Abstract
Time and storage space are the main constraints facing by the current Malaysia composting industries which are handling lignocellulosic food industry waste such as empty fruit bunch (EFB), coffee ground (CG) and palm oil mill sludge (POMS). To develop an efficient accelerated composting method for lignocellulosic food industry waste in Malaysia, a new approach which combined two distinct technologies to achieve rapid composting; (i) co-composting with combination of different lignocellulosic materials and (ii) inoculation of the co-composting with mixture of microorganism were presented in this study. All the mixtures achieved thermophilic condition on the first week of composting, with 50% EFB + 50% CG combination recorded the highest temperature of 56 °C. The mixtures with initial C/N ratios within 25–35 showed the highest reduction in holocellulose, cellulose, hemicellulose and lignin content while compost mixture with initial C/N ratio more than 50 had a minimal reduction. The C/N ratio for 50% EFB + 25% CG + 25% POMS combination was reduced to less than 20 after 4 weeks of co-composting process while the other treatments needed longer composting duration. During thermophilic phase, higher population of bacteria (107–108 CFU/g DW) was observed, followed by fungi (105–106 CFU/g DW) and actinomycetes (105–106 CFU/g DW) populations. However, higher population of actinobacteria (104–106 CFU/g DW) compared to bacterial (104–105 CFU/g DW) and fungal (103–104 CFU/g DW) was found during latter stages. By reducing both the time and space required for composting lignocellulosic food industry waste, this accelerated co-composting method may be a viable option for food industries searching for a long-term, practical solution for solid biowaste disposal issues.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The new approach discussed in this paper combines two distinct technologies to achieve rapid composting of lignocellulosic food industry waste; (i) co-composting with combination of different lignocellulosic materials and (ii) inoculation of the co-composting with mixture of microorganism. To activate an efficient composting process with higher biodegradation rate and better quality of the compost, a few types of materials has to be mixed together to startup the composting process. The observations of physicochemical properties change during co-composting in this study provide significant understanding on the suitability conditions and nutrients sufficiency required by the microbes to achieve effective composting. By reducing both the time and space required for composting lignocellulosic food industry waste, this accelerated co-composting method may be a viable option for food industries searching for a long-term, practical solution for solid biowaste disposal issues.
Introduction
In Malaysia, agriculture residues accounted nearly 70% of the total 70 million tons of the organic waste discharged per year. As one of the world’s largest palm oil producers, the achievement comes at a price. A single ton of crude palm oil generates huge amounts of by-products, which include 1.5 ton of empty fruit bunch (EFB) and 3 ton of palm oil mill effluent (POME) with a total solid content of 0.6% known as palm oil mill sludge (POMS). Instant coffee is also growing industry in Malaysia. In 2018, approximately 900,000 ton of the coffee bean is imported from Indonesia, Vietnam and Brazil with most of these coffee imports are for instant coffee production. About 330–450 kg of instant coffee drink is produced from 1 ton of coffee beans, whereas 550–670 kg known as spent coffee grounds is generated [1]. EFB contains high in potassium (2.4%) while coffee ground (CG) and POMS is a good nitrogen source with total nitrogen of 0.7% and 4.1%, respectively [2]. These lignocellulosic waste from the food industry (EFB, POMS and CG) contained relatively low amounts of heavy metals content when compared with the maximum allowable content specific in MS 1517:2012 standard for organic fertilizer. In the food processing mill, EFB and coffee ground had also gone through a process that involved high pressure and temperature which had probably destroyed most of the plant disease bearing microorganisms or insects. Due to their abundant availability and chemical content, EFB, POMS and coffee ground were judged to be good composting materials as a way to recycle nutrients back into the soil.
However, a lot of local industries in Malaysia fail to do composting in large scale with lignocellulosic biomass as the raw material. Failures have been attributed to many reasons. One of the core reasons is the lignocellulosic biomass composting require very long time to fully decompose. Several researchers reported that the time taken for lignocellulosic biomass to reach maturity stage will take at least four months and above [3,4,5,6]. The composting process which involve long duration of accomplishment will directly affect the cost of the production and reduce the economic value. In addition, huge compost area is required to allocate the compost piles due to the lengthy composting process.
Time to maturity always the main concern in all composting process. Efficient biodegradation in composting is influence by the types of organic compounds (carbon or nitrogen source), chemical composition (alpha-cellulose, hemicellulose and lignin), and concentration of other organic compounds [7]. During composting, some organic compounds such as oil and grease will degrade faster than other highly complex organic compounds, though all will eventually decompose over a length of time. Carbon-to-nitrogen (C/N) ratio is the most important aspect that controls the heat up ability in the compost piles and the quality of the compost produced. A balance of carbon and nitrogen compounds is needed to create the proper environment for composting to occur. If the C/N ratios in the compost materials are not in an appropriate range for composting, longer time is required to achieve maturity [8]. In addition, lignocellulosic materials such as empty fruit bunch (EFB) and coffee grounds are not suitable to be composted alone because it mainly composes of highly complex organic compounds (lignin, cellulose) and with less readily biodegradable organic material.
To activate an efficient composting process with higher biodegradation rate and better quality of the compost, a few types of materials has to be mixed together to startup the composting process. The mixing ratio largely depends on the bioavailability of carbon and nitrogen level in the compost materials. For EFB to be rapidly composted, it is necessary to incorporate second material as compost substrate that have complementary properties, such as a low C/N ratio as well as balancing moisture [9]. Relatively, for POMS and coffee ground to be rapidly composted, high C/N ratio material can be added to increase the carbon content in the compost to induce the microorganism to behave more aggressively in competing for nitrogen which help to increase the microbial activity [9]. Single raw material composting with POMS or coffee ground alone will also create a dense and compact compost pile causes air circulation through the pile is inhibited [2]. This decreases oxygen available to microorganisms within the pile and ultimately decreases the rate of microbial activity. Mixing POMS and CG with materials with a lower bulk density such as EFB may create a compost pile with a sufficient compaction to induce microbial activity and degradation process.
A mixture of two or more types of organic materials in a compost is called co-composting. Five different mixing ratios were tested in this study to determine the effective biodegradation of lignocellulosic biomass from the food industry and at the same time shorten the composting period. A mixture of bacteria and fungi was used as the inoculums to intensify the composting process especially dealing with high oil content food industries waste such as EFB, coffee ground and POMS. The new approach discussed in this paper combines two distinct technologies to achieve rapid composting; (i) co-composting with combination of different lignocellulosic materials and (ii) inoculation of the co-composting with mixture of microorganism.
Materials and Methods
Preparation of Composting Materials
Three lignocelluloses-based materials selected in this study include empty fruit bunch (EFB), palm oil mill sludge (POMS) and spent coffee ground (CG). Shredded EFB and POMS were collected from Seri Ulu Langat Palm Oil Mill, Selangor. EFB was shredded in the palm oil mill using a shredder known as the cutter cum oil extractor which produced EFB fibre with the length of 2″ to 4″. This shredder machine tears, shreds and squeezes the EFB in a single pass. POMS was desludged and collected from the anaerobic or aerobic ponds of the palm oil mill. Whereas spent coffee ground was supplied by All Cosmos Industries Sdn. Bhd., Johor. The physical and chemical properties of EFB, POMS and CG were tested and evaluated prior to the composting process.
Mixture of Microorganism
A commercial microbial inoculant product containing Bacillus subtilis, Aspergillus sp. and actinobacteria: EM·1™ (Effective Microorganism 1) was purchased from All Cosmos Industries Sdn. Bhd., Johor, Malaysia. 10 g of freeze-dried microorganism with 50% Bacillus subtilis, 40% Aspergillus sp. and 10% actinobacteria were weighed and mixed with 1 L distilled water. The mixed culture of microorganism solution was aerated for 24 h using a fish air pump prior the start of the composting process. The mixtures were then sprayed evenly on the compost materials with the ratio 1:500 (w/w). Another batch of microorganism mixture will again be prepared using the same technique on the sixth day and sprayed on the seventh day of composting process.
Co-composting Establishment
To establish effective biodegradation of lignocellulosic materials, EFB as a carbon source material was mixed with materials with higher nitrogen content; coffee grounds and POMS for accelerated co-composting process. Total five mixing ratio of compost materials were fixed in the study (Table 1): (i) 50% EFB mixed with 50% CG; (ii) 50% EFB mixed with 50% POMS; (iii) 70% EFB mixed with 15% CG and 15% POMS; (iv) 50% EFB mixed with 25% CG and 25% POMS; (v) 30% EFB mixed with 35% CG and 35% POMS.
Composting process was performed in the laboratory. Five kilograms of each selected mixture of lignocellulosic food industry waste (Table 1) were placed in each composting boxes (48 cm × 36 cm × 32 cm) made from polystyrene. In order to ensure excess water was able to flow out from the materials and to prevent anaerobic reactions from occurring, sixteen of 1 cm diameter holes were made at the bottom of each composting box. A total of 15 composting boxes were set up with three boxes for each mixtures of composting materials. Manually turning was used in this study as a method of aeration. Turning was done manually once every 6 days using a spade by mingling the compost materials from the bottom to the top of the box. The composting box was labeled accordingly and covered with canvas to reduce heat loss. The composting process was recorded for 60 days.
Temperature, Moisture Content and pH Monitoring
The compost activities were monitored by measuring the temperature, moisture content and pH of the compost. These measurements may provide a basic understanding of the degradation of lignocellulosic materials during the composting process.
Temperature was monitored daily with a digital thermometer probe within the time range of 12 pm to 2 pm. Moisture content and pH of the compost were measured weekly. The pH was measured via suspension of sample in distilled water (5 g/50 ml), after standing for 2 h. In this study, the moisture content of compost was controlled at 40–60% by material weight. The determination of moisture content in the samples was carried via the oven-dry method. Sample was oven dried at 105 °C for 24 h and reweighed. Samples with moisture content lower than 40% will be watered to maintain the moisture content in the range of 40 to 60%.
Evaluation
The organic compound and chemical composition of lignocelluloses-based materials were tested and evaluated prior to the composting. 100 g samples were collected at different locations of the compost: bottom, core and surface. Each sample was oven-dried at 60 °C, grinded and screened through a 2 mm sieve prior for chemical analysis. Each sample was analyzed for the following parameters: total nitrogen, total organic carbon, organic matters, oil and grease, lignin, hemicellulose and alpha-cellulose content. All analyses were done in three replicates. The results shown were mean value of the triplicate.
Organic Compound and Chemical Composition
Determination of oil and grease content was conducted according to EPA method 9071b. Five gram of the dried sample was extracted with 200 ml n-hexane in Soxhlet apparatus for 4 h. The n-hexane extract was concentrated by rotary vacuum evaporator and then air dried. The oil and grease content were defined as the amount of organic matters (oil and grease) recovered in mass compared with the initial amount of compost sample and was presented in percentage (%). Total organic matter was determined via loss-on-ignition method (LOI) by burning dried sample in the muffle furnace at 550 °C for 24 h. The ash that remained was weighed and organic matter was determined by the difference in weight between the original and ignited sample.
Holocellulose, lignin content, hemicellulose and alpha-cellulose content of the samples were determined based on TAPPI (Technical Association of the Pulp and Paper Industry) standard T 203. Acid-insoluble materials consisting majority of lignin were determined by quantitative acid hydrolysis with 72% H2SO4 according to the T-249 em-85. Hemicellulose and alpha-cellulose contents were determined according to T429 cm-10 and Wise method [10]. The analyses on organic compounds were done in ash free basis.
Total Nitrogen, Total Organic Carbon, Organic Matters, Oil and Grease
Biodegradability of carbon in the compost can be measured by determining the changes of total organic carbon during composting process [11]. The total organic carbon (TOC) was determined based on the Walkley and Black wet digestion method [12]. One gram of the oven dried sample was placed into a 500 ml conical flask. 10 ml of 1 N potassium dichromate and 20 ml of concentrated sulphuric acid were added to the conical flask and kept for 30 min. Then it was diluted with 200 ml of distilled water. 10 ml of concentrated orthophosphoric acid and 1 ml of diphenylamine indicator were added to the flask. The above contents were later titrated against standard ferrous ammonium sulphate. The end point was noted when the solution turned from blue to green.
Total nitrogen (TN) was analysed using the Kjeldahl method [13]. The Kjeldahl method entails digestion and distillation. The sample was digested in concentrated sulphuric acid with a catalyst mixture to raise the boiling temperature and to promote the conversion of organic-N to ammonium-N. Then, the ammonium-N from the digestion was obtained by steam distillation, and NaOH was added to increase the pH. The distillate was collected in 2% boric acid indicator solution, and then titrated with dilute sulphuric acid until the colour changes from green to purple. The C/N ratio was determined as TOC/TN.
Colony Forming Unit (CFU) of the Microorganism in the Compost
Colony forming unit (CFU) of the microorganism in the compost was determined to estimate the number of viable microorganisms (bacteria, fungi or actinobacteria) in the compost. Isolation of microorganisms was carried out every 2 weeks throughout the whole composting process. 1 g of compost samples (depending on dried weight) were added to 9 ml of sterilized water. The samples were agitated for 30 min which gave a dilution of 10−1. 1 ml of solution from dilution of 10−1 were then transferred to a universal bottle which contains 9 ml of sterilized water gave a dilution of 10−2. The solution was agitated for homogenize purpose. Tenfold serial dilutions were made up to 10−8. Then, an amount of 0.1 ml from the diluted samples was spread on Nutrient Agar (NA) for bacteria and Actinomycete Isolation Agar (AIA) for actinobacteria by using glass spreader. The media compositions of the NA: peptone (5 g/l), meat extract (1 g/l), yeast extract (2 g/l), NaCl (5 g/l) and agar (20 g/l). The media compositions of the AIA: L-asparagine (0.1 g/l), dipotassium phosphate ferrous sulfate (0.001 g/l), magnesium sulfate (0.1 g/l) and agar (15 g/l). Cycloheximide was added as antibiotic in NA and AIA to avoid contamination by fungi. The fungi species in compost were isolated using Potato Dextrose Agar (PDA). In order to suppress bacterial growth, streptomycin (50 mg/ml) was added to PDA. The petri plates were labeled and sealed with parafilms. Then the petri plates were incubated at 30 °C with 85 ± 5% relative humidity for 24 h for bacteria and 4–5 days for fungi. Standard plate count (SPC) was performed to determine the number of colonies of bacteria, fungi and actinobacteria grown on the respective media [14]. Only the number of colonies in the range of 30–300 was counted.
where: ΣC = sum of all colonies on all plates counted, n1 = number of plates in lower dilution counted, n2 = number of plates in next highest dilution counted, d = dilution from which the first counts were obtained.
Results and Discussion
Temperature
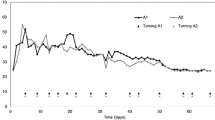
Temperature is a good indicator of the various stages of the composting process. The process is divided into four phases based on temperature. The first stage is the mesophilic stage, where mesophilic organisms generate large quantities of metabolic heat and energy due to availability of abundant nutrients, but gradually this will pave the way for the dominance of thermophiles (> 45 °C). With depletion of food sources, overall microbial activity decreases and temperature falls to ambient, leading to the second mesophilic stage, where microbial growth are slower as readily available food is consumed. Finally, compost material enters the maturation stage. Figure 1 shows the changes of temperature profiles in the five co-compost mixtures during 8 weeks of composting process. Temperature increased sharply at the beginning of the composting process; all compost mixtures exceeded 40 °C in 24 h. The temperature remained above 40 °C for at least 6 days and gradually declined to the level of ambient temperature.
All the mixtures achieved thermophilic condition (>45 °C) on the first week of composting, with Combination 1 recorded the highest temperature of 56 °C, followed by Combination 2 (53.5 °C), Combination 3 (50 °C), Combination 4 (46.5 °C), and Combination 5 (45 °C). High temperatures support degradation of recalcitrant organics such as lignocellulose and elimination of pathogenic and allergenic microorganisms [15]. According to Ishak et al. [15], the optimum biodegradation rate in the compost piles occurred at 45–55 °C while optimum microbial activity occurred at 35–40 °C. Gajalakshimi and Abbasi [16] stated that the organisms which responsible for the composting process can either become inhibited or die if the temperatures in the pile rise above 60 °C.
Temperature increases as a function of metabolic heat evolution of the degrading microflora and heat conservation due to the naturally insulating property of organic waste [17, 18]. Availability of nutrient from organic materials for microbial activity play an important role for the compost to reach thermophilic temperature as temperature of a compost pile is primarily a product of the metabolic heat being generated in the pile from microbial activity [19]. The thermophilic stage continues until the heat production becomes lower than heat dissipation, due to the exhaustion of easily metabolizable substrates. However, compost pile temperatures can also be affected by physical characteristics of the materials being composted (bulk density and particle size), as well as chemical reactions [20]. A sufficiently compact compost pile in correlate to the bulk density and particle size of the raw material will have self-insulating properties which caused higher temperature during thermophilic phase [17, 18]. This is due to the heat generated by the microbial activity is retained in the composting mass. For this reason, temperature is not always a good parameter to predict microbial activity. Especially when comparing different compost pile set up from different combination of raw materials with different thermal properties.
Combination 1 recorded the highest temperature during thermophilic phase. This may due to the high metabolic heat generated in the pile from microbial activity, in conjunction with the high thermal properties from the combination of raw materials. But this did not reflect a higher biodegradation rate of this combination. When compared with the results for single raw material composting of CG, POMS and EFB reported by H’ng et al. [9], the highest temperature recorded in this study were higher than the composting temperature with single raw material [9]. This may due to two reasons: (1) higher microbial activity composting which triggered by the higher accessible form of nitrogen and carbon for the microbes from the mixing of more than one type of materials in the compost,(2) a sufficient compaction of the compost pile in correlate to the bulk density and particle size of the raw material combinations have created a higher self-insulating properties. EFB has the lowest bulk density (66.98 kg/m3), while coffee ground and POMS have the bulk density of 467.38 kg/m3 and 753.43 kg/m3, respectively [2]. The combinations of these raw materials, change the bulk density of the compost pile and indirectly affect the thermal properties. Mukhtar et al. [21] stated that the best range of bulk density at the beginning of composting is between 400 and 600 kg/m3.
pH
The changes of pH profiles during various stages of composting are shown in Fig. 2. Each compost mixture resulted different trends in pH values. The initial pH values of the compost in this research were ranged from 5.72 to 6.94 which were within the optimal range for composting as stated in various published research paper [19, 22, 23]. From different combinations of compost materials, the initial pH of these compost piles varies. It was observed that when EFB incorporated with coffee ground and POMS, the initial pH values were relatively lower compare to 100% EFB (with pH 8.6). This is due to the lower pH of coffee ground (pH 5.1) and POMS (pH 5.2) in nature compared to EFB.
Trends of pH profiles started with a low pH and increased, fluctuated, and then showed slight drop at the end of the process. Generally, the pH of compost piles with different combinations increased at the beginning of composting period and then slight drop with final values ranged from 6.21 to 7.32. After 60 days of co-composting process, the pH values of final compost were fall within the recommended range for organic fertilizer (5.0 to 8.0) according to the fortified organic fertilizer- specification from Department of Standards Malaysia [24]. During composting, pH change is predictable. With a rapid early activity, pH can rise up to approximately 8.5 because of ammonification,mineralization of organic nitrogen converted to ammonia nitrogen [25]. Whereas, a decrease in pH may be caused by increased production of organic acids or increased nitrification. During the fermentation processing that caused oxygen limitation, compost may also slightly drop in pH [25].
Total Organic Carbon, Total Nitrogen and C/N Ratio
Figure 3 shows the changes of total organic carbon, total nitrogen and C/N ratio profiles during 8 weeks of co-composting process. The compost mixtures contained 45.51%, 30.62%, 44.41%, 39.49% and 39.73% of total organic carbon by the end of the process, a carbon-based substance in the form which was largely inaccessible to most organisms.
In general, total nitrogen increase with time (Fig. 4). The compost mixtures initially contained low nitrogen content (0.96 to 1.56%), increased after 8 weeks of composting. This may due to the release of organic acid and nitrification that occurred during the composting process and subsequently accumulate the nitrogen content. Nitrate increased with time during the composting process due to the accumulation of nitrate as a result of the conversion of ammonia to nitrate during the nitrification process. Nitrification is defined as conversion of the most reduced form of nitrogen (NH3) to its most oxidized form (i.e. nitrate) and it is performed in two steps which are carried out by two different groups of microorganisms: the ammonia-oxidizing bacteria or archaea and the nitrite-oxidizing bacteria [26]. Nitrification occurs mainly during the maturation stage due to the elevated temperature and intense biodegradation of organic matter in the active phase of composting [27]. The final nitrogen content in the compost mixtures ranged from 1.9 to 2.87%. In addition, it has been reported that co-composting has better effect in preserving nitrogen content compare to single materials composting [28]. The loss of nitrogen as ammonia to the atmosphere was probably minimal in the study.
Organic matter decomposition is affected by the presence of carbon and nitrogen. Carbon to nitrogen ratio (C/N) is the ratio of the mass of carbon to the mass of nitrogen in a material. In composting, C/N ratio is considered to be the most important parameter, as it reflects the extent of the bio-transformations that took place in the compost in chemical terms [29]. Composting with a low initial C/N ratio could reduce the quantity of bulking agent used, yet require a longer composting period [28]. If carbon is present in excessive amounts relative to nitrogen where the C/N ratio is above the optimal range, microorganisms also need longer time to use the excess carbon and this resulting in slower decomposition. Mixing carbonaceous materials (EFB) with nitrogen rich materials (coffee grounds and POMS) produced co-compost substrates with initial C/N ratio ranged between 27.52 and 53.59. Although the initial C/N ratios in few of the compost mixtures were not within the optimum initial range (C/N ratio 25 to 30) to start the composting process, C/N ratio decreased gradually as time progressed. The reduction in C/N ratio was due to the mineralization of organic matters [30,31,32]. The distinct reduction of carbon content and increase of nitrogen content during co-composting process had caused a marked decline in the C/N ratio as shown in Fig. 5.
The proper balance of nutrients is vital to the composting process. If carbon and nitrogen are present in the proper ratio, the other nutrients also tend to be present in the acceptable amounts. For best performance, the compost pile requires the correct proportion of carbon for energy and nitrogen for protein production of composting microorganisms as mentioned by Guo et al. [16]. An ideal initial C/N ratio can produce good quality compost and reduce decomposing time. In single raw material composting reported by H’ng et al. [9] using EFB, coffee grounds and POMS, the materials were not effectively composted and required an extensive composting period. Even after 8 weeks of composting, the C/N ratio of EFB (C/N ratio 45) and CG (C/N ratio 25) compost pile did not reached lower 20 [9]. Addition of coffee grounds and POMS to EFB showed prominent changes. The C/N ratio was reduced to less than 20 within 5 weeks of co-composting process except Combination 1 and Combination 3. These 2 combinations had a higher initial C/N ratio compared to the other combinations. Microorganisms needed longer time to use to use the excess carbon and this resulting in slower decomposition.
C/N ratio has also been used as the primary indicator of compost maturity and quality. A mature compost should had a C/N ratio below 20/1 [33, 34]. The C/N ratio for treatment 50% EFB + 25% CG + 25% POMS was reduced to less than 20 after 4 weeks of co-composting process while the other treatments needed longer composting duration. The reduction of C/N ratio was greater by composting mixture of lignocellulosic materials as presented in this study when compared to single raw material composting reported by H’ng et al. [9]. Mixing EFB with coffee grounds and POMS has created a favorable condition for microbial growth that caused the rise in temperature of the compost piles. Mixing the compost substrates can facilitate and initiate rapid microbial activity which cause higher rate hydrocarbon substrate breakdown [35].
Holocellulose, Alpha-Cellulose, Hemicellulose and Lignin
Evident changes in holocellulose, alpha-cellulose, hemicellulose and lignin were found during the co-composting (Figs. 6, 7, 8, 9). After 60 days of co-composting, all the compost mixtures showed great loss in holocellulose, alpha-cellulose, hemicellulose and lignin content. Results shows that Combination 4 had the most reduction in hemicellulose and lignin content whilst Combination 2 had the most reduction of holocellulose and alpha-cellulose content. In contrast, compost mixture with 70% EFB showed the lowest degradation in the chemical composition. Compared to the alpha-cellulose and hemicelluloses degradation, the biodegradation of and lignin were considerably lower. Low degradation of lignin is due to its complex structure and association to cell-wall polysaccharides. Therefore, lignin requires longer time to be fully degraded. Lignin is one of the main constituents of plant cell walls, and its complex chemical structure makes it highly resistant to microbial degradation [36].
In general, the biodegradation rate in chemical composition was affected by the initial C/N ratio. The mixtures with initial C/N ratios within 25–35 showed the highest reduction in holocellulose, cellulose, hemicellulose and lignin content while compost mixture with initial C/N ratio more than 50 had a minimal reduction. There is no doubt that co-composting will create higher degradation rate compared to single raw materials composting, thus, proper mixing ratio of EFB, coffee grounds and POMS can create effective biodegradation process by optimizing the microbial activity in the piles and reached maturity in shorter time. Nevertheless, all the compost mixtures were in the range of mature level, C/N ratio < 20 by the end of process except Combination 3 with 70% of EFB mixture.
Microbial Populations During Composting
High microbial activities normally favor faster biodegradation of organic matter. Composting is normally populated by three general categories of microorganisms: bacteria, actinobacteria and fungi. The changes in the bacteria, fungal and actinobacteria population were shown in Tables 2, 3, and 4. The microbial diversity during composting may vary with the selection of composting materials and available nutrient composition [37]. It was found that the microbial populations were increased after mixing with the selected compost materials. Proper mixing enables to balance the carbon and nitrogen content in the compost piles that required by the microorganism to initiate the decomposition process.
In general, there was an increase in the microbial population in the early stages of composting as available nutrients and suitable environmental conditions of the composting were accessible. The bacterial was found to reach high population during early stage until third week of composting with CFU of 107 and 108 cfu/g. Then the bacterial population gradually decreased to 104 and 105 cfu/g and this might probably due to the depletion of accessible organic matters during maturation phase. According to Hassen et al. [38], the bacteria population started to drop mainly due to the thermophilic bacteria were inactivated after the thermophilic stage (temperature of 40–60 °C; while actinobacteria and fungi began to emerge. As shown in the Table 2, bacterial population increased and reached the peak at CFU of 108 CFU/g DW during the second week of composting. The higher the population, the more enzymes produced by bacteria to assimilate the organic matter in the piles thus lead to higher degradation [39]. Bacteria are mostly responsible on initial decomposition and heat generation in the compost. Among all the combinations, Combination 4 (50% EFB + 25% POMS + 25% CG) had the highest bacterial population throughout the composting process ranged from 104 to 108 CFU/g DW. This result was similar to the composting result using agricultural waste reported by Hassen et al. [38] and Chandna et al. [39], with the bacterial population ranged from 104 to 109 CFU/g DW.
The population fungal was lower than bacteria throughout the composting as illustrated in Table 3. Nevertheless, the total fungal load was high in all mixtures during the initial composting with CFU up to 106 CFU/g was recorded. Higher proportion of fungal population were noted on Combination 4 (50% EFB + 25% POMS + 25% CG) throughout the composting process which reached 106 CFU/g DW on week 6. The fungal population was slightly lower than municipal solid waste composting reported by Rebollido et al. [40], where the highest fungal population reported to reach 107 CFU/g DW. The fungal population was then slowly reduced throughout the 8 weeks of composting. As reported by Dashtban et al. [41], the degradation of complex and recalcitrant substrate such as lignin and cellulose are usually carried out by fungi. Ideal compost environment encourages the growth of fungal. A moderately high level of nitrogen is required for fungal growth and low nitrogen content is a limiting factor for the degradation of cellulose [42]. The statement was confirmed when lower fungal population was detected in 70% EFB mixture in the study,the initial C/N ratio of the mixture was more than 50 showed lower degradation rate in hemicellulose, cellulose and lignin throughout 8 weeks compost process.
As shown in Table 4, actinobacteria populations appeared to be high during the initial rose of temperature which happened in the first week with CFU of 106 CFU/g DW and toward the maturation phase of composting. The actinobacteria population increase during maturation phase and were visible on the surface of the compost. Actinobacteria play an important role in the degrading natural polymers process and colonize organic materials after bacteria and fungi have consumed easily degrade fractions during maturation phase [40]. Since actinobacteria were able to tolerate higher temperatures, the initial increase of the population was believed due to the growth of the thermophilic actinobacteria [43]. Although their ability to degrade cellulose and lignin is not as high as fungi, actinobacteria are important agents of lignocellulose degradation during peak heating [41, 44, 45]. Mixture with 70% EFB showed the lowest population of actinobacteria at week 8.
Initially, all the combinations of substrates before microbial inoculation were colonized in major proportion by bacteria (104–106 CFU/g DW), followed by fungi (103–104 CFU/g DW) and in lower number by actinomycetes (102–103 CFU/g DW). The numbers of bacterial CFU were higher than fungal CFU throughout the composting process. Bacteria are nutritionally also the most diverse group of compost microorganisms, using a broad range of enzymes to chemically degrade a variety of organic materials, as a result, bacterial population are usually much higher than number of other microorganisms [42]. Consequently, bacteria are responsible for the most of initial decomposition and heat generation in compost, provide the major growth requirements are met.
The increase in bacterial and fungal population demonstrated during the mesophilic phase, was influenced fundamentally by temperature. Mesophilic fungi and bacteria are the dominant active degraders of fresh organic materials. The high surface/volume ratio of bacteria allows a rapid transfer of soluble substrate into a cell [46]. During thermophilic phase (compost reaching highest temperature), higher population of bacteria (107–108 CFU/g DW) was observed, followed by fungi (105–106 CFU/g DW) and actinomycetes (105–106 CFU/g DW) populations. Nevertheless, actinobacteria are commonly identified as one the main groups responsible for organic matter conversion during latter stages of composting with the highest population of 105 CFU/g DW according to Rebollido et al. [42]. The higher population of actinobacteria (104–106 CFU/g DW) compared to bacterial (104–105 CFU/g DW) and fungal (103–104 CFU/g DW) during latter stages was also reflected in this study.
Conclusion
Conclusively, the observations of physicochemical properties change with different ratio combinations of EFB, coffee grounds and POMS provide significant understanding on the suitability conditions and nutrients sufficiency required by the microbes to achieve effective composting. The composting duration for the lignocellulosic materials was reduced through the accelerated co-composting method which combines two distinct technologies; (i) co-composting with combination of different lignocellulosic materials and (ii) inoculation of the co-composting with mixture of microorganism. All the mixtures achieved thermophilic phase on the first week of composting, with Combination 1 (50% EFB + 50% CG) recorded the highest temperature of 56 °C. The mixtures with initial C/N ratios within 25–35 showed the highest reduction in holocellulose, cellulose, hemicellulose and lignin content while compost mixture with initial C/N ratio more than 50 had a minimal reduction. The C/N ratio for treatment 50% EFB + 25% CG + 25% POMS was reduced to less than 20 after 4 weeks of co-composting process while the other treatments needed longer composting duration. During thermophilic phase, higher population of bacteria (107–108 CFU/g DW) was observed, followed by fungi (105–106 CFU/g DW) and actinomycetes (105–106 CFU/g DW) populations. However, higher population of actinobacteria (104–106 CFU/g DW) compared to bacterial (104–105 CFU/g DW) and fungal (103–104 CFU/g DW) was found during latter stages. By reducing both the time and space required for composting lignocellulosic food industry waste, this accelerated co-composting method may be a viable option for food industries searching for a long-term, practical solution for solid biowaste disposal issues. Further studies should be conducted on the thermal properties of the compost materials with different sizes especially on the effect on microbial activity and degradation rate.
References
Acevedo, F., Rubilar, M., Scheuermann, E., Cancino, B., Uquiche, E., Garcés, M., Inostroza, K., Shene, C.: Bioactive compounds of spent coffee grounds, a coffee industrial residue. Presented at the III Symposium on Agricultural and Agroindustrial Waste Management, 12–14 Mar, Sao Pedro, Brazil (2013)
Chai, E.W., H’ng, P.S., Peng, S.H., Wan-Azha, W.M., Chin, K.L., Chow, M.J., Wong, W.Z.: Compost feedstock characteristics and ratio modelling for organic waste materials co-composting in Malaysia. Environ. Technol. 34, 2859–2866 (2013)
Lopez-Gonzalez, J.A., Vargas-García, M.D.C., Lopez, M.J., Suarez-Estrella, F., Jurado, M., Moreno, J.: Enzymatic characterization of microbial isolates from lignocellulose waste composting: chronological evolution. J. Environ. Manag. 145, 137–146 (2014)
Paradelo, R., Moldes, A.B., Barral, M.T.: Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 116, 18–26 (2013)
Oviasogie, P.O., Aisueni, N.O., Brown, G.E.: Oil palm composted biomass: a review of the preparation, utilization, handling and storage. Afr. J. Agric. Res. 5, 1553–1571 (2010)
Thambirajah, J.J., Zulkafli, M.D., Hashim, M.A.: Microbiological and biochemical changes during the composting of oil palm empty fruit bunches Effect of nitrogen supplementation on the substrate. Bioresour. Technol. 52, 133–134 (1995)
Averous, L., Pollet, E.: Biodegradable polymers. In: Averous, L., Pollet, E. (eds.) Environmental Silicate Nano-biocomposites, pp. 13–39. Springer, Houten (2012)
Gao, J., Yang, J.P., Yang, H.: Optimal C/N ratio of pig manure-rice straw mixture for its composting with earthworm and maturity assessment of the mixture compost. Ying Yong Sheng Tai Xue Bao 23, 765–771 (2012)
H’ng, P.S., Chin, K.L., Chai, E.W., Peng, S.H., Wan-Azha, W.M., Halimatun, I., Go, W.Z., Khoo, P.S., Lee, C.L., Raja-Nazrin, R.A., Ashikin, S.N.: Evolution of organic matter within sixty days of composting of lignocellulosic food industry waste in Malaysia. Compost Sci. Util. 26, 16–26 (2018)
Wise, L.E., Murphy, M., Daddieco, A.A.: Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 122(2), 35–43 (1946)
Sudharsan, V.V., Ajay, S.K.: Composting of municipal solid waste (MSW) mixed with cattle manure. Int. J. Environ. Sci. 3, 2068–2079 (2013)
Nelson, D.W., Sommer, L.E.: Total Carbon, Organic Carbon and Organic Matter Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties, 2nd edn, pp. 595–599. ASA-SSSA, Madison (1982)
Bremner, J.M.: Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33 (1960)
Pelczar, M.J.J.R., Chan, E.C.S., Krieg, N.R.: Microbiology, 5th edn. Tata McGraw Hill Publishing Company Limited, New Dehli (2003)
Ishak, N.F., Ahmad, A.L., Ismail, S.: Feasibility of anaerobic co-composting empty fruit bunch with activated sludge from palm oil mill wastes for soil conditioner. J. Phys. Sci. 25, 77–92 (2014)
Gajalakshimi, S., Abbasi, S.A.: Solid waste management by composting: state of the art. Crit. Rev. Environ. Sci. Technol. 38, 311–400 (2008)
Haug, R.T.: The Practical Handbook of Compost Engineering. Lewis Publishers, Florida (1993)
Ahn, H.K., Richard, T.L., Choi, H.L.: Mass and thermal balance during composting of a poultry manure-wood shavings mixtures at different aeration rates. Proc. Biochem. 42, 215–223 (2007)
Wu, D.L., Liu, P., Luo, Y.Z., Tian, G.M., Mahmood, Q.: Nitrogen transformations during co-composting of herbal residues, spent mushrooms, and sludge. J. Zhejiang Univ. Sci. B 11(7), 497–505 (2010)
Ahn, H.K., Sauer, T.J., Richard, T.L., Glanville, T.D.: Determination of thermal properties of composting bulking materials. Bioresour. Technol. 100(17), 3974–3981 (2009)
Mukhtar, S., Kalbasi, A., Ahmed, A.: Composting: A Comprehensive Review. Kansas State University, National Agricultural Biosecurity Center (2004)
Guo, R., Li, G., Jiang, T., Schuchardt, F., Chen, T., Zhao, Y., Shen, Y.: Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 112, 171–178 (2012)
Dewes, T.: Effect pH, temperature, amount of litter and storage density on ammonia emission from stable manure. J. Agric. Sci. 127, 501–509 (1996)
Buijsman, E., Maas, H.F.M., Asman, W.A.H.: Anthropogenic NH3 emissions in Europe. Atmos. Environ. 21, 1009–1022 (1987)
Miller, F.C.: Composting as a process based on the control of ecologically selective factors. In: Metting, F.B. (ed.) Soil Microbial Ecology: Applications in Agricultural and Environmental Management, pp. 515–576. Marcel Dekker Inc, New York (1993)
Cáceres, R., Malińska, K., Marfà, O.: Nitrification within composting: A review. Waste Manage. 72, 119–137 (2018)
Salah, M., El-Haggar, P.E.: Sustainability of agricultural and rural waste management. In: Sustainable Ind. Des. Waste Manage., pp 223–260. Elsevier, Amsterdam (2007)
Li, Z., Lu, H., Ren, L., He, L.: Experimental and modeling approaches for food waste composting: a review. Chemosphere 93(7), 1247–1257 (2013)
Saber, M., Mohammed, Z., Badr-el-Din, S., Awad, N.: Composting certain agricultural residues to potting soils. J. Ecol. Nat. Environ. 3(3), 78–84 (2011)
Department of Standards Malaysia: Draft Malaysian Standard: Fortified Organic Fertilizers—Specification (2013)
Heilskov, A.C., Holmer, M.: Effects of benthic fauna on organic matter mineralization in fish-farm sediments: importance of size and abundance. ICES J. Mar. Sci. 58, 427–434 (2001)
Grigatti, M., Ciavatta, C., Gessa, C.: Evolution of organic matter from sewage sludge and garden trimming during composting. Bioresour. Technol. 91, 163–169 (2004)
Saad, N.F.M., Ma’min, N.N., Zain, S.M., Basri, N.E.A., Zaini, N.S.M.: Composting of mixed yard and food wastes with effective microbes. Jurnal Teknologi (Sci. & Eng.) 65(2), 89–95 (2013).
Mangkoedihardjo, S.: Revaluation of maturity and stability indices for compost. J. Appl. Sci. Environ. Manag. 10(3), 83–85 (2006)
Kala, D.R., Rosenani, A.B., Fauziah, C.I., Thohirah, L.A.: Composting oil palm wastes and sludge sewage for use in potting media of ornamental plants. Malaysia J. Soil Sci. 13, 77–91 (2009)
Nutongkaew, T., Duangsuwan, W., Prasertsan, S., Prasertsan, P.: Effect of inoculum size on production of compost and enzymes from palm oil mill biogas sludge mixed with shredded palm empty fruit bunches and decanter cake. Songklanakarin J. Sci. Technol. 36, 275–281 (2014)
Chen, Y.H., Chai, L.Y., Zhu, Y.H., Yang, Z.H., Zheng, Y., Zhang, H.: Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J. Appl. Microbiol. 112, 900–906 (2012)
Hassen, A., Belguith, K., Jedidi, N., Cherif, M., Boudabous, A.: Microbial characterization during composting of municipal solid waste. In: Proceedings of International Symposium on Environmental Pollution Control and Waste Management, Tunis (EPCOWM’2002), pp. 357–368 (2002)
Chandna, P., Nain, L., Singh, S., Kuhad, R.C.: Assessment of bacterial diversity during composting of agricultural byproducts. BMC Microbiol. 13, 99 (2013)
Rebollido, R., Martínez, J., Aguilera, Y., Melchor, K., Koerner, I., Stegmann, R.: Microbial populations during composting process of organic fraction of municipal solid waste. Appl. Ecol. Environ. Res. 6(3), 61–67 (2008)
Dashtban, M., Schraft, H., Qin, W.: Fungal bioconversion of lignocellulosic residues opportunities and perspectives. Int. J. Biol. Sci. 5, 578–595 (2009)
Tuomela, M., Vikman, M., Hatakka, A., Itavaara, M.: Biodegradation of lignin in a compost environment: a review. Bioresour. Technol. 72, 169–183 (2000)
Cross, T.: Thermophilic actinomycetes. J. Appl. Bacteriol. 31, 36–53 (1968)
Godden, B., Ball, A.S., Helvenstein, P., McCarthy, A.J., Penninckx, M.J.: Towards elucidation of the lignin degradation pathway in actinomycetes. J. Gen. Microbiol. 138, 2441–2448 (1992)
Crawford, J.H.: Composting of agricultural wastes: a review. Process Biochem. 18, 14–18 (1983)
Wang, L., D'Odorico P.: Decomposition and mineralization. In: Reference Module in Earth Systems and Environmental Sciences, Encyclopedia of Ecology, 2nd edn, vol. 2, pp. 280–285 (2013).
Acknowledgements
The authors are grateful for the financial support given by the Ministry of Education Malaysia (MOE) under the Higher Institution Centre of Excellence (HICoE) project at the Institute of Tropical Forestry and Forest Products. The authors also sincerely thank the postgraduate students that participated in the field sampling exercise
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chin, K.L., H’ng, P.S., Chai, E.W. et al. Valorization of Lignocellulosic Food Industry Waste in Malaysia by Accelerated Co-composting Method: Changes in Physicochemical and Microbial Community. Waste Biomass Valor 11, 4871–4884 (2020). https://doi.org/10.1007/s12649-019-00825-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00825-4