Abstract
The cellulase and xylanase productions from the pretreated empty fruit bunch as the low-cost substrate in a submerged fermentation were investigated. The objectives of this study were selected the local strain that produced high cellulase and xylanase and enhanced the enzymes production by the selected strain. Ten Streptomyces strains were cultivated in the complex medium [(g/l); CaCl2·2H2O 0.1, MgSO4·7H2O 0.1, KH2PO4 0.5, K2HPO4 1.0, NaCl 0.2, NH4NO3 1.0, yeast extract 5.0 and Tween 80 0.5 with alkaline peroxide pretreated empty fruit bunch (APEFB) 20 in distilled water, pH 7.0] in an incubator shaker at 150 rpm and 45 °C for 120 h. The strain TC13W gave the highest cellulase and xylanase activities (280 and 878 U/g APEFB, respectively). This strain was identified by 16S rDNA method as Streptomyces thermocoprophilus (96% similarity). Cellulase and xylanase productions by S. thermocoprophilus TC13W in the optimized medium with 1% (w/v) APEFB and 0.5% (w/v) yeast extract, pH 6.5 at 150 rpm and 40 °C for 120 h gave the maximum cellulase and xylanase activities of 925 and 1796 U/g APEFB, respectively. The increasing of cellulase and xylanase activities in the optimized medium was 3.30 and 2.04 folds, respectively in comparison to the original medium. These in-house enzymes could be used as the promising candidate enzyme for produce various value-added products via an enzymatic hydrolysis of the lignocellulosic waste.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This is the first report using EFB for cellulase and xylanase productions by Streptomyces thermocoprophilus TC13W. The high cellulase and xylanase activities were obtained after the optimization step.
Introduction
Palm oil industry is one of the major agro-industry in Southeast Asia. The palm oil industry has developed very fast in the last few years due to the use of palm oil as a raw material for biodiesel production in addition to direct consumption. This increased not only palm oil production but also its wastes. The standard palm oil milling process has a lot of wastes consisting of 20–28% (w/w) empty fruit bunch (EFB), 11–13% (w/w) palm pressed fiber (PPF) and wastewater or palm oil mill effluent (POME) 0.87–1.05 m3/ton fresh fruit bunch (FFB) [1]. As a lignocellulosic material, EFB could be used as a renewable and low-cost substrate for the cellulase and xylanase productions and for transformation into fermentable sugars and other high value-added chemicals. Thus, EFB may be used as an alternative renewable bioresource to replace more expensive substrates such as carboxymethylcellulose (CMC), Avicel, and Solka-Floc for the induction of cellulase production by microorganisms [2]. If this biomass is properly used, it will be able to solve the waste problem and can create value added products [3].
Cellulase and xylanase are the most wildly used enzymes in food processing, pulp and paper industry, textile industry, laundry detergents and cellulosic ethanol production [2]. Cellulase and xylanase are enzymes that breaks down cellulose and xylan, the main component of the cell walls of plant biomass. These enzymes are produced mainly by fungi and bacteria [4,5,6,7]. There are several reports on the production of cellulase and xylanase using lignocellulose as the substrate such as sugar cane bagasse [8, 9], wheat straw [10, 11], palm oil trunk [12] and palm oil EFB [3, 13]. This enzymatic system is responsible for the hydrolysis of lignocellulosic biomass and its production is the most important step in the economic production of bioethanol, single cell proteins and other chemicals [14].
The cellulase and xylanase productions are the expensive step during ethanol production from lignocellulosic biomass. It cost approximately 40% of the total cost [15]. The use of cheaply available agro-industrial wastes as a substrate to reduces the cost of the fermentation process had been reported [8, 12, 13, 16,17,18]. Many studies have been used EFB as a carbon source for cellulase and xylanase productions by bacteria and fungi, but the amounts of the produce enzyme were low [2, 13, 19, 20]. Actinomycetes especially Streptomyces spp. also produces a significant amount of many industrially important enzymes [21]. Cellulase, xylanase, alkaline protease, chitosanase, and ligninase are secreted in the culture medium of various Streptomyces strains and are implicated in biomass recycling. Unlike filamentous fungi, very scarce information on the application of enzymes from actinomycetes in biofuel production from different feedstocks has been published. Streptomyces are prokaryote and could grow at a faster growth rate. They are more amenable to genetic manipulation. Hence, they can prove to be a good candidate for the production of cellulolytic enzymes [22].
This study selected the native Streptomyces and used for cellulase and xylanase production using an alkaline peroxide pretreated EFB (APEFB) as a cheap carbon source.
Materials and Methods
EFB Preparation
EFB fiber was kindly provided by Thai Tallow and Oil Co., Ltd., Surat Thani Province, Thailand. The EFB fiber was sun-dried and reduced to an average length of 2 mm by grinding using a cross beater mill, then was ground to pass through 18 mesh screen [23]. The EFB was pretreated by soaking in 15% NaOH solution with 3% H2O2 for 4 h and autoclaved at 121 °C for 5 min. After cooling, the samples were washed several times with tap water to neutralize the pH followed by a final rinse in distilled water. Then they were oven dried at 105 °C overnight. The cellulose, hemicellulose, lignin, and ash contents of the APEFB were analyzed [24] to be 74.46, 15.72, 6.40 and 0.22% (w/w), respectively. The APEFB was kept in a plastic bag and stored at room temperature.
Microorganisms
Ten Streptomyces strains isolated from the lignocellulosic compost, were obtained from the Division of Biotechnology, Faculty of Agro-Industry, Chiang Mai University, Thailand. The basal medium used was the carboxymethylcellulose (CMC) agar [(g/l); CaCl2·2H2O 0.1, MgSO4·7H2O 0.1, KH2PO4 0.5, K2HPO4 1.0, NH4NO3 1.0, yeast extract 1.0, peptone 1.0, CMC 10 and agar 13 in distilled water, pH 7.0]. The starter culture was prepared by transferred 1 loop of Streptomyces strains from the CMC agar into 250 ml Erlenmeyer flasks containing 50 ml of the CMC medium without agar and cultivated by shaking at 150 rpm and 45°C for 48 h (OD660 = 1.0).
Culture Media
The complex medium used in this study was consisting of (g/l); CaCl2·2H2O 0.1, MgSO4·7H2O 0.1, KH2PO4 0.5, K2HPO4 1.0, NaCl 0.2, NH4NO3 1.0, yeast extract 5.0, Tween 80 0.5 and 2 mm APEFB 20 in distilled water. The initial pH was adjusted to 7.0 with 1.0 N HCl before autoclaving at 121 °C for 15 min. 10% (v/v) [25] each starter culture was inoculated in 250 ml Erlenmeyer flasks containing 50 ml of the complex medium and shaking at 150 rpm and 45 °C for 120 h. The samples were taken periodically and centrifuged at 15,000 rpm and 4 °C for 30 min. The supernatants were collected and examined cellulase and xylanase activities.
Strain Identification
The strain which produced the highest cellulase and xylanase activities was subjected to molecular identification by analyzing 16S rDNA sequence and was selected for optimization of the enzyme production. The sequence was compared to the different sequence of the Streptomyces species deposited in the Genbank (NCBI). Then the phylogenetic tree was reconstructed by the neighbor-joining method.
Enzyme Production
Effect of EFB Concentration
The selected strain was cultivated in the complex medium. 10% (v/v) starter culture of the selected strain was inoculated into the complex medium with 1–5% APEFB (2 mm) at an initial pH 7.0 and shaking at 150 rpm and 45 °C for 120 h.
Effect of Initial pH
The medium with optimized concentrations of the APEFB was used for further study. The initial pH of the complex medium was adjusted to pH 4.5–8.0.
Effect of Temperature
The effect of temperature on the enzyme productions was studied by cultivating the selected strain in the medium at 30–50 °C.
Effect of Nitrogen Source and Selected Nitrogen Source Concentrations
The effect of nitrogen source was studied by adding 0.5% (w/v) of NH4NO3, tryptone, peptone, yeast extract, meat extract, or malt extract to the basal medium without nitrogen sources. Furthermore, the concentration of selected nitrogen source was studied.
Effect of APEFB Size
The effect of APEFB size on enzyme production was studied by cultivating the selected strain in the medium supplemented with 2, 5 and 10 mm APEFB and cultivated at the optimum conditions.
Enzyme Assays
The culture supernatant was prepared by centrifugation the culture broth at 15,000 rpm and 4 °C for 30 min. Cellulase activity was assayed by adding 0.5 ml of appropriately diluted enzyme source to 1.0% (w/v) CMC (Fluka) in 50 mM sodium acetate buffer (pH 4.8) 0.5 ml and incubated at 500 rpm and 50 °C for 10 min. The amount of reducing sugar released during the reaction was measured by DNS method [26] and glucose was used as a standard. One unit of cellulase was defined as the amount of enzyme that liberated 1 µmol of glucose equivalent per min under the assay conditions. Xylanase activity was determined at 500 rpm and 50 °C by using 0.5% (w/v) beechwood xylan (Sigma) dissolved in 50 mM sodium acetate buffer (pH 4.8) 0.5 ml as a substrate. The amount of reducing sugar released during the reaction was measured by DNS method [26] and xylose was used as a standard. One unit of xylanase activity was defined as the amount of enzyme that released 1 µmol of xylose under the experimental conditions.
Statistical Analysis
The experimental design was a completely randomize design with three replications. Data are presented as mean values with standard deviations. One-way analysis of variance (ANOVA) was carried out and comparisons of means were done by Duncan’s New Multiple Range Test, with significance threshold p < 0.05. Statistical analyses were performed with the statistical program SPSS (version 16.0).
Results and Discussion
Selection of Microorganism
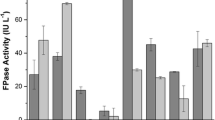
Ten Streptomyces strains were cultivated in the complex medium contained APEFB as a carbon source with shaking at 150 rpm and 45 °C for 120 h. The results showed that all Streptomyces strains could produce cellulase and xylanase in this complex medium. The strain TC13W gave the highest cellulase of 280 U/g APEFB (p < 0.05) and xylanase of 878 U/g APEFB (p > 0.05) activities in comparison with other strains (Fig. 1). Many reports showed that pretreated EFB could be used as a sole carbon source for cellulase and xylanase productions. Ariffin [2] reported that Bacillus pumilus EB3 produced 6.3 U/g EFB of cellulase in the alkaline pretreated EFB medium at 37 °C for 5 days. Cellulase production by Chaetomium globosum was 140 U/g EFB in the acidic pretreated EFB medium at 30 °C for 12 days [13]. Alam et al. [14] found that Trichoderma harzianum gave the cellulase activity of 8.2 U/g EFB in the EFB medium at 32 °C for 4 days, while Aspergillus niger EB4 gave the cellulase activity of 6.4 U/g EFB in the EFB medium at 40 °C for 6 days [27]. In addition, Streptomyces strain TC13W that showed the highest enzyme production was selected for optimization of cellulase and xylanase productions.
Identification of Selected Strain
The selected strain TC13W was identified by the Thailand Institute of Scientific and Technological Research (TISTR), Pathum Thani as Streptomyces thermocoprophilus and was included in the collection at the DNA Data Bank of Japan (DDBJ) Center (National Institute of Genetics Research Organization of Information and Systems, Mishima, Shizuoka, Japan) with accession number LC060712.1. The 16S rDNA gene sequences of the strain TC13W was determined and showed the highest similarity to the type strain of S. thermocoprophilus B19 (96%). The phylogenetic tree based on the neighbor-joining method showed that the strain TC13W conformed to branch with S. thermocoprophilus B19 (NR025291.1) and S. thermocoprophilus (AB249938.1) (Fig. 2).
Streptomyces sp. could be isolated from various sources such as soil, compost and water [28,29,30]. For the degradation of cellulose, hemicellulose and lignin, different strains of Streptomyces sp. have been studied and found to be good producers of cellulase [31,32,33,34,35,36].
Enzyme Production by Streptomyces thermocoprophilus TC13W
Effect of EFB Concentration
The effect of APEFB concentration on cellulase and xylanase productions by S. thermocoprophilus TC13W was studied in the complex medium supplementing with 1–5% APEFB and incubated at 150 rpm and 45 °C for 120 h. The result showed that the highest cellulase and xylanase activities of 597.4 and 1204.8 U/g APEFB, respectively (p < 0.05) were found in the medium with 1% APEFB (Fig. 3). Further increase in the concentration of APEFB did not increase the enzyme activities. It might be that high concentration of APEFB affects the oxygen mass transfer between the substrate, medium, and microorganism [37]. Bahrin et al. [37] reported that the EFB concentration higher than 4% did not enhance the cellulase production by Botryosphaeria rhodina while Aspergillus terreus could produce more cellulase in the medium with 1% EFB [38].
Effect of Initial pH
The effect of an initial pH on cellulase and xylanase productions by S. thermocoprophilus TC13W was studied by using 10% starter culture in the complex medium with 1% APEFB and adjusted an initial pH between 4.5 and 8.0. The result shows that S. thermocoprophilus TC13W gave the highest cellulase and xylanase activities of 766.5 and 1553.4 U/g APEFB, respectively (p < 0.05) in the medium with an initial pH 6.5 (Fig. 4). The enzyme productions were drastically reduced at acidic pH and no enzyme production was noticed in the medium at pH 4.5–5.5. The result shows that S. thermocoprophilus TC13W produced cellulase and xylanase activities at a wide range of pH (between 6.0 and 8.0). However, when it was grown in the medium with an alkaline at pH 7.5–8.0 the cellulase and xylanase productions were slightly decreased. The initial pH has been observed to influence the transport of enzymes across the cell membrane [39, 40]. A pH range of 6.5–7.0 has been observed to be optimum for cellulase and xylanase productions by Streptomyces spp. [32, 35]. In contrast, Saratale et al. [36] reported that the optimum pH for cellulase production by Streptomyces sp. MDS was pH 5.0.
Effect of Temperature
The temperature is an important parameter that influenced enzyme activity and production. The effect of temperature on cellulase and xylanase productions by S. thermocoprophilus TC13W was studied by using 10% starter culture in the medium with 1% APEFB at an initial pH 6.5. S. thermocoprophilus TC13W gave the highest cellulase and xylanase activities of 917.3 and 1789.9 U/g APEFB, respectively at 40 °C (Fig. 5). The other studies also show the optimum temperature for the production 40C such as S. albidoflavus SAMRC-UFH5 [41] and Streptomyces sp. MDS [36]. On the other hand, S. drozdowiczii [33], Streptomyces T3-1 [32] and Streptomyces sp. SLBA-08 [42] had the optimum temperature for cellulase and xylanase productions at 50 °C while Streptomyces sp. AMT-3 [43], Streptomyces sp. BRC1; BRC2 [34], Streptomyces sp. F2621 [44], Streptomyces sp. J2 [45] and Streptomyces M23 [31] had an the optimal condition at 26–35 °C.
Effect of Nitrogen Source and Concentration
Various nitrogen sources were proved to have different influences on enzyme production [13, 46]. The effect of nitrogen sources on cellulase and xylanase productions by S. thermocoprophilus TC13W was studied by adding 0.5% (w/v) of different nitrogen sources in the complex medium instead of NH4NO3 and yeast extract with 1% APEFB at an initial pH 6.5 and incubated at 150 rpm and 40 °C for 120 h. The result shows that S. thermocoprophilus TC13W gave the highest (p < 0.05) cellulase and xylanase activities of 904.8 and 1785.6 U/g APEFB in the medium with only yeast extract as a nitrogen source (Fig. 6a). The result was not significantly different (p > 0.05) with the control medium with added NH4NO3 and yeast extract. Yeast extract is a very important ingredient in most media designed for actinomycetes, being a source of nitrogen and other nutrients and has been used and recommended as a nitrogen source for cellulase and xylanase productions by many Streptomyces [31, 33, 34, 41, 44, 47].
Effect of nitrogen source (a) and yeast extract concentrations (b) on cellulase and xylanase productions by Streptomyces thermocoprophilus TC13W in the complex medium with 1% APEFB, pH 6.5 at 150 rpm and 40 °C for 120 h. (Control: (g/l); CaCl2 0.1, MgSO4·7H2O 0.1, KH2PO4 0.5, K2HPO4 1.0, NaCl 0.2, NH4NO3 1.0, yeast extract 5.0, Tween 80 0.5 and APEFB 10 in distilled water, initial pH 6.5)
The effect of yeast extract concentration on the enzyme productions was studied by adding 0–1% (w/v) yeast extract into the medium. The result shows that adding 0.5% yeast extract produced the highest cellulase and xylanase productions by S. thermocoprophilus TC13W (Fig. 6b) but was not significantly different from adding 0.75% and 1.0% yeast extract. The increase of yeast extract concentration from 0.3 to 1.0% was not beneficial to the cellulase production by S. drozdowiczii [33].
Effect of APEFB Size
The purpose of this experiment was to reduce the cost of the grinding process for EFB preparation. The result shows that the 2 mm pretreated EFB gave the highest production of cellulase (924.8 U/g APEFB) and xylanase (1795.6 U/g APEFB) in the medium with 1% APEFB and 0.5% yeast extract, an initial pH 6.5 at 150 rpm and 40 °C for 120 h (Fig. 7). The enzyme productions were decreased in the medium with APEFB size of 5 and 10 mm. The small particle size of the APEFB showed increasing the surface area and gave more space which was contacted by air (oxygen) resulting enhancement for the growth of the microorganism [48].
Effect of Time Course
Time course of cellulase and xylanase productions by S. thermocoprophilus TC13W in the optimized medium and conditions (1% APEFB with an initial pH 6.5 at 40 °C) was compared with in the original medium and conditions (2% APEFB with an initial pH 7.0 at 45 °C) (Fig. 8). The results show that this strain could increase the cellulase and xylanase activities in the optimized medium higher than in the original medium by 3.30 folds and 2.04 folds, respectively. This result confirmed that the pretreated EFB can be used as a low cost of carbon source for cellulase and xylanase productions by this strain.
Many studies used CMC, cellulose, oat spelt xylan and agro-industrial wastes such as bagasse, corn cob and wood straw as a carbon source for cellulase and xylanase productions [31,32,33,34, 36, 41,42,43,44,45]. Most researchers showed the results that produced cellulase and xylanase less than the use of AFEFB by S. thermocoprophilus TC13W (Table 1). Only the use of CMC by Streptomyces T3-1 [32] produced more cellulase and the use of oat spelt xylan by Streptomyces sp. F2621 [44] produced xylanase higher than the cellulase and xylanase productions using APEFB by S. thermocoprophilus TC13W. However, CMC and oat spelt xylan are more expensive than APEFB. In addition, many studies used different pretreated EFB for cellulase and xylanase productions by various microorganisms (Table 2) [2, 3, 13, 19, 27, 37, 38]. It is noted that the use of APEFB by S. thermocoprophilus TC13W produced much higher amounts of cellulase and xylanase than the use of other pretreated EFB by other bacteria and fungi. Therefore, the productions of cellulose and xylanase by S. thermocoprophilus TC13W using APEFB as a carbon source is a promising step to reduce the cost of these enzyme productions.
Conclusion
The local strain, S. thermocoprophilus TC13W isolated from the compost was used as a producer of cellulase and xylanase enzymes. This strain produced much higher amounts of cellulase and xylanase in the medium composed of yeast extract and APEFB in comparison to the productions by other microorganisms using CMC, oat spelt xylan and agro-industrial wastes including the pretreated EFB by other methods. EFB is a cheap and largely available biomass that could be used for an enzyme production by microorganisms instead of expensive raw materials. The cost of cellulase and xylanase productions by S. thermocoprophilus TC13W would be reduced using APEFB as a carbon source. These enzymes could be used in pulp and paper industry as well as hydrolysis of EFB and other biomass to produce sugars for ethanol production and other biorefined products. In addition, the use of this agro-industrial waste will add value to EFB and will be beneficial to the environment as well.
Data Availability
All data generated or analyzed during this study are included this published article and its supplementary information files.
References
Prasertsan, S., Prasertsan, P.: Biomass residues from palm oil mills in Thailand: an overview on quantity and potential usage. Biomass Bioenergy 11, 387–395 (1996)
Ariffin, H., Hassan, M.A., Shah, U.K., Abdullah, N., Ghazali, F.M., Shirai, Y.: Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci. Bioeng. 106, 231–236 (2008)
Harun, N.A.F., Baharuddin, A.S., Zainudin, M.H.M., Bahrin, E.K., Naim, M.N., Zakaria, R.: Cellulase production from treated oil palm empty fruit bunch degradation by locally isolated Thermobifida fusca. BioResources 8, 676–687 (2012)
Baldrian, P., Valásková, V.: Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 32, 501–521 (2008)
Bayer, E.A., Chanzy, H., Lamed, R., Shoham, Y.: Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 8, 548–557 (1998)
Fontes, C.M.G.A., Gilbert, H.J.: Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79, 655–681 (2010)
Lynd, L.R.: Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu. Rev. Energy Environ. 21, 403–465 (1996)
Adsul, M.G., Bastawde, K.B., Varma, A.J., Gokhale, D.V.: Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresour. Technol. 98, 1467–1473 (2007)
Camassola, M., Dillon, A.J.P.: Biological pretreatment of sugar cane bagasse for the production of cellulases and xylanases by Penicillium echinulatum. Ind. Crops Prod. 29, 642–647 (2009)
Katapodis, P., Christakopoulou, V., Kekos, D., Christakopoulos, P.: Optimization of xylanase production by Chaetomium thermophilum in wheat straw using response surface methodology. Biochem. Eng. J. 35, 136–141 (2007)
Yang, C., Shen, Z., Yu, G., Wang, J.: Effect and aftereffect of γ radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresour. Technol. 99, 6240–6245 (2008)
Ang, S.K., Shaza, E.M., Adibah, Y., Suraini, A.A., Madihah, M.S.: Production of cellulase and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48, 1293–1302 (2013)
Umikalsom, M.S., Ariff, A.B., Shamsuddin, Z.H., Tong, C.C., Hassan, M.A., Karim, M.I.A.: Production of cellulase by a wild strain of Chaetomium globosumusing delignified oil palm empty-fruit-bunch fibre as substrate. Appl. Microbiol. Biotechnol. 47, 590–595 (1997)
Alam, M.Z., Mamun, A.A., Qudsieh, I.Y., Muyibi, S.A., Salleh, H.M., Omar, N.M.: Solid state bioconversion of oil palm empty fruit bunches for cellulase enzyme production using a rotary drum bioreactor. Biochem. Eng. J. 46, 61–64 (2009)
Ahamed, A., Vermette, P.: Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem. Eng. J. 40, 399–407 (2008)
Archana, A., Satyanarayana, T.: Xylanase production by thermophilic Bacillus licheniformis A99 in solid-state fermentation. Enzyme Microb. Technol. 21, 12–17 (1997)
Bhushan, B., Pal, A., Jain, V.: Isolation, screening and optimized production of extracellular xylanase under submerged condition from Aspergillus flavus Mtcc 9390. Enzyme Eng. 1, 1–6 (2012)
Elegir, G., Szakács, G., Jeffries, T.W.: Purification, characterization, and substrate specificities of multiple xylanases from Streptomyces sp. strain B-12-2. Appl. Environ. Microbiol. 60, 2609–2615 (1994)
Kim, S., Kim, C.H.: Production of cellulase enzymes during the solid-state fermentation of empty palm fruit bunch fiber. Bioprocess Biosyst. Eng. 35, 61–67 (2012)
Ting, A.S.Y., Hermanto, A., Peh, K.L.: Indigenous actinomycetes from empty fruit bunch compost of oil palm: evaluation on enzymatic and antagonistic properties. Biocatal. Agric. Biotechnol. 3, 310–315 (2014)
Gilbert, M., Morosoli, R., Shareck, F., Kluepfel, D.: Production and secretion of proteins by Streptomycetes. Crit. Rev. Biotechnol. 15, 13–39 (1995)
Vasavada, S.H., Thumar, J.T., Singh, S.P.: Secretion of a potent antibiotic by salt-tolerant and alkaliphilic actinomycete Streptomyces sannanensis strain RJT-1. Curr. Sci. 91, 1393–1397 (2006)
Kamcharoen, A., Champreda, V., Eurwilaichitr, L., Boonsawang, P.: Screening and optimization of parameters affecting fungal pretreatment of oil palm empty fruit bunch (EFB) by experimental design. Int. J. Energy Environ. Eng. 5, 303–312 (2014)
A.O.A.C.: Official Methods of Analysis of the Association of Official Analytical Chemists, 15th edn. A.O.A.C., Washington, D.C. (1990)
Jang, H.-D., Chang, K.-S.: Thermostable cellulases from Streptomycessp.: scale-up production in a 50-l fermenter. Biotechnol. Lett. 27, 239–242 (2005)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Baharuddin, A.S., Razak, N.A.A., Rahman, N.A.A., Budiatman, S., Shirai, Y., Hassan, M.A.: Bioconversion of oil palm empty fruit bunch by Aspergillus niger EB4 under solid-state fermentation. Pertanika J. Trop. Agric. Sci. 32, 143–151 (2009)
Cuesta, G., García-de-la-Fuente, R., Abad, M., Fornes, F.: Isolation and identification of actinomycetes from a compost-amended soil with potential as biocontrol agents. J. Environ. Manag. 95(Suppl), 280–284 (2012)
Kang, S.W., Park, Y.S., Lee, J.S., Hong, S.I., Kim, S.W.: Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91, 153–156 (2004)
Rateb, M.E., Houssen, W.E., Harrison, W.T.A., Deng, H., Okoro, C.K., Asenjo, J.A., Andrews, B.A., Bull, A.T., Goodfellow, M., Ebel, R., Jaspars, M.: Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 74, 1965–1971 (2011)
Semêdo, L.T.A.S., Gomes, R.C., Bon, E.P.S., Soares, R.M.A., Linhares, L.F., Coelho, R.R.R.: Endocellulase and exocellulase activities of two Streptomyces strains isolated from a forest soil. Appl. Biochem. Biotechnol. 84–86, 267–276 (2000)
Jang, H.D., Chen, K.S.: Production and characterization of thermostable cellulases from Streptomyces transformant T3-1. World J. Microbiol. Biotechnol. 19, 263–268 (2003)
Grigorevski de Lima, A.L., Pires do Nascimento, R., da Silva Bon, E.P., Coelho, R.R.R.: Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme Microb. Technol. 37, 272–277 (2005)
Chellapandi, P., Jani, H.M.: Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation. Braz. J. Microbiol. 39, 122–127 (2008)
Prasad, P., Bedi, S., Singh, T.: In vitro cellulose rich organic material degradation by cellulolytic Streptomyces albospinus (MTCC 8768). Malays. J. Microbiol. 8, 164–169 (2012)
Saratale, G.D., Saratale, R.G., Oh, S.E.: Production and characterization of multiple cellulolytic enzymes by isolated Streptomyces sp. MDS. Biomass Bioenergy 47, 302–315 (2012)
Bahrin, E., Ibrahim, M., Abd Razak, M.N., Abd-Aziz, S., Md Shah, U.K., Alitheen, N., Salleh, M.: Improved cellulase production by Botryosphaeria rhodina from OPEFB at low level moisture condition through statistical optimization. Prep. Biochem. Biotechnol. 42, 155–170 (2012)
Shahriarinour, M., Wahab, M.N.A., Mohamad, R., Mustafa, S., Ariff, A.B.: Effect of medium composition and cultural condition on cellulase production by Aspergillus terreus. Afr. J. Biotechnol. 10, 7459–7467 (2011)
Bakri, Y., Jawhar, M., Arabi, M.: Improvement of xylanase production by Cochliobolus sativus in solid state fermentation. Braz. J. Microbiol. 39, 602–604 (2008)
Goyal, V., Mittal, A., Bhuwal, A.K., Singh, G., Yadav, A., Aggarwal, N.K.: Parametric optimization of cultural conditions for carboxymethyl cellulase production using pretreated rice straw by Bacillus sp. 313SI under stationary and shaking conditions. Biotechnol. Res. Int. 2014, 1–7 (2014)
Fatokun, E.N., Nwodo, U.U., Okoh, A.I.: Classical optimization of cellulase and xylanase production by a marine Streptomyces species. Appl. Sci. 6, 1–14 (2016)
Macedo, E.P., Cerqueira, C.L.O., Souza, D.A.J., Bispo, A.S.R., Coelho, R.R.R., Nascimento, R.P.: Production of cellulose-degrading enzyme on sisal and other agro-industrial residues using a new Brazilian actinobacteria strain Streptomyces sp. SLBA-08. Braz. J. Chem. Eng. 30, 729–735 (2013)
Nascimento, R.P., Coelho, R.R.R., Marques, S., Alves, L., Gı́rio, F.M., Bon, E.P.S., Amaral-Collaço, M.T.: Production and partial characterisation of xylanase from Streptomyces sp. strain AMT-3 isolated from Brazilian cerrado soil. Enzyme Microb. Technol. 31, 549–555 (2002)
Tuncer, M., Kuru, A., Isikli, M., Sahin, N., Celenk, F.G.: Optimization of extracellular endoxylanase, endoglucanase and peroxidase production by Streptomyces sp. F2621 isolated in Turkey. J. Appl. Microbiol. 97, 783–791 (2004)
Jaradat, Z., Dawagreh, A., Ababneh, Q., Saadoun, I.: Influence of culture conditions on cellulase production by Streptomyces sp. (strain J2). Int. J. Life Sci. Med. Res. 3, 141–146 (2008)
Narasimha, G., Sridevi, A., Buddolla, V., Subhosh, C.M., Rajasekhar, R.B.: Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr. J. Biotechnol. 5, 472–476 (2006)
Chaiyaso, T., Kuntiya, A., Techapun, C., Leksawasdi, N., Seesuriyachan, P., Hanmoungjai, P.: Optimization of cellulase-free xylanase production by thermophilic Streptomyces thermovulgaris TISTR1948 through Plackett-Burman and response surface methodological approaches. Biosci. Biotechnol. Biochem. 75, 531–537 (2011)
Pandey, A.: Effect of particle size of substrate of enzyme production in solid-state fermentation. Bioresour. Technol. 37, 169–172 (1991)
Acknowledgements
The authors would like to thank the Office of the Higher Education Commission (Strategic Scholarships of Frontier Research Network, specific for the Southern region), the National Research Council of Thailand and the Graduate School of Prince of Songkla University for supporting this study.
Funding
This study was funded by Strategic Scholarships of Frontier Research Network (Specific for the Southern region), the Office of the Higher Education Commission (Grant No. 022/2012), National Research Council of Thailand (Grant year 2014), and the Graduate School of Prince of Songkla University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sinjaroonsak, S., Chaiyaso, T. & H-Kittikun, A. Optimization of Cellulase and Xylanase Productions by Streptomyces thermocoprophilus TC13W Using Low Cost Pretreated Oil Palm Empty Fruit Bunch. Waste Biomass Valor 11, 3925–3936 (2020). https://doi.org/10.1007/s12649-019-00720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00720-y