgal biomass saccharification in the autoclave. The total carbohydrate yields were increased with increasing acid concentration during the hydrolysis processes with H2SO4 pretreatment in the autoclave at 121 °C for 15 min as shown in Figure 1. The highest carbohydrate yields due to acid hydrolysis were 252.84 mg/g DW for defatted biomass cultivated mixotrophically on SBAE obtained with 5.0% (v/v) H2SO4 concentration with respect to 207.41 mg/g DW for defatted biomass cultivated autotrophically (defatted control treatment). Regarding the reducing sugar, results shown in Figure 1 revealed that increasing acid concentration from 1.0 (v/v) to 4.0% (v/v) increased the concentration of H+ to a level that was optimum for hydrolyzing the biomass. The highest level of reducing sugars due to acid hydrolysis (4.0% (v/v) H2SO4) was 157.47 mg/g DW for defatted biomass cultivated mixotrophically compared to 135.30 mg/g DW for defatted control treatment.
Effect of enzymatic hydrolysis on total carbohydrates and total reduced sugar content
The synergistic effect of enzymatic hydrolysis coupled with acid pretreatment was investigated and shown in Fig. 2. The highest carbohydrate yield was obtained after a 15-min pretreatment with 2.0% sulfuric acid at 121 °C. It was recorded as 268. 53 and 177.73 mg/g DW for defatted biomass cultivated mixotrophically and for defatted biomass cultivated autotrophically treatment, respectively. However, the highest levels of reducing sugars were 232.39 mg/g obtained with 3.0% (v/v) H2SO4 concentration for defatted biomass cultivated mixotrophically and 150.75 mg/g for the defatted control treatment (Fig. 2). By the same respect, 8.56 and 4.25 mg/g were obtained with 0.0% H2SO4 digestion comparing with 40.39 and 32.17 mg/g of 0.5% (v/v) H2SO4 digestion or with 1.0% H2SO4 digestion that resulted in 66.25 and 45.09 mg/g. Whereas pretreatment with 2.0%(v/v) H2SO4 digestion gave 152.39 and 87.68 mg/g for the defatted biomass cultivated mixotrophically on SBAE and control treatment, respectively. Therefore, combination with acidic pretreatment increased the enzymatic scarification performance of algal biomass.
Sugar composition of the polysaccharides
Enzymatic hydrolysis after pretreatment with 3.0% (v/v) H2SO4 yielded the highest saccharification rates of 92.2% and 90.6% for defatted biomass cultivated mixotrophically on SBAE and control treatment, respectively. At high acid hydrolysis concentration 4.0% (v/v), both defatted biomasses were more resistant than enzymatic hydrolysis with released sugar yields of 76.29% and 75.17% for defatted biomass cultivated mixotrophically on SBAE and control treatment, respectively. Thus, both treatments were subjected to HPLC analysis of hydrolase products before bioethanol production. Results in Table 1 and Fig. SI 1 A and B showed the sugar composition of the defatted biomass of N. oculata pretreated with 4.0% (v/v) H2SO4; unexpectedly, acid hydrolysis of the algal biomass grown mixotrophically or autotrophically (control) gave low glucose yield (22.5% and 18.2%, respectively).
However, the enzymatic hydrolysis coupled with acid pretreatment showed a higher saccharification ratio (total reducing sugar yield to total carbohydrates) for the defatted biomass of N. oculata grown mixotrophically or autotrophically (control treatment). Both the defatted biomasses showed the same suite of sugars. Glucose was the principal polysaccharide sugar (62.49% and 60.7%) followed by galactose, fucose, rhamnose, xylose, arabinose, and mannose (Table 1, Fig. SI 1C and D). Figure SI 2 showed the HPLC chromatograms of hydroxymethyl furfural (HMF): (A) standard and (B) sample of N. oculata extracts from acid enzyme hydrolysis.
Ethanol productivity
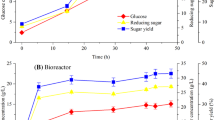
The algae hydrolysates were then used as a substrate for the producer microorganisms to convert to bioethanol. After every 12 h, samples were taken from the fermentation media and were analyzed for alcohol contents through the GC method. Also, the reduction in sugar content was simultaneously measured. Based on chromatogram analysis by comparing ethanol standard and sample, ethanol production from hydrolysates of defatted N. oculata biomass treated with acid hydrolysis and acid coupled enzyme hydrolysis is shown in Fig. 3. Among the samples, the ethanol yield of the acid pretreated defatted biomass samples was only 0.86 g/L (0.062 g/g sugar consumed) for defatted biomass grown autotrophically (control) and 1.17 g/L (0.069 g/g sugar consumed) for SBAE mixotrophic. However, the highest ethanol yield of 6.17±0.47 g/L (0.26±0.11 g/g sugar consumed) was obtained at the samples from defatted biomass grown mixotrophically (SBAE mixotrophic) pretreated with acid coupled enzyme hydrolysis (AEH). Whereas the initial reducing sugar concentration was 24.33±1.41 g/L at 48 h corresponding to 64.50% of theoretical fermentation yield. The reducing sugar in N. oculata hydrolysate was not fully consumed after 72 h and it was 2.47±1.63 g/L.
Discussion
Results of acid hydrolysis clearly indicate that when 5.0% (v/v) H2SO4 was added, the sugar release values were decreased. Increasing the acid concentration to 5.0% (v/v) H2SO4 led to lower pretreatment efficiency with excessive H+ degrading the sugar progressively into inhibitors. These results correspond to the findings of Miranda et al. (2012), which found that a decrease in reducing sugars is observed when a concentration higher than 2 N (5.4% v/v) H2SO4 was applied for Scenedesmus oblique.
The highest levels of reducing sugars were obtained with 4% H2SO4 in accordance with that reported by Hernández et al. (2015) in which pretreatments for Chlorella sorokiniana, Nannochloropsis gaditana, and Scenedesmus almeriensis were carried out using H2SO4 at different concentrations (4%, 7%, and 10% (v/v)) at 121 °C for 30 min. They reported that the highest reducing sugar was obtained with 7% H2SO4 concentration and values of 84, 93, and 55 mg/g DW for C. sorokiniana, N. gaditana, and Scenedesmus almeriensis, respectively, with small differences between 4% and 7%.
Results in Figure 2 confirmed that the enzymatic saccharification efficiency of algal biomass was enhanced by coupling with 3.0% H2SO4 pretreatments. The higher saccharification rate is obtained due to the greater exposure of algal biomass to enzyme degradation. Nannochloropsis cells have a high cell rupture resistance with a structurally complex and mechanically rigid cell wall consisting of an outer layer of algaenan and a cellulose layer in the inner layer (∼75 wt% of the cell wall) (Ajjawi et al. 2017). Therefore, cell wall conferred microalgal cells with structural rigidity as well as resistance to cell rupture and chemical digestion (Gerken et al. 2013; Scholz et al. 2014; Angles et al. 2017; Baudelet et al. 2017). In general, our results revealed that samples of defatted biomass cultivated mixotrophically had relatively higher carbohydrate contents than photoautotrophic condition (control).
Under heterotrophic and mixotrophic conditions, energy storage molecules such as lipids and carbohydrates (starch and glycogen) are strongly accumulated. Consequently, the biomass content of these compounds is higher than that under photo-autotrophic conditions (Choix et al. 2012).
Concerning to the polysaccharide composition of N. oculata biomass, HPLC chromatogram results of the polysaccharide composition showed that acid hydrolysis was not sufficient to hydrolyze high amounts of cellulosic materials. The existence of galactose may be attributed to the high concentrations of galactolipids (e.g., MGDG, DGDG) that compose the photosynthetic membranes of actively growing cells (Harwood et al. 1998) or to the 1-6 linked galactans that decorate the cell wall glycoproteins (Noda et al. 1996). Murano et al. (1997) evaluated the effect of H2SO4 concentration and reaction time on the hydrolysis efficiency of the biomass from Solieria filiformis seaweed, and they obtained similar effects of acid on galactose. An increase in acid concentration up to 0.2 M resulted in a sharp increase in galactose. Most galactose presences in hydrolysates are confirmed by the high contents of the pure iota-carrageenan of the Solieria filiformis (Harwood et al. 1998). Nannochloropsis has complex cell walls that comprise an outer algaenan layer and an inner layer of cellulose (Ajjawi et al. 2017). Nannochloropsis algaenans appeared to be much the same biopolymer as the cutan found in drought-resistant land plants such as Agave and Clivia (Blokker et al. 1998; Gupta et al. 2006). Moreover, the presence of a high amount of HMF (Table 1 and Fig. 3) is due to the degradation of glucose to HMF. Whereas HMF was presented in low concentrations in defatted biomass pretreated with 3.0% H2SO4 followed by enzymatic hydrolysis biomass grown mixotrophically and autotrophically. Appropriate pretreatment methods are required to overcome the recalcitrance of lignocellulosic materials such as dilute 0.5–2.5% H2SO4 performed at temperatures from 100 to 200 °C (Sun et al. 2016).
Results of monosaccharide analysis after enzymatic hydrolysis coupled with acid pretreatment (AEH) were in relative agreement with that obtained by Volkman et al. (1993), which also showed glucose (45.2–66.2% of total sugars) to be the most dominant monosaccharide in Nannochloropsis biomass. Other sugars included fucose, galactose, mannose, rhamnose, ribose, and xylose (2.0–14.0). The most prominent monosaccharide in Nannochloropsis sp. is glucose, fucose, galactose, mannose, ribose, and xylose (Templeton et al. 2012). The acid coupled enzyme hydrolysis introduces a leap in bioethanol production from defatted biomass of N. oculata. Bioethanol yields of Nannochloropsis gaditana grown in various municipal wastewaters (0, 30, 60, and 100%) ranged between 70.3±2.4 mg/g biomass and 94.3±5.5 mg/g biomass (Onay 2018). The maximum bioethanol production and yield by the microalgal hydrolysate were found to be 4.84 g/L and 0.37 g/g, respectively (Lee et al. 2019) when Brettanomyces custersii H1-603 was used to convert Nannochloropsis gaditana hydrolysate.
In the present study, the ethanol production rate and fermentation efficiency of the acid hydrolysate (AH) were low, due to the presence of galactose as the main reducing sugar in the dilute acid hydrolysate, and the fact that ethanol yield from galactose is lower than glucose (Hong et al. 2011; Hessami et al. 2019). D-galactose undergoes conversion via the Leloir pathway; basically, in this pathway, a five-step enzymatic pathway converts D-galactose to glucose-6-phosphate (Timson 2007). Therefore, lower fermentation yield from galactose compared with glucose is due to higher energy consumption in galactose metabolism. All the biofuel sources seem to be more expensive compared with fossil oil, and the ambient world conditions obligate producers to use vegetables and edible plants sources. Algae production seems to be of high infrastructure costs, and reducing these costs took place (El-Sayed et al. 2015, 2017) besides the ambient Egyptian conflations. Otherwise, integration of a fuel matrix was also studied to get the maximum full gain of inducing biodiesel, followed by bioethanol from defatted algae. It will then orient the rest dry biomass for the production of the main products in terms of bio-stimulators, fodder, and bio-control agents.
Concerning economic productivity of bioethanol from algae, the used biomass after oil extraction could serve as wastes, and to increase the beneficial force of such wastes, production of bioethanol and biogas might be useful. Further studies will be focused on the increasing of reduced sugars of algae. The local production cost is approximately US $2.0 (35 L.E.) of dried biomass. The produced matrix could be summarized as 40–60% of unsaturated fatty acids with a high marketing value, about 50% on the average saturated fatty acid which must be oriented to biodiesel production. Biogas reached 263 mL/g with sludge (Adam and Shanableh 2017) and about 750 g.kg−1 of bio-stimulator or bio-control agent which represented the main goal. Accordingly, any quantity of the obtained ethanol would increase the commercial gain of the algal matrix. Further research might increase the initial content of the reduced sugars and consequently the net ethanol.
Conclusion
In general, this is the first study describing bioethanol and carbohydrate productions from defatted biomass of Nannochloropsis oculata grown mixotrophically on SBAE. The comparison between different chemical pretreatment methods has shown that acid treatment combined with subsequent enzyme treatment is a suitable method for the conversion of cellulose to sugar. Up to 92% of sugar yield could be observed after this method. Acid hydrolysis was not sufficient to hydrolyze large quantities of celluloses. The maximum ethanol production was obtained from defatted biomass hydrolyzed by acid coupled with enzyme hydrolysis of defatted biomass. The acid coupled enzyme hydrolysis introduces a leap in bioethanol production from microalgae such as N. oculata. However, this method recommended efficient improvement in fermentation technology to make it an industrially feasible feedstock for bioethanol production in terms of economic viability.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abdelsalam E, Samer M, Attia Y, Abdel-Hadi MA, Hassan HE, Badr Y (2019) Effects of laser irradiation and Ni nanoparticles on biogas production from anaerobic digestion of slurry. Waste and Biomass Valor. 10(11):3251–3262
Adam N, Shanableh A (2017) Combined production of three bioenergy resources from Nannochloropsis sp. microalgae. Int. J. Energy Environ. Eng. 7(4):114–119
Ajjawi I, Verruto J, Aqui M, Soriaga L, Coppersmith J, Kwok K, Peach L, Orchard E, Kalb R, Xu W, Carlson TJ, Francis K, Konigsfeld K, Bartalis J, Schultz A, Lambert W, Schwartz AS, Brown R, Moellering ER (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol 35(7):647–652
Angles E, Jaouen P, Pruvost J, Marchal L (2017) Wet lipid extraction from the microalga Nannochloropsis sp.: disruption, physiological effects and solvent screening. Algal Res. 21:27–34
Aristizábal-Marulanda V, Solarte-Toro JC, Cardona Alzate CA (2021) Study of biorefineries based on experimental data: production of bioethanol, biogas, syngas, and electricity using coffee-cut stems as raw material. Environ Sci Pollut Res. 28:24590–24604
Attia YA, Samer M, Moselhy MA, Arisha AH, Abdelqader AA, Abdelsalam E (2021) Influence of laser photoactivated graphitic carbon nitride nanosheets and nickel nanoparticles on purple non-sulfur bacteria for biohydrogen production from biomass. J. Clean. Prod 299:126898
Baudelet PH, Ricochon G, Linder M, Muniglia L (2017) Grand challenges in algae biotechnology. Algal Res. 25:333–371
Blokker P, Schouten S, Vanen EH, De Leeuwm JW, Hatcher PG, Damste JSS (1998) Chemical structure of algaenans from the fresh water algae Tetraedron minimum, Scenedesmus communis and Pediastrum boryanum. Org Geochem 29:1453–1468
Ceron-Garcia MC, Sanchez MA, Fernandez SJM, Molina GE, Garcia CF (2005) Mixotrophic growth of the microalga Phaeodactylum tricornutum: influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem 40(1):297–305
Chaudhary L, Priya P, Soni N, Singh P, Tiwari A (2014) Algae as a feedstock for bioethanol production: new entrance in biofuel world. Int J Chemtech Res 6(2):1381–1389
Cheah WY, Show PL, Juan JC, Chang JS, Ling TC (2018) Enhancing biomass and lipid productions of microalgae in palm oil mill effluent using carbon and nutrient supplementation. Energy Convers Manag 164:188–197
Choi HJ, Lee SM (2015) Biomass and oil content of microalgae under mixotrophic conditions. Environ Eng Res 20(1):25–32
Choix FJ, De-Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzyme Microb Technol 51(5):300–309
Demiray E, Karatay SE, Dönmez G (2019) Improvement of bioethanol production from pomegranate peels via acidic pretreatment and enzymatic hydrolysis. Environ Sci Pollut Res 26:29366–29378
Dong T, Knoshaug EP, Davis R, Laurens LML, Van Wychen S, Pienkos PT, Nagle N (2016) Combined algal processing: a novel integrated biorefinery process to produce algal biofuels and bioproducts. Algal Res. 19:316–323
Dragone G, Fernandes B, Vicente A, Teixeira JA (2010) Third generation biofuels from microalgae, current research, technology and education. Appl Microbiol Biotechnol 2:1355–1368
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356
El-Sayed AB, Battah MG, El-Sayed EW (2015) Utilization efficiency of artificial carbon dioxide and corn steam liquor by Chlorella vulgaris. Biolife 3(2):391–403
El-Sayed AB, Nashwa AHF, Magda K (2017) Bioethanol production from Egyptian alga Enteromorpha sp. Middle East J Appl Sci 7(1):216–225
El-Sayed AB, Nashwa AHF, Ibrahim FM, Fayed SA, Sadik MW (2020) Application of bagasse extract in economic Nannochloropsis oculata mass production. Egypt J Chem 63(12):5183–5192
El-Sheekh MM, Gheda SF, El-Sayed AEKB, Abo Shady AM, El-Sheikh ME, Schagerl M (2019) Outdoor cultivation of the green microalga Chlorella vulgaris under stress conditions as a feedstock for biofuel. Environ Sci Pollut Res 26:18520–18532
Gerken HG, Donohoe B, Knoshaug EP (2013) Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237(1):239–253
Giang TT, Lunprom S, Liao Q, Reungsang A, Salakkam A (2019) Improvement of hydrogen production from Chlorella sp. biomass by acid-thermal pretreatment. PeerJ 7:6637.
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Charley MM (eds) Culture of marine invertebrates’ animals. Plenum, New York, pp 29–60
Gupta NS, Collinson ME, Briggs DEG, Evershed RP, Pancost RD (2006) Reinvestigation of the occurrence of cutan in plants: implications for the leaf fossil record. Paleobiology 32:432–449
Harwood JL, Siegenthaler PA, Murata N (eds) (1998) Lipids in photosynthesis: structure, function and genetics. Springer, Netherlands, p 53
Hernández D, Riaño B, Coca M, García-González MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945
Hessami MJ, Cheng SF, Ambati RR, Yin YH, Phang SM (2019) Bioethanol production from agarophyte red seaweed, Gelidium elegans, using a novel sample preparation method for analyzing bioethanol content by gas chromatography. J Biotechnol 3:9–25
Hijazi O, Abdelsalam E, Samer M, Amer BMA, Yacoub IH, Moselhy MA, Attia YA, Bernhardt H (2020a) Environmental impacts concerning addition of the trace metals in the process of biogas production from anaerobic digestion of slurry. J. Clean. Prod. 243:118593
Hijazi O, Abdelsalam E, Samer M, Attia YA, Amer BMA, Amer MA, Badr M, Bernhardt H (2020b) Life cycle assessment of the use of nanomaterials in biogas production from anaerobic digestion of manure. Renew. Energy 148:417–424
Hong KK, Vongsangnk W, Vemuri GN, Nielsen J (2011) Unravelling evolutionary strategies of yeast for improving galactose utilization through integrated systems level analysis. PNAS 108(29):12179–12184
Knoshaug EP, Dong T, Spiller R, Nagle N, Pienkos PT (2018) Pretreatment and fermentation of salt-water grown algal biomass as a feedstock for biofuels and high-value biochemicals. Algal Res. 36:239–248
Koyande AK, Show P, Guo R, Tang B, Ogino O, Chang J (2019) Bio-processing of algal bio-refinery: a review on current advances and future perspectives. Bioengineered 10(1):574–592
Lee JH, Lee HU, Lee JH, Lee SK, Yoo HY, Park C, Kim SW (2019) Continuous production of bioethanol using microalgal sugars extracted from Nannochlorpsis gaditana. Korean J Chem Eng 36:71–76
Malhotra M, Suman SK (2021) Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing obstacles in energy generation. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13283-0
Matsumoto H, Hamasaki A, Sioji N, Ikuta Y (1997) Influence of CO2, SO2 and NO in flue gas on microalgae productivity. JCEJ 30:620–624
Mesa L, González E, Cara C, González M, Castro E, Mussatto SI (2011) The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chem Eng J 168:1157–1162
Miller GL (1959) Use of dinitrosalicylic acid for determination of reducing sugar. Anal Chem 31:426–428
Miranda JR, Passarinho PC, Gouveia L (2012) Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Biores Technol 104:342–348
Mirsiaghi M, Reardon KF (2015) Conversion of lipid-extracted Nannochloropsis salina biomass into fermentable sugars. Algal Res 8:145–152
Murano E, Toffanin R, Cecere E, Rizzo R, Knutsen SH (1997) Investigation of the carrageenans extracted from Solieria filiformis and Agardhiella subulata from Mar Piccolo, Taranto. Mar Chem 58(3-4):319–325
Nanda S, Rana R, Sarangi PK, Dalai AK, Kozinski JA (2018) A broad introduction to first-, second-, and third-generation biofuels. In: Sarangi P, Nanda, Mohanty P (eds) Recent advancements in biofuels and bioenergy utilization. Springer, Singapore. https://doi.org/10.1007/978-981-13-1307-3_1
Nguyen HC, Su CH, Yu YK, Huong DTM (2018) Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalgae Schizochytrium sp. Ind Crops Prod 121:99–105
Nobre BP, Villalobos F, Barragan BE, Oliveira AC, Batista AP, Marques PASS, Mendes RL, Sovova L, Palavra AF, Gouveia L (2013) A biorefinery from Nannochloropsis sp. microalga—extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Biores Technol 135:128–136
Noda K, Ohno N, Tanaka K, Kamiya N, Okuda M, Yadomae T, Nomoto K, Shoyama Y (1996) A water-soluble antitumor glycoprotein from Chlorella vulgaris. Planta Med 62(5):423–426
Onay M (2018) Bioethanol production from Nannochloropsis gaditana in municipal wastewater. Energy Procedia 153:253–257
Osman MEH, Abo-Shady AM, Elshobary ME, Abd El-Ghafar MO, Abomohra A (2020) Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ Sci Pollut Res 27:32481–32493
Phwan CK, Chew KW, Sebayang AH, Ong HC, Ling TC, Marlinda AM, Ho Y, Show PL (2019) Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol Biofuels 12:191
Rodríguez-Valderrama S, Escamilla-Alvarado C, Rivas-García P, Magnin J-P, Alcalá-Rodríguez M, García-Reyes RB (2020) Biorefinery concept comprising acid hydrolysis, dark fermentation, and anaerobic digestion for co-processing of fruit and vegetable wastes and corn stover. Environ Sci Pollut Res 27:28585–28596
Sanchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Biores Technol 99(13):5270–5295
Scholz MJ, Weiss TL, Jinkerson RE, Jing J, Roth RU, Goodenough MCP, Gerken HG (2014) Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell 13:450–1464
Sipauba TLH, Scardoeli TB, Fenerick DC, Tedesque MG (2020) Comparison of photoautotrophic and mixotrophic cultivation of microalgae Messastrum gracile (Chlorophyceae) in alternative culture media. Braz J Biol Sci:7–23
Siqueira G, Várnai A, Ferraz A, Milagres AMF (2013) Enhancement of cellulose hydrolysis in sugarcane bagasse by the selective removal of lignin with sodium chlorite. Applied Energy 102:399–340
Sivasankar P, Dhandayuthapani K, Shanthi K (2017) Production of ethanol using hydrolysates derived from acid pretreated defatted biomass Nannochloropsis limnetic. Int J Recent Sci 8(8):19392–19395
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric. Nat Resour 51(6):512–519
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Biores Technol 199(1):49–58
Swana J, Yang Y, Behnam M, Thompson R (2011) An analysis of net energy production and feedstock availability for biobutanol and bioethanol. Bioresource Technology 102(2):2112–2117
Timson DJ (2007) Galactose metabolism in Saccharomyces cerevisiae. Dyn Biochem Process Biotechnol Mol Biol 1(1):63–73
Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ (2012) An overview of the organic and inorganic phase composition of biomass. Fuel 94:1–33
Volkman JK, Brown MR, Dunstan GA, Jeffrey SW (1993) The biochemical composition of marine microalgae from the class Eustigmatophyceae. J Phycol 29:69–78
Walker GM (2011) Fuel alcohol: current production and future challenges. J Inst Brew 117(1):3–22
Wen X, Tao H, Peng X, Wang Z, Ding Y, Xu Y, Liang L, Du K, Zhang A, Liu C, Geng Y, Li Y (2019) Sequential phototrophic-mixotrophic cultivation of oleaginous microalgae Grasiella sp. WBG-1 in a 1000 m2 open raceway pond. Biotechnol. Biofuels 12(1):27
Wyman C (1996) Handbook on bioethanol. Routledge, New York. https://doi.org/10.1201/9780203752456
Yao P, You S, Qi W, Su R, He Z (2020) Investigation of fermentation conditions of biodiesel by-products for high production of β-farnesene by an engineered Escherichia coli. Environ Sci Pollut Res 27:22758–22769
Yoswathana N, Phuriphipat P, Treyawutthiwat P, Eshtiaghi MN (2010) Bioethanol production from rice straw. Energy Res J 1(10):26–31
Acknowledgements
The authors acknowledge the members of the Faculty of Agriculture—Cairo University for their supports.
Funding
This work was financially supported by the Academy of Scientific Research & Technology program “Scientists for Next Generation”.
Author information
Authors and Affiliations
Contributions
MWS, ABE, and YAA conceived, designed research, analyzed data, wrote the manuscript, read, and approved the manuscript. FMI and NAHF conducted experiments and contributed with reagents and test methods and analytical tools. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 472 kb)
Rights and permissions
About this article
Cite this article
Fetyan, .A.H., El-Sayed, A.EK.B., Ibrahim, F.M. et al. Bioethanol production from defatted biomass of Nannochloropsis oculata microalgae grown under mixotrophic conditions. Environ Sci Pollut Res 29, 2588–2597 (2022). https://doi.org/10.1007/s11356-021-15758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15758-6