Abstract
This study attempted to find potential effective thermotolerant microorganisms producing complex enzymes for use in the hydrolyzing empty fruit bunch (EFB) to reduce cost of enzyme and enhance the efficiency of saccharification. The enrichment process at 45 °C was employed as a strategy to obtain four effective thermotolerant microorganisms. Streptomyces thermocarboxydus ME742, Bacillus subtilis ME751 and Bacillus amyloliquefaciens ASB/TRE produced the highest activity of xylanase (226.2 U/mL), CMCase (3.84 U/mL) and FPase (69.55 U/mL), respectively, while Aspergillus fumigatus A4112 exhibited the highest specific activity of xylanase (637.9 U/mg), CMCase (5.55 U/mg) and FPase (21.58 U/mg). Xylanase of isolated ME742 and A4112, CMCase of isolated ASB/TRE, FPase of isolated ME742, ME751 and ASB/TRE possessed thermostability with 80% remaining activity at 60 °C after 1 h incubation. These four strains were capable to reduce 49–78% (w/w) lignin in raw EFB with simultaneous enzyme production. The EFB residue was reused as substrate for saccharification with the highest amount of reducing sugar using the crude enzymes from S. thermocarboxydus ME742 (9.24 mg/g EFB). The sugar was 3.76 and 3.61 fold higher than that obtained from saccharification of acid- and alkaline-pretreated EFB, respectively. Moreover, the crude enzymes from A. fumigatus A4112 and B. amyloliquefaciens ASB/TRE hydrolyzed palm oil mill effluent (POME) to generate high yield of reducing sugar (61.01–64.63 mg/g TS-POME). Therefore, these selected strains were considered as the potent biological tool applicable in the bioconversion of oil palm biomass to fermentable reducing sugars.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Empty fruit bunch (EFB) and palm oil mill effluent (POME) represent significant and most abundant solid and liquid wastes of palm oil mill, respectively. This work initiated the biomass valorization for production of enzymes from EFB using the selected thermotolerant (45 °C) bacteria and fungi isolated through enrichment strategy. The enzymes were thermostable at 65 °C for 1 h, hence, this condition was applied for saccharification on raw and pretreated (chemical vs biological) EFB and POME. The results demonstrated high potential of the thermotolerant isolates for production of thermostable enzymes with high xylanase and cellulase activities that could be applied for saccharification of the oil palm biomass to obtain sugars as feedstock for production of many valuable products.
Introduction
Oil palm (Elaeis guineensis) is one of most important economic crops in Southern Thailand. The process of extracting crude oil from palm fresh fruit bunches (FFB) generates a lot of lignocellulosic biomass wastes from the oil palm industries. The empty fruit bunch (EFB) is major residue of the process which is about 20% of FFB weight and equivalent to 1.1 metric ton of EFB per ton of oil production [1]. A large amount of residual EFB creates a serious disposal problem. Recycle of residual biomass is the fundamental principles for waste management [2]. Currently, EFB is used as raw material for many fermentative productions of renewable bioenergy and other valuable products since they contain a potency polysaccharides; cellulose (24–65%) and hemicellulose (21–34%), which can be degraded to fermentable sugars [3]. In recent year, biological conservations by microbial and enzymatic operations are considered as sustainable process for releasing fermentable sugars from lignocellulosic material because it is environmental friendly and easier controllable approach. However, the complexity of this raw material is a main bottleneck of its utilization. The efficient degradation of lignocellulosic biomass requires the complex enzymes including cellulolytic enzymes such as endo β-1,4-glucanase, exo β-1,4-glucanase, and β-1,4-glucosidase and hemi-cellulolytic enzymes such as endo-β-1,4-xylanase and β-xylosidase [4, 5]. At the industrial scale, large amounts of desired enzymes are required. For example, about 100 g of cellulase was needed for saccharification of cellulosic biomass to yield one gallon of ethanol [6] and the cost of cellulase was estimated to be $0.50–$1.47 per gallon ethanol [7]. Such high cost of cellulase makes the production not economically feasible.
Since approximately 30% of total production cost of microbial enzyme is attributed to feedstock for growth of microorganisms [8], researchers have developed several procedures to reduce the cost of enzyme production, for instance, using agricultural wastes as a nutrient source [9, 10]. EFB is one of promising feedstock and a number of microorganisms have been reported for their capacity to produce enzymes from EFB [11, 12]. However, the commercial potential of lignocellulolytic enzymes has major impediments as yield. In fact, non-genetically modified microorganisms produce crude cellulolytic enzyme less than 100 g per liter of fermented medium [13]. Therefore, the enzyme concentration is required in downstream processing and consequently leads to an increase of enzyme cost. The enzyme production still is restricted by the need for special culturing and induction conditions to achieve high level of enzyme. It is well-known that cellulolytic and hemi-cellulolytic enzymes are inducible enzyme and lignin is a physical barrier prohibiting the access of microbe to surface of cellulose and hemicellulose act as inducer for enzyme synthesis. Unlike rice bran and wheat bran, EFB contains higher lignin content (14–31%), thus cellulolytic and xylanolytic enzymes normally are produced at lower yield by microorganism from EFB. Nowadays, technological challenges are required to improve the enzyme production from EFB. Pretreatment of EFB by physical and chemical process to remove lignin content efficiently could increase enzyme yield [14, 15]. Nevertheless, these pre-process might be inconvenient at large scale production. Therefore, screening new microorganisms with excellent capacity to efficiently degrade EFB simultaneous with enzyme production remains a hot topic in the fundamental research and industrial application. The enrichment process with complex crystal substrate was created as promising strategy to success of best microorganism screening [16].
From a practical point of view, stability of enzyme in process must be considered. Thermo-stability is a primary important in hydrolysis process because of high temperature gives several advantages such as enhance enzyme accessibility [17], lower viscosity of substrate [18], facilitate velocity of enzyme which benefit for enzymatic hydrolysis as well as decrease risk of contamination. Among the microorganisms, thermotolerant microorganisms are known as potential producer of thermotolerant enzyme because its products usually exhibit high activity and stability at the elevated temperature.
Therefore, the aim of the present study is to isolate thermotolerant microorganism which can effectively utilize EFB, followed by selection of the potent stains as a producer of thermotolerant lignocellulolytic enzymes, mainly cellulolytic and xylanolytic enzymes. The enrichment process was used as a strategy for microorganism screening. Moreover, the experiments were conducted to evaluate the potential of the produced enzyme for future practical applications in palm oil industry.
Materials and Methods
Culture Media and Pretreatment of EFB Fiber
Mandel and Andreotti (MA) medium was used for isolation, screening and enzyme production. The medium contained 10 g/L carbon source (carboxymethycellulose (CMC) or empty fruit bunch (EFB)), 3 g/L KH2PO4, 1.5 g/L K2HPO4·12H2O, 0.5 g/L NaCl, 0.5 g/L (NH4)2SO4, 0.2 g/L MgSO4·6H2O and 2.0 g/L yeast extract. The initial pH was adjusted to 5 and 7 for isolation of fungi and bacteria, respectively [19].
EFB fiber and POME were kindly provided by Nam Hong Palm Oil Co., Ltd. in Krabi Province, Thailand. The EFB fiber was cut to particle size of 5–10 cm and stored in airtight container to maintain the moisture content less than 10% until use in each experiment. Raw POME was kept in a freezer (− 20 °C) until use. Pretreatment of EFB was performed as following steps. Dried EFB was soaked in 1% (w/v) NaOH and 1% (v/v) H2SO4 in the ratio of 1:10 (w/v) and autoclaved (121 °C, 15 min) to obtain autoclaved-alkaline and autoclaved-acid pretreated EFB, respectively. These pretreated EFB was washed with water until pH reached 7.0 [19].
Selection and Enrichment of Culture Source
Soil around manure’s herbivore farm including cow, horse, goat and sheep farms located in Hat Yai, Thailand were sampled at 10–20 cm depth using sterile hoe and hand trowel. The samples were taken from 5 spots in each site and then mixed together for a good representative samples [20]. Moreover, the fresh and dry manure of herbivores and compost were also collected. Samples from 5 L biogas reactor in our laboratory using seed sludge from two palm oil mills (Namhong Co., Ltd, in Krabi Province and Suksomboon Co., Ltd, in Chonburi Province, Thailand) were collected at the surface (0 cm depth), middle (22.5 cm depth) and bottom (45 cm depth) of the reactor and mixed to obtain the representative samples and kept in 50 mL-falcon tubes.
The populations of lignocellulose-degrading microbes in collected samples were enhanced through enrichment process with raw materials [16]. Five gram of each sample was transferred into 250 mL Erlenmeyer flask containing 50 mL MA medium using 10 g/L filter paper (Whatman No.1) or raw EFB fiber as sole carbon source and incubated at 45 °C with shaking speed at 150 rpm for 7 days. The changes of appearance of filter paper and raw EFB during cultivation was observed to preliminary confirm cellulose degradation ability of microorganisms in the samples.
Isolation and Primary Screening of Thermotolerant Lignocellulose Degrading Microorganisms
The culture sources showing the highest digestion of filter paper or raw EFB fiber in enriched media were selected for isolation. The sample was diluted to various concentrations (10–1–10–5) by using 0.85% (w/v) NaCl. The diluted sample (100 µL) was spread onto MA agar containing 10 g/L CMC, adjusted pH to 5 and 7 for isolating fungi and bacteria, respectively. The plates were incubated at 45 °C for 2 days to grow bacteria and 7 days to grow fungi [19].
Primary screening of all isolates was performed by observation of clear zone from CMC hydrolysis around each colony. After incubation at 45 °C for 48 h, the agar plates with growth of isolates were flooded with 0.1% (w/v) Congo red and destained by 1 M NaCl [21]. The isolates with clear zone were selected and inoculated into 10 mL MA medium containing 10 g/L EFB. The cell culture was grown on a shaker at 150 rpm, incubated at 45 °C for 2 days to grow bacteria and 5 days to grow fungi. Afterwards, the supernatant was separated by centrifugation (10,000 rpm for 10 min), and used as crude enzyme to determine the activities of cellulase (CMCase and FPase) and xylanase.
Selection of Cellulase- and Xylanase-Producing Thermotolerant Microorganism from EFB
The isolates were further screened for their ability to produce enzymes. Each seed culture, 108 spore/mL of fungal spore and 1% (v/v) of bacterial culture with optical density at 600 nm (OD600) reached 0.6 (108 CFU/mL), were transferred into 250 mL Erlenmeyer flask containing 50 mL MA medium using 50 g/L raw EFB as sole carbon source. Prolonged incubation time was required to allow microorganism to access to xylan and cellulose inside of EFB, thus the bacterial and fungal cultures were incubated at 45 °C for 4 days and 15 days, respectively. After centrifugation, the supernatant was used to determine activities of cellulase (CMCase and FPase), xylanase and calculated for their enzyme productivity. In this case, the enzyme production was followed up every day and 2 days interval for bacteria and fungi, respectively.
Molecular Identification of the Selected Isolates
Genomic DNA of the selected microorganisms was isolated and purified using DNA purification kit (Promega). 16S rDNA and ITS fragment of bacteria and fungi were identified, respectively. They were amplified using DNA polymerase with the universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) for bacteria and F63-Forward (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and LR3-Reverse (5′-GGTCCGTGTTTCAAGACGG -3′) for fungi. The PCR products were sequenced and compared with sequences variable in GenBank of NCBI (National Centre for Biotechnology Information, https://www.ncbi.nlm.nih.gov/) using BLAST (Basic Local Alignment Search Tool) analysis.
Production and Determination of Thermostability of the Lignocellulolytic Enzymes from the Selected Microorganisms
The cultivation was carried out in MA broth containing 50 g/L EFB at 45 °C for optimal incubation time of each strain obtained from the previous experiment. The culture both of each selected microorganisms was collected and centrifuged (10,000 rpm, 4 °C for 10 min). The supernatant was used for determination of thermostability of the enzymes. Crude enzymes with the concentration of 0.18 mg-protein/mL was incubated at 40–80 °C for 1 h, then immediately put in ice box for 5 min before analyzing for the residual enzyme activities. EFB residues were collected for determination of chemical composition and reused as substrate for saccharification.
Saccharification Capacity of the Crude Enzyme of the Selected Strain on Palm Oil Mill Wastes
The solid wastes (raw EFB, chemical pretreated EFB) and liquid waste (POME) of a palm oil mill were used for saccharification by the crude enzyme from the selected strain. The EFB residues from enzyme production by the four selected isolates were used as biological pretreated EFB. Two grams of raw EFB and pretreated EFBs were hydrolyzed with 50 mL of crude enzyme (0.18 mg-protein/mL).
For POME hydrolysis, the crude enzyme (0.18 mg-protein/mL) of the selected strains was added into sterilized POME in a ratio 1:100 in 250 mL Erlenmeyer flask and incubated at 60 °C for 72 h. The supernatant was separated from insoluble particles by centrifugation at 10,000 rpm at 4 °C for 10 min. The amount of reducing sugar liberated in the supernatant was measured through DNS method [22].
Quantitative Analysis of Chemical Composition of EFBs
The fermented EFB, alkaline- and acid-pretreated EFB were determined for cellulose, hemicellulose and lignin content using standard method [23]. Two grams of fiber was boiled in ethanol (4 times) for 15 min, washed thoroughly with distilled water and dried in oven at 40 °C overnight. The dried fiber was divided into two parts in which one part was designed as fraction A. The other part was further treated with 24% NaOH for 4 h at 25 °C, washed thoroughly with distilled water and dried at 80 °C overnight. This dried sample was taken as fraction B. Fraction A was again treated with 72% H2SO4 for 3 h to hydrolyze the cellulose and refluxed with 5% H2SO4 for 2 h. H2SO4 was removed completely by washing it with distilled water, dried at 80 °C in oven overnight and dry weight taken as fraction C [24]. The contents of cellulose, hemicellulose and lignin were calculated using the following equation.
Enzyme Activity Assay
The cellulase and xylanase activity of each culture was measured according to the method described by Miller [22]. The reaction mixture contained 0.5 mL of culture filtrate and 0.5 mL of 1% (w/v) solubilized CMC in 50 mM sodium citrate buffer (pH 4.8) was used to measure cellulase activity. After incubation at 50 °C for 30 min, 2.0 mL DNS reagent was added and boiled at 100 °C for 10 min. The reaction mixture was put in ice box for 5 min before measure absorbance at 520 nm by spectrophotometer Spectroquant® Pharo 300. One unit of cellulase activity was determined as amount of enzyme releases 1 µmol of glucose per min under the assay condition. Xylanase activity was determined using the method of which is similar to assay of cellulase. However, the mixture was replaced CMC by xylan and incubated at 50 °C for 10 min. One unit of xylanase activity was determined as amount of enzyme releases 1 µmol of xylose per min.
Filter paper activity (FPase) was determined by adding 0.5 mL of culture filtrate into filter paper (Whatman No. 1) strip (1 × 6 cm; 50 mg) immersed in 1 mL 50 mM sodium citrate buffer (pH 4.8). After incubation at 50 °C for 1 h, the released reducing sugar was estimated by DNS method [22] and measured absorbance at 520 nm. One unit of filter paper (FPase) activity was defined as the amount of enzyme releasing 1 µmol of reducing sugar from filter paper per ml per min.
Determination of Protein Concentration
Protein concentration of crude enzyme was determined using Lowry method [25] with bovine serum albumin (BSA) as the standard protein.
Statistical Analyses
All experiments in this study were conducted in triplicate. The variance was analyzed with one-way ANOVA followed by Duncan’s multiple range test (DMRT) comparison of means at 0.05 significance level. The statistical analysis was evaluated with SPSS software package (SPSS 16.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Isolation and Primary Screening of Thermotolerant Lignocellulose Degrading Microorganisms
Total 22 samples with potential degradation of lignocellulosic material were collected from different sites. Prior to isolation, the population of thermotolerant microorganisms in the samples were increased through enrichment in MA medium supplemented with filter paper (Whatman No.1) and EFB as sole carbon source and incubated at 45 °C for 7 days. The potential source for isolation was selected based on the degree of degrading substances expressed as (+) until (+++) level observed during enriched cultivation (Supplementary Fig. 1). Among 22 sources, 12 samples gave positive results (data not shown) while only 7 samples exhibited EFB degradation. The compost, seed sludge and manure from herbivores were found to be the potent sources of lignocellulolytic microorganisms. A similar observation was reported by Mori et al. [16] who proved that the population of lignocellulolytic microorganism was improved more than 3 times of raw soil by enrichment cultivation with complex cellulose (microcrystalline cellulose (Avicel®) and unbleached hardwood (kraft pulp). The nineteen enriched sources of cultures were primary subjected for isolation and screening of cellulose-assimilating thermotolerant microorganisms on MA agar with CMC as carbon source. A total of 200 isolates were obtained from filter paper degrading cultures and 87 isolates from degradation of EFB. Among the overall number of 287 isolates, 36 bacterial isolates and 18 fungal isolates showed clear zone around colony with diameter of 0.2–5.0 mm (data not shown). Thus, these 54 isolates were further screened for their ability to produce cellulase and xylanase using EFB.
Selection of Cellulase- and Xylanase-Producing Thermotolerant Microorganisms from EFB
Fifty-four isolates from primary screening were screened for their ability to use EFB as feedstock for production of enzymes; cellulases (CMCase and FPase) and xylanase. The preliminary experiment (data not shown) indicated that microorganisms required longer time for accessing to xylan and cellulose inside EFB, thus the experiment was extended to 4 days for bacteria and 15 days for fungi. The activity of CMCase (0.1–2.6 U/mL), FPase (3.0–13.0 U/mL) and xylanase (33–141 U/mL) were obtained from the cell culture supernatant of five bacteria and six fungal isolates (Fig. 1). Noteworthy, almost bacterial isolates tend to produce high activity of FPase (3.35–4.34 U/mL), whereas fungal isolates exhibited the capacity to produce CMCase (1.10–2.43 U/mL) and xylanase (33.01–44.87 U/mL) at similar level.
In order to select the potent producer of FPase, CMCase and xylanase, the screening was performed from viewpoint of productivity. The enzyme production by five bacterial and six fungal isolates were followed up for 7 days and 15 days, respectively. The results (Table 1) indicated that the isolate ME742 was dominant for xylanase production (226.2 U/mL or 4523 U/g EFB) after 6 days cultivation, giving the productivity of 37.70 U/mL/day. The xylanase activity from strain ME742 was about 113 folds higher than those obtained from other bacterial isolates and about 2 folds higher than those from the fungal isolates. On the other hand, the isolate ME751 exhibited the highest CMCase production (3.84 U/mL or 76.81 U/g EFB) at 3 days cultivation resulted in the highest productivity of 1.28 U/mL/day. In addition, the isolate ASB/TRE was found to be a potent producer of FPase, with the maximum FPase activity (69.55 U/mL or 1391 U/g EFB) as well as productivity (13.19 U/mL/day). This was 7–10 folds higher than those of other tested microorganisms.
Among six fungal strains, the isolate A4112 was a promising source for production of xylanase (115.7 U/mL or 2315 U/g EFB) and CMCase (1.01 U/mL or 20.15 U/g EFB) with the highest specific activity of 637.9 and 5.55 U/mg-protein, respectively. Besides, the isolate A4112 generated the highest xylanase and CMCase productivity of 12.86 and 0.11 U/mL/day, respectively. Furthermore, the isolate MR511 possessed the highest FPase production (18.04 U/mL or 360.9 U/g EFB) as well as productivity (2.00 U/mL/day). All of these values, except the specific activity, from the six fungal isolates were much lower than those from the three bacterial isolates. The highest specific activities of both xylanase and CMCase were achieved from the fungal isolate A4112 which were 3.22 and 1.95 folds higher than those of the bacterial isolate ME742. From these results, all potent enzyme producers with three bacterial isolates and one fungal isolate A4112 were selected for further studies.
According to the homologous analysis based on the sequences of bacterial 16s rDNA and fungal ITS region, the isolates ASB/TRE, ME742, ME751, and A4112 were identified as Bacillus amyloliquefaciens ASB/TRE, Streptomyces thermocarboxydus ME742, Bacillus subtilis ME751, and Aspergillus fumigatus A4112, respectively (Fig. 2). The gene sequences of them were deposited in the GenBank under accession number MN216230, MN216231, MN216232 and MN243565, respectively.
Phylogenetic tree revealing the relationship between selected bacteria (a) and fungi (b) with related species. The trees (neighbor-joining tree) were conducted using ClustalX Omega software based on sequences of 16S rDNA for bacteria and ITS for fungi. Numbers after the species name are gene accession code
Indigenous microorganism isolated from decay EFB, for instance, genus Aspergillus and Trichoderma produced large quantities of xylanase and CMCase but lower level of FPase which is most important property of cellulase system in the saccharification of lignocellulosic material [26, 27]. Through several steps of screening, four most promising isolates were S. thermocarboxydus ME742, B. subtilis ME751, B. amyloliquefaciens ASB/TRE and A. fumigatus A4112 producing remarkable level of xylanase, CMCase and FPase from EFB. These results were in agreement with Cheng et al. [28], Li et al. [29] and Mori et al. [16] who suggested that the enrichment culture process with nature form of substrates as lignocellulosic material and avicel help in finding microorganism with high xylanase and FPase in addition of CMCase.
Comparison of activities of lignocellulolytic enzymes from untreated EFB produced by the four isolates of this study with those from the other strains is given in Table 2. Xylanase activity obtained from crude enzymes of S. thermocarboxydus ME742 (226.2 U/mL) and A. fumigatus A4112 (115.7 U/mL) were significantly higher than those from Trichoderma koningii D-64 (35.4 U/mL), Aspergillus niger DSM 26641 (0.02 U/mL), and S. flavogriseus AE64X (0.6 U/mL) [30,31,32]. The highest CMCase activity of B. subtilis ME751 (3.84 U/mL) was higher than those from Cellvibrio japonicas (0.141 U/mL) [21], B. pumilus EB3 (0.063 U/mL) [33] and Thermobifida fusca (0.07 U/mL) [15] but lower than CMCase produced from fungi. B. amyloliquefaciens ASB/TRE produced lower quantity of both xylanase (0.86 U/mL) and CMCase (0.08 U/mL), but large amount of FPase (69.55 U/mL) which was about 18.30, 40.91 and 88.03 times higher than that from fungi such as T. koningii D-64 (3.8 U/mL), A. fumigatus UPM2 (1.7 U/mL) and T. virens UKM1 (0.79 U/mL) [10, 30, 34]. Additionally, FPase activities of S. thermocarboxydus ME742 (9.35 U/mL) and B. subtilis ME751 (6.76 U/mL) also were greater than those produced by reference strains in Table 2. With regarding to FPase activity, which is a most effective on crystalline cellulose as nature cellulose, the selected strains in this study have potential to produce this enzyme from EFB better than the reported strains. Therefore, S. thermocarboxydus ME742 was considered as a source of lignocellulolytic enzymes rich in xylanase and FPase, B. amyloliquefaciens ASB/TRE was a dominant generates high level of FPase and B. subtilis ME751 was a producer of cellulase; CMCase and FPase.
In comparison with the studies using other lignocellulolytic substrates, the production of CMCase activity in U/g substrate from EFB using B. subtilis ME751 (76.81 U/g substrate) was greater than that of Paenibacillus xylanexedens (4.00 U/g substrate) [35] using the combination of orange juice processing waste and CMC, and T. koninggiopsis TM3 (4.44 U/g substrate) from OPT [36], but smaller than CMCase of T. reesei RUT C-30 (959.53 U/g substrate) using sorghum stover [37]. The FPase activity of B. amyloliquefaciens ASB/TRE (1391 U/g substrate) was remarkably higher than the value obtained from T. koninggiopsis TM3 (2.13 U/g substrate) using OPT [36], and co-culture of A. niger ATCC 16404 and T. reesei DSMZ 970 (80.00 U/g substrate) from potato peel residue [38]. The xylanase activity of S. thermocarboxydus ME742 (4523 U/g substrate) and A. fumigatus A4112 (2315 U/g substrate) were obviously larger than that T. koninggiopsis TM3 (56.46 U/g substrate) from oil palm trunk (OPT) [36].
Aspergillus fumigatus A4112 was one of the attractive isolate that gave the considerable level of specific activity of xylanase (637.9 U/mg), CMCase (5.55 U/mg) and FPase (21.58 U/mg) with greatest value of enzyme specificity (Table 1). This strain was chosen for production of enzymes with high specificity of xylanase and cellulase. Interestingly, specific activity of A. fumigatus A4112 xylanase (637.9 U/mg) was larger than that reported for Pseudomonas psychrotolerans or P. oryzihabitans (280.0 U/mg) cultured on orange juice processing waste and xylan from corn crop [39], which gave higher activity than xylanase of S. thermocarboxydus ME742 (197.8 U/mg). However, it should be noted that the activity reported in this study was obtained from cultivation using untreated or raw EFB before optimization of factors affecting enzyme production. Therefore, higher enzyme production could be expected after further optimization.
Thermostability of Lignocellulolytic Enzymes Produced from the Selected Strains
Maximum saccharification of cellulose and xylan could be achieved at elevated temperature (50–60 °C) and extended reaction time more than 72 h. Thermostability is one of the most interesting properties of the enzymes in hydrolysis that required for industrial application in order to save time, lower cost and give better reaction yields [39,40,41,42]. Thus, the thermostability of the crude enzymes from the four selected strains, S. thermocarboxydus ME742, B. subtilis ME751, B. amyloliquefaciens ASB/TRE and A. fumigatus A4112 were evaluated (Fig. 3). Xylanase from all selected strains could retain more than 60% of initial activity after incubation at 65 °C for 1 h, except from B. amyloliquefaciens ASB/TRE that dropped sharply at temperature over 40 °C. The crude enzyme from B. subtilis ME751 could maintain its activity over 60% until 75 °C, while all sources lost their activities completely at 80 °C (Fig. 3a). The thermal stability of crude xylanase from S. thermocarboxydus ME742, B. subtilis ME751, and A. fumigatus A4112 was greater than that reported for S. flavogriseus AE64X [32], B. amyloliquefaciens CH51 [43] and A. fumigatus N2 [44]. On the other hand, CMCase and FPase were relatively stable over high temperature at 70–80 °C (Fig. 3b, c). The result revealed good stability of crude cellulase from the four selected strains that was comparable to known thermostable cellulases produced from Bacillus sp. SMIA-2 [41] and S. thermocerradoensis I3 [45].
Chemical Composition of EFBs and Delignification Efficiency of the Selected Strains
Chemical composition of raw EFB, pretreated EFBs; fermented EFBs, alkaline- and acid-pretreated EFB was determined in order to investigate the potential of the four selected strains in decomposing lignin of EFB. The composition of raw EFB was 51.96 ± 1.95% (w/w) of cellulose, 23.98 ± 1.54% (w/w) of hemicellulose and 17.50 ± 0.42% (w/w) of lignin. As shown in Table 3, the reduction of lignin content and increment of cellulose were found in all pretreated EFBs. The lignin content remained in all fermented EFB was approximately 3–5 folds lower than raw EFB. B. subtilis ME751 exhibited higher delignification (78.86% w/w-dry basis) than A. fumigatus A4112, B. amyloliquefaciens ASB/TRE and S. thermocarboxydus ME742 (65.60, 65.14 and 49.37% w/w-dry basis), respectively. These values were comparable with those of alkaline and acid pretreated EFBs with 52.80 and 84.69% w/w-dry basis lignin removal, respectively. The results suggested that the four culture supernatants containing enzymes related in delignification of EFB. In contrast, cellulose content in fermented EFB was increased to 79.78–84.78% w/w-dry basis. The obtained results were insignificant difference from the value detected in chemical treated EFB but 1.6 times higher than raw EFB. Hemicellulose content in fermented EFB was lower than that detected in chemical treated EFB. Some part of hemicellulose might be used for microbial growth and enzyme production.
Many processes have established for delignification to facilitate the enzyme hydrolysis toward cellulose and/or hemicellulose in saccharification process. With the growing concerns over environmental aspects, however, the chemical process is environmentally unfriendly and costly due to it not only uses acid or base but also generates a huge amount of water-effluent necessary to further management. Thus, biological delignification has attracted the attention in recent years. The delignification efficiency toward EFB achieved by the four strains in this study was comparable to the process using Phanerochaete chrysosporium ATCC 32629 [46] and higher than the results obtained from Stenotrophomonas sp. S2 (45% w/w) and B. subtilis S11Y (20% w/w) after 7 day incubation [47]. These finding results indicated the attractive potential of the four selected microorganisms in lignin removal of raw EFB simultaneous with enzyme production.
Saccharification Capacity of Palm Oil Mill Wastes by Crude Enzyme of the Selected Strains
Oil palm wastes including EFB and POME were known as potential source of fermentable sugar for biofuel production. However, because of complex structure of lignocellulose material, conversion of such biomass to fermentable sugar necessitate enzymatic cocktail process carried out at high temperature for long period [39]. Therefore, this experiment was conducted to examine the ability of enzyme produced by the four selected microorganism to hydrolyze EFB and POME at 60 °C for 72 h, which were the conditions from the previous study [34].
As shown in Fig. 4, crude enzyme of S. thermocarboxydus ME742 could generate the highest reducing sugar about 5.70 mg/g raw EFB. Crude enzyme of A. fumigatus A4112 released the amount of reducing sugar of 2.34 mg/g raw EFB, and B. subtilis ME751 and B. amyloliquefaciens ASB/TRE, liberated a comparable amount of 1.42 and 1.62 mg/g raw EFB, respectively. In nature, EFB contains lignin (14–31% w/w-dry basis) which acts as barrier to prevent the assessment of enzyme to xylan and cellulose. The capacity to hydrolyze EFB was often found to increase by pretreatment of this substrate. In present work, the level of reducing sugar from hydrolysate of 1% (w/v) NaOH (alkaline) pretreated EFB was not significantly increased as compared with the results from raw EFB. On the other hand, the yield of reducing sugar sharply increased by hydrolysis of EFB pretreated with 1% (v/v) H2SO4 (acid). The maximum reducing sugar of 7.43 mg/g-treated EFB was obtained from hydrolysis by crude enzyme of B. amyloliquefaciens ASB/TRE, followed by crude enzyme of S. thermocarboxydus ME742 (7.21 mg/g-treated EFB). The amount of reducing sugar from hydrolysis by crude enzyme of A. fumigatus A4112 and B. subtilis ME751 was in the range of 5.64 and 5.52 mg/g-treated EFB, respectively. This result indicated that four crude enzymes can be used in saccharification of diluted acid pretreated EFB. This result was in agreement with the study by Alrumman et al. [48] who showed that acid treatment of EFB increased enzymatic hydrolysis yield (19.57%) by cellulase from Geobacillus stearothermophilus Y-1.
The amount of reducing sugar from saccharification of raw and pretreated (chemical vs biological) empty fruit bunch (EFB). Raw EFB, EFB pretreated by 1% w/v NaOH (EFB-NaOH), 1% v/v H2SO4 (EFB-H2SO4) and residue EFB after enzyme production by S. thermocarboxydus ME742 (EFB-ME742), B. subtilis ME751 (EFB-ME751), B. amyloliquefaciens ASB/TRE (EFB-ASB/TRE) and A. fumigatus A4112 (EFB-A4112)
Nevertheless, pretreatment of EFB may cause inconvenient for the scale-up of sugar production. It is not only time consuming but also non-economic process [49] as described above, so finally it made the large scale production costly. It is interesting that the lignin content detected in fermented EFB collected after enzyme production was significantly reduced whereas cellulose content was consequently increased (Table 3). Therefore, we attempted to reuse fermented EFB as substrate in hydrolysis process again. The result in Fig. 4 also clearly showed that fermented EFBs were promising substrate for saccharification. The larger amount of reducing sugar could be obtained from hydrolysis of fermented EFBs by crude enzyme of the selected microorganism. The crude enzyme of S. thermocarboxydus ME742 generated the highest amount of reducing sugar 9.24 mg/g fermented EFB by B. amyloliquefaciens ASB/TRE and 8.48 mg/g fermented EFB by S. thermocarboxydus ME742 (itself). The other crude enzymes, except for that from A. fumigatus A4112, also liberated considerable amount of reducing sugar from fermented EFBs. These results emphasized the potential use of the four selected strains not only in enzyme production but also on biological pretreatment of EFB.
Besides EFB, crude enzyme from A. fumigatus A4112 and B. amyloliquefaciens ASB/TRE, which exhibited high FPase activity, effectively hydrolyzed POME and gave the amount of reducing sugar of 64.63 and 61.01 mg/g-TS of POME, respectively (Fig. 5). Enzymatic pre-treatment of POME was found to be important process to increase fermentable sugar content before use as feedstock for biofuel fermentation process as reported by Prasertsan et al. [34] and Kamal et al. [50]. This report indicates the feasibility for application of B. amyloliquefaciens ASB/TRE and A. fumigatus A4112 in hydrolyzing POME and conversion it into reducing sugar. Nevertheless, the yield of reducing sugar was lower than the value reported previously by other studies. In order to maximize the yield of hydrolysis products, the factors involved in hydrolytic process will be optimized in further work.
Conclusion
This study employed the enrichment process to enhance the opportunity for finding out certain microorganism from natural sources. As the result, we obtained four strains including S. thermocarboxydus ME742, B. subtilis ME751, B. amyloliquefaciens ASB/TRE and A. fumigatus A4112 as the candidate microorganisms for producing complex enzymes involved in saccharification of lignocellulosic wastes from palm oil industry. Moreover, the produced enzyme exhibited thermal stability over the range of operating temperature in industrial process (60–80 °C). In addition, the present study also indicates the attractive potential of the four selected microorganisms in biological pretreatment of raw EFB simultaneous with enzyme production. The obtained EFB also can be used as a substrate for sugar production.
References
Palamae, S., Dechatiwongse, P., Choorit, W., Chisti, Y., Prasertsan, P.: Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohyd. Polym. 155, 491–497 (2017)
Abdullah, N., Sulaiman, F., Gerhauser, H.: Characterization of oil palm empty fruit bunches for fuel application. Int. J. Phys. Sci. 22(1), 1–24 (2011)
Onoja, E., Chandren, S., Razak, F.I.A., Mahat, N.A., Wahab, R.A.: Oil Palm (Elaeis guineensis) Biomass in Malaysia: The Present and Future Prospects. Waste Biomass Valoriz. 10(8), 2099–2117 (2018)
Gaur, D., Jain, P.K., Sisodia, Y.S., Bajpai, V.: Estimation of extracellular lipase enzyme produced by thermophilic Bacillus sp. isolated from arid and semi-arid region of Rajasthan, India. Nat. Proc. (2012). https://doi.org/10.1038/npre.2012.7072.1
Fatriasari, W., Anita, S.H., Risanto, L.: Microwave assisted acid pretreatment of oil palm empty fruit Bunches (EFB) to enhance its fermentable sugar production. Waste Biomass Valoriz. 8(2), 379–391 (2016)
Zhang, X.Z., Zhang, Y.H.P.: Cellulases: characteristics, sources, production, and applications. bioprocessing technologies in biorefinery for sustainable production of fuels. Chem. Polym. 1, 131–146 (2013)
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B.A., Blanch, H.W.: The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 109, 1083–1087 (2012)
Ravindran, R., Jaiswal, A.K.: Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering. 3(4), 30 (2016)
Kitcha, S., Cheirsilp, B.: Bioconversion of lignocellulosic palm byproducts into enzymes and lipid by newly isolated oleaginous fungi. Biochem. Eng. J. 88, 95–100 (2014)
Ngikoh, B., Karim, A.A., Jahim, J., Bakar, F.D.A., Murad, A.M.A.: Characterisation of cellulases and xylanase from Trichoderma virens UKM1 and its potential in oil palm empty fruit bunch (OPEFB) saccharification. J. Phys. Sci. 28, 171–184 (2017)
Ting, A.S.Y., Tay, H., Peh, L., Tan, W.S., Tee, C.S.: Novel isolation of thermophilic Ureibacillus terrenus from compost of empty fruit bunches (EFB) of oil palm and its enzymatic activities. Biocatal. Agric. Biotechnol. 2, 162–164 (2013)
Ajijolakewu, A.K., Leh, C.P., Abdullah, W.N.W., Lee, C.K.: Optimization of production conditions for xylanase production by newly isolated strain Aspergillus niger through solid state fermentation of oil palm empty fruit bunches. Biocatal. Agric. Biotechnol. 11, 239–247 (2017)
Ellilä, S., Fonseca, L., Uchima, C., Cota, J., Goldman, G.H., Saloheimo, M., Sacon, V., Siika-aho, M.: Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol. Biofuels 10, 30 (2017)
Umikalsom, M.S., Ariff, A.B., Zulkifli, H.S., Tong, C.C., Hassan, M.A., Karim, M.I.A.: The treatment of oil palm empty fruit bunch fiber for subsequent use as substrate for cellulase production by Chaetomium globosum Kunze. Bioresour. Technol. 62, 1–9 (1997)
Harun, N.A.F., Baharuddin, A.S., Zainudin, M.H.M., Bahrin, E.K., Naim, M.N., Zakaria, R.: Cellulase production from treated oil palm empty fruit bunch degradation by locally isolated Thermobiffida fusca. BioResources 8(1), 676–687 (2013)
Mori, T., Kamei, I., Hirai, H., Kondo, R.: Identification of novel glycosyl hydrolases with cellulolytic activity against crystalline cellulase from metagenomic libraries constructed from bacterial enrichment cultures. Springer Plus 3(365), 1–7 (2014)
Ping, L., Wang, M., Yuan, X., Cui, F., Huang, D., Sun, W., Zou, B., Wang, H.: Production and characterization of a novel acidophilic and thermostable xylanase from Thermoascus aurantiacu. Int. J. Biol. Macromol. 109, 1270–1279 (2018)
Olajuyigbe, F.M., Fatokun, C.O.: Oyelere OM (2018) Biodelignification of some agro-residues by Stenotrophomonas sp CFB-09 and enhanced production of ligninolytic enzymes. Biocatal. Agric. Biotechnol. 15, 120–130 (2018)
Dashtban, M., Buchkowski, R., Qin, W.: Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. Int. J. Biochem. Mol. Biol. 2, 274–286 (2011)
Mareckova, M.S., Cermak, L., Novotna, J., Plhackova, K., Forstova, J., Kopecky, J.: Innovative methods for soil DNA purification tested in soils with widely differing characteristics. Appl. Environ. Microbiol. 74, 2902–2907 (2008)
Oke, M.A., Annuar, M.S.M., Simarani, K.: Mixed lignocellulosic biomass degradation and utilization for bacterial cellulase production. Waste Biomass Valoriz. 8, 893–903 (2017)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugars. J. Anal. Chem. 31, 426–428 (1959)
Moubasher, M.H., Abdel-Hafez, S.I.I., Abdel-Fattah, H.M., Mohanram, A.M.: Direct estimation of 484 cellulose, hemicellulose and lignin. J. Agric. Res. 46, 1467–1476 (1982)
Brindha, D., Vinodhini, S., Alarmelumangai, K., Malathy, N.S.: Physico-chemical properties of fibers from banana varieties after scouring. Indian J. Fundam. Appl. Life Sci. 2, 217–221 (2012)
Lowry, O.H.N.G., Rosebrough, N.J.J., Farr, A.L., Randall, R.J.R.: Protein measurement with folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951)
Khaw, T.S., Ariff, A.B.: Optimization of enzymatic saccharification of palm oil mill effluent solid and oil palm fruit fiber to fermentable sugars. J. Trop. Agric. Food Sci. 37, 85–94 (2009)
Ezeilo, U.R., Wahab, R.A., Tin, L.C., Zakaria, V., Huyop, F., Mahat, N.A.: Fungal-assisted valorization of raw oil palm leaves for production of cellulase and xylanase in solid state fermentation media. Waste Biomass Valoriz (2019). https://doi.org/10.1007/s12649-019-00653-6
Cheng, Y.F., Edwards, J.E., Allison, G.G., Zhu, W.Y., Theodorou, M.K.: Diversity and activity of enriched ruminal cultures of anaerobic fungi and methanogens grown together on lignocellulose in consecutive batch culture. Bioresour. Technol. 100(20), 4821–4828 (2009)
Li, H., Xu, X., Chen, H., Zhang, Y., Xu, J., Wang, J., Lu, X.: Molecular analyses of the functional microbial community in composting by PCR–DGGE targeting the gene of the β-glucosidase. Bioresour. Technol. 134, 51–58 (2013)
Wang, Z., Ong, H.X., Geng, A.: Cellulase production and oil palm empty fruit bunch saccharification by a new isolate of Trichoderma koningii D-64. Process Biochem. 47, 1564–1571 (2012)
Ottenheim, C., Meier, K., Zimmermann, W., Wu, J.C.: Isolation of filamentous fungi exhibiting high endoxylanase activity in lignocellulose hydrolysate. Appl. Biochem. Biotechnol. 175, 2066–2074 (2015)
Pennacchio, A., Ventorino, V., Cimini, D., Pepe, O., Schiraldi, C., Faraco, M.V.: Isolation of new cellulase and xylanase producing strains and application to lignocellulosic biomasses hydrolysis and succinic acid production. Bioresour. Technol. 259, 325–333 (2018)
Ariffin, H., Abdullah, N., Umikalsom, M.S., Shirai, Y., Hassan, M.A.: Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci. Bioeng. 106, 231–236 (2008)
Prasertsan, S., Khangkhachit, W., Duangsuwan, W., Mamimin, C., O-Thong, S.: Direct hydrolysis of palm oil mill effluent by xylanase enzyme to enhance biogas production using two-steps thermophilic fermentation under non-sterile condition. Int. J. Hydrog. Energy. 42(45), 277759–277766 (2017)
Zerva, I., Remmas, N., Ntougias, S.: Biocatalyst potential of cellulose-degrading microorganisms isolated from orange juice. Process. Waste. Beverges. 5(21), 1–11 (2019)
Nutongkaew, T., Prasertsan, P., Leamdum, C., Sattayasamitsathit, S., Noparat, P.: Bioconversion of oil palm trunk residues hydrolyzed by enzymes from newly isolated fungi and use for ethanol and acetic acid production under two-stage and simultaneous fermentation. Waste Biomass Valoriz. 11, 1333–1347 (2019)
Idris, A.S.O., Pandey, A., Rao, S.S., Sukumaran, R.K.: Cellulase production through solid-state tray fermentation, and its use for bioethanol from sorghum stover. Bioresour. Technol. 242, 265–271 (2017)
Taher, I.B., Bennour, H., Fickers, P., Hassouna, M.: Valorization of potato peels residues on cellulase production using a mixed culture of Aspergillus niger ATCC 16404 and Trichoderma reesei DSMZ 970. Waste Biomass Valoriz. 8, 183–192 (2017)
Zerva, I., Remmas, N., Ntougias, S.: Diversity and biotechnological potential of xylan-degrading microorganisms from orange juice processing waste. Water 11(274), 1–13 (2019)
Rigoldi, F., Donini, S., Redaelli, A., Parisini, E., Gautieri, A.: Review: engineering of thermostable enzymes for industrial applications. APL Bioeng. 2, 1–18 (2018)
Zamost, B.L., Nielsen, H.K., Starnes, R.L.: Thermostable enzymes for industrial application. J. Ind. Microbiol. 8, 71–81 (1991)
Ladeira, S.A., Cruz, E., Delatorre, A.B., Barbosa, J.B., Martins, M.L.L.: Cellulase production by thermophilic Bacillus sp SMIA-2 and its detergent compatibility. Electron. J. Biotechnol. 18, 110–115 (2015)
Tehei, M., Zaccai, G.: Adaptation to extreme environments: Macromolecular dynamics in complex systems. Biochem. Biophys. Acta. 1724, 404–410 (2005)
Kalawong, R., Wakayama, M., Anuntalabhochai, S., Wongsawad, C., Sangwijit, K.: Comparison and characterization of purified cellulase and xylanase from Bacillus amyloliquefaciens CX1 and Bacillus subtilis B4. Chiang Mai J. Sci. 45, 92–105 (2018)
Lin, C., Shen, Z., Qin, W.: Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw. Appl. Biochem. Biotechnol. 182, 559–569 (2017)
Britio-Cunha, C.C.Q., Gama, A.R., Jesuino, R.S.O., Faria, F.P., Bataus, L.A.M.: Production of cellulases from a novel thermophilic Streptomyces thermocerradoensis I3 using agricultural waste residue as substrate. J. Agric. Environ. Sci. 4(1), 90–99 (2015)
Hamisan, A.F., Abd-Aziz, S., Kamaruddin, K., Shah, UKMd, Shahab, N., Hassan, M.A.: Delignification of oil palm empty fruit bunch using chemical and microbial pretreatment methods. Int. J. Agric. Res. 1(8), 250–256 (2009)
Alrumman, S.A.: Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 47, 110–119 (2016)
Tsegaye, B., Balomajumder, C., Roy, P.: Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull. Natl. Res. Centre. 43, 1–16 (2019)
Kamal, S.A., Jahim, J.M., Anuai, N., Hassan, O., Daud, W.R.W., Mansor, M.F., Rashid, S.S.: Pre-treatment effect of palm oil mill effluent (POME) during hydrogen production by a local isolate Clostridium butyricum. Int. J. Adv. Sci. Eng. Inf. Technol. 2, 2088–5334 (2012)
Acknowledgements
This research was financially supported by the Agro-Industry Practice School of Prince of Songkla University, and Graduate School of Prince of Songkla University and Thailand Research Fund through Grant Number RTA6080010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khangkhachit, W., Suyotha, W., O-Thong, S. et al. Selection of Microorganisms Possessing Thermostable Lignocellulolytic Enzymes and Application of the Enzymes for Saccharification of Pretreated Palm Oil Mill Wastes. Waste Biomass Valor 12, 711–724 (2021). https://doi.org/10.1007/s12649-020-01027-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01027-z




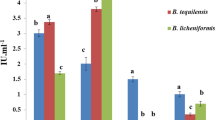



 ), B. subtilis ME751 (
), B. subtilis ME751 ( ), B. amyloliquefaciens ASB/TRE (
), B. amyloliquefaciens ASB/TRE ( ) and A. fumigatus A4112 (
) and A. fumigatus A4112 ( ) cultivated in 50 g/L of EFB at 45 °C for the optimal incubation time of each strain
) cultivated in 50 g/L of EFB at 45 °C for the optimal incubation time of each strain
