Abstract
Fucoidanase is a promising biocatalyst for several biotechnological applications. Crude fucoidanase production by Dendryphiella arenaria was optimized using a natural low-cost medium composed of Cystoseira trinodis and natural seawater. The results showed that seaweed biomass concentration and incubation period were the most significant factors affecting fucoidanase production. At the optimized conditions [seaweed biomass (4.25% w/v), seawater concentration (100% v/v), and incubation period (2 days)], the fucoidanase production was 3.43 U/mL. The crude fucoidanase exhibited a wide pH (3–9) stability with residual activity > 58%. The enzyme showed a good thermostability at 40 and 50 °C with half-lives of 239.02 and 115.52 min, respectively. Several parameters of thermal inactivation kinetics and thermodynamics were calculated, and suggested that the enzyme would be thermostable. Additionally, enzymatic extract containing fucoidanase was used for the enzymatic saccharification of the brown algal biomass in terms of seaweed particle size, solid/liquid ratio, and enzyme dosage. The maximum reducing sugars obtained was 57.11 mg/g. To the best of our knowledge, this is the first report regarding fungal fucoidanase optimization mediated saccharification of a brown seaweed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fucoidans are fucose containing sulphated polysaccharides synthesized by marine brown macroalgae. The steadily increasing research on fucoidans is primarily due to their various biological activities such as anticancer, antioxidant, anticoagulant, antidiabetic, anti-inflammatory, and immunomodulatory activities [1]. However, fucoidans are heterogenous polysaccharides with large molecules which restrict their application as new drug [2]. Thus, preparing low molecular weight fucoidans by enzymatic depolymerization may solve this problem, and could obviously decrease side effects and antigenicity caused by fucoidan [3]. Enzymatic degradation of fucoidan provides a substantial tool for the production of oligosaccharides as well as studying the structural properties of fucoidans.
Fucoidan-hydrolyzing enzymes are known as fucoidanases (EC 3.2.1.44) [2, 4]. Endo-fucoidanase catalyze the cleavage of the glycoside bonds in the core of fucoidan chain, leading to a rapid reduction in the molecular weight. On the other hand, exo-fucoidanase active over fucoidanase ends, releasing oligosaccharides with a little decrease in the molecular weight [4, 5].
Generally, there are a few studies have been performed regarding fucoidanase production, isolation, and characterization [2]. Fucoidanases are mainly produced by marine invertebrates [6], marine bacteria [7], and marine fungi [3, 8]. However, fucoidanases produced by these producers have low activity. Thus, an optimization study to enhance fucoidanase production would be of interest. Additionally, the production of fucoidanase by a fungal strain growing on a natural substrate will be of industrial demand for the cost-efficient bioprocessing of the renewable macroalgal biomass and the crude enzymes can be used directly. Crude enzymes have been used by many researchers to study the efficiencies of the preparations, and/or to investigate the kinetic and thermostability parameters [9,10,11].
Dendryphiella arenaria is an obligate marine fungus, that generally found associated with marine macroalgae [12, 13], and was reported as a potential source of fucoidanase [3, 10]. The aim of the present study was to develop a low-cost fermentation for the production of fucoidanase by D. arenaria in a natural medium composed of natural seawater and Cystoseira trinodis (a brown alga) as a low-cost, renewable, and environmentally friendly biomass. The effects of seaweed biomass concentration, seawater concentration, and incubation period were optimized using Box–Behnken experimental design. The pH stability, thermostability, and thermal inactivation kinetics and thermodynamics were determined for the crude fucoidanase. Additionally, the crude fucoidanase was evaluated for potential enzymatic saccharification of the macroalgal biomass. To the best of our knowledge, this is the first report on the optimization of fungal fucoidanase production using a natural low-cost medium.
Materials and Methods
Seaweed Material
Naturally growing benthic macroalga C. trinodis (Forsskål) C. Agardh (Phaeophyceae) was collected during summer from the Red Sea coast of Egypt. The seaweed biomass was air-dried and milled in a home blender.
Microorganism and Cultivation Conditions
The marine algicolous fungus D. arenaria Nicot was isolated from the marine red alga Palisada perforata collected from the Red Sea, Egypt [10]. D. arenaria was cultivated on potato dextrose agar plates prepared with natural seawater at 28 °C for 9 days. A fungal inoculum was prepared by collecting the fungal spores in sterilized natural seawater, and a final inoculum concentration of about 1 × 107 propagules/mL was counted by a haemocytometer [13].
Semi-solid State Fermentation of Macroalgal Biomass
Semi-solid state media were developed using autoclaved (121 °C, 20 min) natural seawater (salinity 42 PSU), and oven sterilized (120 °C, 90 min) macroalgal biomass in 40 mL glass bottles. The fermentation media were inoculated by 100 µL of fungal inoculum, and incubated at 28 °C with continuous shaking (120 rpm).
Box–Behnken Experimental Design (BBD)
Box–Behnken experimental design with three factors (each with three levels) was developed to optimize and investigate the influence of the selected variables (seaweed biomass, seawater concentration, and incubation period) on fucoidanase production in semi-solid state fermentation. The natural seawater was diluted using distilled water to develop three different levels: 100, 75, and 50% (v/v). Seaweed biomass was added at three concentrations: 1, 3, and 5% (w/v). The culture media were incubated for 2, 4, and 6 days. The levels of the three variables were coded as − 1, 0, and + 1 for low, medium, and high levels, respectively. A total of 17 experimental runs was carried out in duplicate and the average fucoidanase activity was assigned as response (Table 1). BBD is based on a second order polynomial model:
where Y is the predicted response; Xi and Xj are the independent variables; βo is the intercept (regression coefficient of the model); βi, βii, and βij are the linear, quadratic and interaction coefficients, respectively.
Determination of the Fucoidanase Activity
The fermentation broth was separated by centrifugation (6000 rpm, 10 min), and used for the determination of extracellular fucoidanase activity. Fucoidanase activity was determined by measurement of reducing sugars production from fucoidan using 3,5-dinitrosalicylic acid (DNS) method [14]. A 100 µL of the crude enzyme solution was mixed with 900 µL of fucoidan (extracted from Sargassum sp. [1], 0.5% w/v, dissolved in 50 mM citric acid-sodium citrate buffer, pH 6.0). The mixture was incubated at 50 °C for 30 min, and then the reaction was stopped by adding dinitrosalicylic acid reagent. The fermentation broth was mixed directly with DNS and used as control. The mixture was heated at 90 °C for 10 min, and then the tubes were cooled down, and the developed color was measured at 540 nm using UV–Vis spectrophotometer. The absorbance values were calculated using fucose as standard. One unit of fucoidanase activity (1 U) was defined as the amount of enzyme that released 1 µmol of reducing sugars per minute under the conditions of the proposed assay.
Validation of the Developed Model
Numerical optimization technique was applied to the results of BBD to define the most desirable combination of the fermentation variables to maximize fucoidanase production. Triplicate experiments were conducted under the optimum conditions predicted by the model. The experimental values were compared to the predicted results to confirm the validity and accuracy of the model.
Effect of pH on Fucoidanase Stability
The stability of crude fucoidanase as a function of pH was determined by mixing the fungal crude enzyme (1:1 v/v) with different buffer solutions. The pH buffer systems were citrate (pH 3, 4, and 5), phosphate (pH 6, 7, and 8), and glycine–NaOH buffer (pH 9, 10). The concentration of each buffer was 0.1 M. The mixture was incubated for 120 min at 5 °C, and then the residual enzyme activity was measured under standard assay conditions.
Effect of Temperature on Fucoidanase Stability
The thermal stability of crude fucoidanase was determined by incubating the fungal crude enzyme in a water bath at different temperatures from 40 to 70 °C, in the absence of the substrate, for 30 and 60 min. Then, the residual fucoidanase activity was measured under standard assay conditions.
Kinetics and Thermodynamics During Thermal Inactivation of Fucoidanase
The values of fucoidanase residual activity at each temperature were used to calculate the thermal inactivation rate constant (Kd min−1) from the slope of the curves in the first-order plot of ln (% residual activity, RA) versus time (t, min) according to the following equation [15,16,17]:
The half-lives of fucoidanase (t1/2, time where residual activity reaches 50%) were calculated as:
The D-values (the decimal reduction time), or the time required to maintain 10% RA was calculated as:
The Z-value (the temperature required to reduce the D-value by one logarithmic cycle) was calculated from the slope of the plot between log (D) versus temperature (°C) using the equation:
An Arrhenius plot (ln Kd vs. 1/T, T, absolute temperature) was used to estimate the value of deactivation energy (Ed) from the Arrhenius equation:
where R (gas constant) = 8.3145 J/mol/K.
The values of kd, and Ed were used to calculate the variation of enthalpy of inactivation (ΔH°, KJ/mol), Gibbs free energy (ΔG°, KJ/mol), and variation in entropy (ΔS°, J/mol/K) using the following equations:
where h is the Planck constant (11.04 × 10−36 J/min−1), and KB is the Boltzmann constant (1.3806 × 10−23 J/K).
Enzymatic Saccharification of the Macroalgal Biomass
Crude fucoidanase produced by D. arenaria under optimized conditions was used for the production of fermentable sugars from C. trinodis biomass without any chemical pretreatment. The macroalgal biomass was powdered and sieved into different particle sizes (1, 0.6, 0.2, and < 0.15 mm using mesh no. 18, 30, 70, and 100, respectively). A 100 mg of mechanically pretreated macroalgal biomass was mixed with 3 mL of sodium citrate buffer (pH 5.0, 0.1 M) and 100 µL of crude fucoidanase. The enzymatic reaction was incubated at 40 °C with agitation (100 rpm). After 24 h, the enzymatic hydrolysate was separated by centrifugation (6000 rpm, 20 min), and was used for the quantification of reducing sugars using DNS method. Similarly, the effects of different solid/liquid ratios (1:20, 1:30, 1:40,1:50, and 1:60 g/mL), and different enzyme loadings (1:1, 2:1, 3:1, 4:1, 5:1, 6:1, 7:1, 8:1 mL/g) were optimized.
Data Analysis
The statistical analysis was carried out using Design Expert 7.0.0 statistical software (Stat-Ease Inc., Minneapolis, USA). The experimental results were analyzed by multiple regression analysis, and analysis of variance (ANOVA). The significance was checked by the F-test at probability levels (p < 0.05). The values of enzymatic saccharification were expressed as mean ± SD. Multiple comparisons of means were performed by the Tukey’s B test.
Results and Discussion
The fucoidan degrading enzymes, or fucoidanases, produced by either micro- or macro-organisms have an extremely low activity [18]. In the context of fungi, marine algal-inhabiting fungi show superior fucoidanase activity in comparison to terrestrial fungi [10]. Thus, the marine algicolous fungus D. arenaria was used in the present study for fucoidanase optimization. A natural media composed of C. trinodis and seawater as renewable resources were evaluated for cost-effective enzymatic production. The fucoidan content of C. trinodis was determined as described by Hifney et al. [1] and was 6.89 ± 0.22% w/w.
Box–Behnken Design for Fucoidanase Optimization
Three important variables like seaweed biomass content, natural seawater concentration, and incubation period were considered as critical parameters and optimized through Box–Behnken design (BBD). Batch experiments were carried out with different combinations of the model parameters (each with three levels) in order to find the optimum combination of these parameters toward fucoidanase production. BBD matrix for actual and coded factors along with experimental and predicted values for fucoidanase production is shown in Table 1.
The empirical relationship between agarase activity and the three independent factors was obtained by backward elimination of insignificant variables, and represented by the following second order polynomial equation:
where A, B, C are coded levels of seaweed biomass, seawater concentration, and incubation period, respectively.
The goodness of fit of the model was checked by coefficient of determination (R2). The R2 value of 93.63%, indicates that the model is adequate and that only 6.37% of the total variation is not explained by the model (Table 2). The adjusted-R2 corrects R2 value according to the sample size and number of terms in the model. Adjusted- R2 value (89.80%) was found to be close to R2 value. Similarly, predicted-R2 (73.01%) is in reasonable agreement with adjusted-R2 value and reflected a good adjustment between the experimental and predicted results [1]. Adequate precision is a measure of the contrast in predicted response relative to its associated error, and its desired value is 4 or more [19]. Adequate precision of 12.96 is high, and indicted adequate signal. The Lack of fit test measures the failure of the model to represent experimental data at point which is not included in the regression analysis. Lack of fit test was non-significant (p > 0.05, Table 2), and it suggests that the model equation was adequate and could predict fucoidanase activity under any sets of combination of the tested variables.
The significance of each variable was checked by p-value (p < 0.05). The developed model appeared to be highly significant for the experimental results at very low probability value (p < 0.0001, Table 2). Seaweed biomass as the sole carbon source was definitely the most critical factor for fucoidanase synthesis, and exhibited low p-value (p = 0.0006, Table 2). The results showed that dilution of natural seawater with distilled water have no effect on fucoidanase production (p > 0.05), and indicted that the concentration of minerals in the natural seawater after dilution is suitable for the fungal growth as well as enzyme synthesis. Increasing incubation period showed significant positive effects on fucoidanase production (p = 0.0039, Table 2). However, fucoidanase production was negatively influenced with the interaction of incubation period and seaweed biomass (p = 0.03). The quadratic effect of the model parameters B2 and C2 were also found significant, suggesting little variation of their respective values will affect fucoidanase synthesis markedly.
Graphical Representation of the Response Surface Model
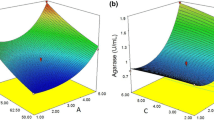
Three-dimensional response surface curves were plotted to explain the interaction of variables and to determine the optimum for maximum fucoidanase activity. Figure 1 depicts the response surface plots between each two factors, while the third factor was held constant in terms of its central value.
Figure 1a illustrates the effect of varying seaweed biomass concentration, and incubation period on fucoidanase activity at fixed level of seawater concentration (75% v/v). It suggested that fucoidanase production was improved as the percentage of seaweed biomass increased. The macroalgal biomass acts as a source of fucoidan for fucoidanase production. The enzymatic degradation of fucoidan satisfies the fungal requirements for carbon, sulfur, and energy [10]. In the current study maximum fucoidanase activity occurred at 2 or 6 days (Fig. 1a, b). The fungal growth and degradation of the macroalgal cell wall contributes to the release of algal metabolites into the fermentation medium such as polyphenolic compounds. Brown algal Phenolic compounds were recently reported as fucoidanase inhibitors [20], which may contribute to the week fucoidanase production after 4 days of incubation. On the other hand, the effect of seawater concentration (not statistically significant as reported by the model) on fucoidanase production was not evident (Fig. 1c).
Validation of the Optimized Conditions
In order to maximize fucoidanase production, a Derringer’s desirability function method was applied to detect the overall most desirable fermentation conditions [21]. Therefore, in the numerical optimization step, the goal for seaweed biomass (A), seawater concentration (B), and incubation period (C) was assigned as in range. A weight factor of 3 (equal importance) was chosen for all the factors.
Applying the methodology of the desired function, the optimum level for various fermentation parameters was: seaweed biomass (4.25% w/v), Seawater concentration (99.77% v/v), and incubation period (2 days), which give 3.45 U/mL of fucoidanase with an overall desirability value of 1.0 (Fig. 2). Triplicate experiments were carried out under the aforementioned conditions, and the fucoidanase activity was found to be 3.43 ± 0.4 U/mL. The experimental results are very close to the data obtained from optimization analysis using desirability functions, which indicates the adequacy of the developed quadratic models. Thus, BBD in corporate with desirability functions could be effectively used to optimize fucoidanase production from the marine fungus D. arenaria.
It is worth to mention here that the proposed method for fucoidanase production provides some advantages can be resumed as follows: decreasing the number of medium components, decreasing of fermentation time, enhancement of fucoidanase production, and decreasing the cost of the production medium. Additionally, the obtained fucoidanase value at optimized conditions (3.43 ± 0.4 U/mL) is higher than previously reported values. A maximum fucoidanase activity of 9.62 U/L (0.00962 U/mL) was reported by the terrestrial fungus Mucor sp. 3P grown on autohydrolyzed Fucus vesiculosus [5]. On the other hand, some algicolous fungi were able to utilize Sargassum sp. (autoclaved and filtered) in submerged fermentation and fucoidanase production was lower than 50 U/L (0.05 U/mL) [10].
In the current study, the purification of crude fucoidanase will increase the cost of the whole process, thus, pH stability, thermostability and biomass saccharification were performed on the crude extract of the enzyme.
Effect of pH on Fucoidanase Stability
Figure 3a depicts the pH stability of crude fucoidanase produced extracellularly in the fermentation broth in semi-solid state fermentation. The fucoidanase was highly stable in a wide range of pH, showing > 50% residual activity after preincubation in the pH range 3.0–9.0 for 120 min (Fig. 3a). At pH 6.0 and 7.0 full activity was regained for fucoidanase, while more than 80% of the initial activity remained at pH 5.0 and 8.0. Additionally, the residual activity of fucoidanase at pH 10.0 was more than one-third (37.93%). Wu et al. [3] reported that fucoidanase from D. arenaria TM94 in solid-state fermentation retained 60.8 and 80.5% of the maximum activity at optimum pH, at pH 3.0 and 8.0, respectively. Similarly, at pH 5.0 and 7.0, the enzyme in solid-state culture of Fusarium sp. LD8, retained 68.2 and 86.4% of the enzyme activity at pH 6, respectively [8]. Thus, fucoidanase produced in the current study through semi-solid state fermentation exhibited promising pH stability, and could be a potential candidate for different applications requiring broader pH stability ranges.
Effect of Temperature on Fucoidanase Stability
The effect of various temperature treatment on crude fucoidanase activity along time is depicted in Fig. 3b. The values of fucoidanase residual activity at different temperatures were used to obtain different kinetic and thermodynamic parameters. The enzyme showed higher activity after incubation at 40 °C for 30 and 60 min (Fig. 3b). At 50 °C fucoidanase exhibited moderate thermostability (residual activity = 69.7%) after 60 min of thermal pretreatment. However, there was a downscaling in the fucoidanase activity to 14.15 and 8.89% at 60 and 70 °C, respectively (Fig. 3b). Fucoidanase obtained in the present work lost its activity very slowly below 50 °C, while above 60 °C there was a remarkable increase in the rate of denaturation. Thus, at 40 °C, 239.02 min were necessary to reduce fucoidanase activity to 50% (t1/2), while at 70 °C only 17.2 min of treatment caused the same reduction (Table 3). The t1/2 value of fucoidanase at 50 °C was 115.52 min. Previous studies reported that at 50 or 56 °C, fucoidanases produced by Fusarium sp. LD8 and D. arenaria TM94 reached 50% inactivation after 1 h [3, 8]. A half-life of fucoidanase from a marine mollusk Lambis sp. was 20 min at 54 °C [6]. Additionally, fucoidanase from the marine bacterium Formosa algae was completely inactivated after 40 min of incubation at 55 °C [7]. Thus, fucoidanase from D. arenaria appeared to have a short range of thermostability, but was more thermostable than previously reported fungal fucoidanases.
From the results, it is clear that fucoidanase is less thermostable at higher temperature, thus, low t1/2, D-values, and higher rate constant (Kd) were obtained (Table 3). The representation of the log D-values against temperature allows to calculate Z-value. Generally, the Z-value is used to determine which the enzyme is sensitive to temperature increase (low Z-value) or more sensitive to the duration of thermal treatment (high Z-value). Therefore, the Z-value of 24.03 °C indicates that fucoidanase of D. arenaria is more sensitive to duration rather than temperature increase.
The activation energy (Ed) for thermal denaturation was 85.81 KJ/mol, which indicates that high energy is required for thermal denaturation of fucoidanase. The Ed value for fucoidanase is quite similar to some reported microbial enzymes [16]. The Ed value was used to calculate the thermodynamic values of variation of enthalpy (ΔH°). Positive ΔH° values were obtained with the investigated temperatures (Table 3), indicating the endothermic nature of the denaturation reaction. The ΔH° values are relatively high, suggesting that the enzyme would be thermostable. A slight decrease in enthalpy with increasing temperature is correlated to the disruption of the non-covalent linkages, including hydrophobic interactions in the enzyme structure [22]. Lower ΔH° at higher treatment temperature showed that the thermal inactivation of fucoidanase is easier. The variation in Gibbs free energy (ΔG°) is a more reliable measure of enzyme thermostability. The ΔG° values are significantly higher at 40 and 50 °C than at 60 and 70 °C. The high ΔG° values indicated high thermostability.
During thermal denaturation of enzymes, opening up of the enzyme structure is accompanied by an increase in the disorder or entropy (ΔS°), and thus positive values of ΔS° is obtained [17]. Since the ΔS° values were negative (Table 3), the native form of fucoidanase is more in ordered state. In addition, it indicates that there is a significant process of protein aggregation during denaturation [15]. Generally, when ΔH° is positive and ΔS° is negative, the process is not spontaneous at any temperature. Thus, deactivation of fucoidanase can be reversible between 40 and 70 °C. This result is similar to many fungal enzymes [11, 16]. However, no report has been found describing the thermodynamic properties of fucoidanase.
Enzymatic Saccharification of Macroalgal Biomass
Fungal fermentation broth containing crude fucoidanase produced at optimized conditions by D. arenaria in semi-solid state cultures was used for the enzymatic treatment of powdered C. trinodis biomass. The enzymatic saccharification was optimized in terms of macroalgal particle size, solid/liquid ratio (S/L), and enzyme loadings. The enzymatic reaction was performed at optimum pH 5.0 and temperature 40 °C was selected based on enzyme thermostability.
Figure 4a depicts the influence of particle size on the production of reducing sugars of the macroalgal biomass. The results revealed that particle size has a significant impact on enzymatic hydrolysis. The maximum reducing sugar yield was obtained at particle size 0.4 mm (Fig. 4a). Fucoidans in the brown algal cell wall are known to form complexes with polyphenolic compounds known as phlorotannins [1], which recently recognized as fucoidanase inhibitors [20]. Thus, brown algal metabolites liberated at smaller particle size might contribute to the decrease in the saccharification efficiency of fucoidanase.
Optimization of enzymatic saccharification of C. trinodis biomass using crude enzyme extract containing fucoidanase produced by D. arenaria in semi-solid state fermentation. Different values are mean ± SD. Different letters within a column indicate significant differences between samples at the level of p < 0.05
S/L ratio showed a little effect on the enzymatic treatment (Fig. 4b). The release of reducing sugars was significantly increased from 32.92 mg/g at S/L ratio of 1:20 g/mL to 39.11 mg/g at S/L ratio of 1:30 g/mL, but no further significant increase was observed (Fig. 4b). A S/L ratio of 1:60 g/mL was considered optimum, and was used for further optimization of the effect of the enzyme loadings. The maximum concentration of reducing sugars (57.11 mg/g) was recorded at enzyme loading of 6:1 mL/g. However, at higher enzyme loadings (7:1 and 8:1 mL/g, Fig. 4c), a significant decrease in the enzymatic hydrolysis was observed.
However, the use of fungal broth containing fucoidanases in the production of reducing sugars from the brown algae is not reported yet. In the present study, the maximum reducing sugars yield was 57.11 ± 0.33 mg/g using crude fucoidanase produced by D. arenaria. It was reported that acidic pretreatment combined with enzymatic hydrolysis using commercial enzyme blends yielded 120, and 200.8 mg/g biomass. Since brown algal cells contain different polysaccharides, the use of different enzyme blends of fucoidanase, alginate lyase, cellulase, laminarinase is important to obtain high sugar yields. Additionally, acidic pretreatment at high temperature causes the formation of toxic byproducts such as hydroxymethylfurfural, and formic acid, which act as inhibitors for the microbial fermentation [23]. Moreover, the use of acid increases the cost and time consumed for the whole process.
On the other hand, hydrothermal pretreatment does not involve hazardous or expensive chemicals, and may be applied before enzymatic hydrolysis. To test this theory, the macroalgal biomass was mixed with distilled water (1:30 g/mL) and autoclaved (121 °C, and 20 min) prior to enzymatic hydrolysis. Then crude fucoidanase at different enzyme loadings in sodium citrate buffer was mixed with the hydrothermally pretreated samples. The amount of reducing sugars after hydrothermal pretreatment was 5.5 ± 0.38 mg/g, which was increased to 42.38 ± 0.21 mg/g by enzymatic hydrolysis (enzyme loading: 7:1 mL/g; data not shown). The enzymatic treatment of the macroalgal sample without hydrothermal pretreatment yielded higher sugar concentration (57.11 ± 0.11 mg/g) than the hydrothermally pretreated sample. This result is mainly attributed to the inhibition of fucoidanase by the macroalgal metabolites released during hydrothermal pretreatment. Thus, the enzymatic conversion of the brown algal biomass into reducing sugars requires no hydrothermal pretreatment.
Conclusion
The aim of the present study was to develop a simple and cost effective crude fucoidanase production process by D. arenaria that could be utilized in the context of biosugars production. The production medium was based on C. trinodis and natural seawater as a low-cost, renewable, and environment friendly components. The crude fucoidanase produced may contribute to adding an economic value for the macroalgal biomass. Besides enzyme cost, the production of fucoidanase was enhanced to 3.43 U/mL using seaweed biomass (4.25% w/v), seawater concentration (100%), and incubation period (2 days). The enzyme showed promising pH stability and thermostability, which make fucoidanase in its crude form a potential candidate for many biotechnological applications. Additionally, the crude fucoidanase contributed to the production of fermentable sugars, which could be subsequently used for the production of fermentative chemicals such as bioethanol.
References
Hifney, A.F., Fawzy, M.A., Abdel-Gawad, K.M., Gomaa, M.: Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll. 54, 77–88 (2016)
Kusaykin, M.I., Silchenko, A.S., Zakharenko, A.M., Zvyagintseva, T.N.: Fucoidanases. Glycobiology 26, 3–12 (2015)
Wu, Q., Zhang, M., Wu, K., Liu, B., Cai, J., Pan, R.: Purification and characteristics of fucoidanase obtained from Dendryphiella arenaria TM94. J. Appl. Phycol. 23, 197–203 (2010)
Gurpilhares, D.D.B., Moreira, T.R., Bueno, J.D.L., Cinelli, L.P., Mazzola, P.G., Pessoa, A., Sette, L.D.: Algae’s sulfated polysaccharides modifications: potential use of microbial enzymes. Process Biochem. 51, 989–998 (2016)
Rodríguez-Jasso, R.M., Mussatto, S.I., Sepúlveda, L., Agrasar, A.T., Pastrana, L., Aguilar, C.N., Teixeira, J.A.: Fungal fucoidanase production by solid-state fermentation in a rotating drum bioreactor using algal biomass as substrate. Food Bioprod. Process. 91, 587–594 (2013)
Silchenko, A.S., Kusaykin, M.I., Zakharenko, A.M., Menshova, R.V., Khanh, H.H.N., Dmitrenok, P.S., Isakov, V.V., Zvyagintseva, T.N.: Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B 102, 154–160 (2014)
Silchenko, A., Kusaykin, M., Kurilenko, V., Zakharenko, A., Isakov, V., Zaporozhets, T., Gazha, A., Zvyagintseva, T.: Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, Formosa algae. Mar. Drugs 11, 2413–2430 (2013)
Qianqian, W., Shuang, M., Hourong, X., Min, Z., Jingmin, C.: Purification and the secondary structure of fucoidanase from Fusarium sp. LD8. Evid. Based Complement. Alternat. Med. 2011, 1–8 (2011)
Saqib, A.A., Hassan, M., Khan, N.F., Baig, S.: Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF). Process Biochem. 45, 641–646 (2010)
Gomaa, M., Hifney, A.F., Fawzy, M.A., Issa, A.A., Abdel-Gawad, K.M.: Biodegradation of Palisada perforata (Rhodophyceae) and Sargassum sp. (Phaeophyceae) biomass by crude enzyme preparations from algicolous fungi. J. Appl. Phycol. 27, 2395–2404 (2015)
Han, W., Lam, W.C., Melikoglu, M., Wong, M.T., Leung, H.T., Ng, C.L., Yan, P., Yeung, S.Y., Lin, C.S.K.: Kinetic analysis of a crude enzyme extract produced via solid state fermentation of bakery waste. ACS Sustain. Chem. Eng. 3, 2043–2048 (2015)
Abdel-Gawad, K.M., Hifney, A.F., Issa, A.A., Gomaa, M.: Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea Egypt. Hydrobiologia 740, 37–49 (2014)
Hifney, A.F., Fawzy, M.A., Abdel-Gawad, K.M., Issa, A.A., Gomaa, M.: In vitro comparative evaluation of antioxidant activity of hydrophobic and hydrophilic extracts from algicolous fungi. J. Aquat. Food Prod. Technol. 26, 124–131 (2017)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Marín, E., Sanchez, L., Perez, M., Puyol, P., Calvo, M.: Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. J. Food Sci. 68, 89–93 (2003)
Pal, A., Khanum, F.: Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: characterization of immobilized enzyme. Process Biochem. 46, 1315–1322 (2011)
Mohapatra, B.R.: Kinetic and thermodynamic properties of alginate lyase and cellulase co-produced by Exiguobacterium species Alg-S5. Int. J. Biol. Macromol. 98, 103–110 (2017)
Kusaykin, M., Bakunina, I., Sova, V., Ermakova, S., Kuznetsova, T., Besednova, N., Zaporozhets, T., Zvyagintseva, T.: Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 3, 904–915 (2008)
Abdel-Gawad, K.M., Hifney, A.F., Fawzy, M.A., Gomaa, M.: Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 63, 593–601 (2017)
Silchenko, A.S., Imbs, T.I., Zvyagintseva, T.N., Fedoreyev, S.A., Ermakova, S.P.: Brown alga metabolites—inhibitors of marine organism fucoidan hydrolases. Chem. Nat. Compd. 53, 345–350 (2017)
Fawzy, M.A., Gomaa, M., Hifney, A.F., Abdel-Gawad, K.M.: Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 157, 1903–1912 (2017)
Daniel, R.: The upper limits of enzyme thermal stability. Enzyme Microb. Technol. 19, 74–79 (1996)
Ravanal, M.C.A.D.C., Pezoa-Conte, R., Schoultz, S.V., Hemming, J., Salazar, O., Anugwom, I., Jogunola, O., Mäki-Arvela, P., Willför, S., Mikkola, J.-P., Lienqueo, M.E.: Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Res. 13, 141–147 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hifney, A.F., Gomaa, M., Fawzy, M.A. et al. Optimizing a Low-Cost Production Process of Crude Fucoidanase by Dendryphiella arenaria Utilizing Cystoseira trinodis (Phaeophyceae) and Enzymatic Hydrolysis of the Brown Algal Biomass. Waste Biomass Valor 10, 2773–2781 (2019). https://doi.org/10.1007/s12649-018-0333-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0333-7