Abstract
Dendryphiella arenaria TM94 is an obligate marine fungus. Fucoidanase expressed by TM94 by solid state fermentation was purified. The fermented solid medium was extracted with citric acid buffer, and the extracts were precipitated by acetone and separated on Sephadex G-100 chromatography. The specific fucoidanase activity of purified enzyme was 27-fold than that of the crude enzyme. The recovery of the enzyme was 17.69%. SDS-PAGE was used to identify the purity and the molecular weight of the fucoidanase. A single band appeared on SDS-PAGE gel which suggested that relatively pure fucoidanase has been obtained. The molecular weight of fucoidanase is 180 kDa and the isoelectric point was about pH 4.4. The purified fucoidanase appeared to have the maximum enzymatic activity at pH 6.0. KM and the maximum velocity of the enzyme was 6.56 mg·mL−1 and 6.55 mg·mL−1·min−1 by using fucoidan from Fucus vesiculosus as substrate. The enzyme may be a type of endo-fucoidanase which could hydrolyze high molecular weight fucoidan to low molecular weight fucoidan rather than to fucose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fucoidan, a sulfated polysaccharide, is extracted from brown algae. Studies have indicated that fucoidan has some biological activities, such as anticoagulant (Albuquerque et al. 2004; Mourao 2004), antithrombotic/antithrombin activity (Nishino et al. 2000), antiviral (Schaeffer and Krylov 2000), and other activities (Maruyama et al. 2003).
Fucoidan, a heterogeneous polysaccharide, has a high molecular weight and is highly sulfated. As the fucoidan molecule is too large to be used as clinical drugs, investigators are interested in preparing low molecular weight fucoidan (LMWF) that could obviously decrease side effects and antigenicity caused by fucoidan. LMWF activities depend on the molecular mass, sulfate content, and sugar constituents (Nishino et al. 1994; Chevolot et al. 1999).
LMWF can be prepared from fucoidan by enzymolysis or acidolysis but it is difficult to control the size of LMWF and its sulfated degree by the latter. The enzymatic method may be better. Fucoidanase can hydrolyze fucoidan to produce sulfated LMWF without removal of its side substitute groups because of its substrate hydrolysis property. So it could additionally be used as a tool for elucidating the structure of fucoidan.
Fucoidanase can be extracted from the hepatopancreas of invertebrates (Fujikawa et al. 1979; Kitamura et al. 1992), marine bacteria (Yaphe and Morgan 1959; Furukawa et al. 1992a,b; Bakunina et al. 2002; Sakai et al. 2002, 2003a,b,c,d; Wang et al. 2004; Urvantseva et al. 2006), and marine fungi (Wu et al. 2002; 2004; Rodríguez Jasso et al. 2008).
There are papers focusing on fermentation conditions of fucoidanase from marine fungi (Rodríguez Jasso et al. 2008) and marine bacteria (Sakai et al. 2004; Descamps et al. 2006), but few papers concerning fucoidanase purification. Only purifications of fucoidanase from hepatopancreas (Fujikawa et al. 1979) and marine bacteria have been reported (Furukawa et al. 1992a,b; Sakai et al. 2003a,b,c,d)
Our study focused on investigating the purification and characteristics of endo-fucoidanase from the marine fungus Dendryphiella arenaria TM94.
Materials and methods
Fucoidan from Fucus vesiculosus was obtained from Sigma Chemical Co. Different molecular weight of standard dextrans (5,000, 25,000, 80,000, 150,000, and 270,000 Da) were purchased from Sigma.
Dendryphiella arenaria TM94 was isolated from the sand of Baltic Sea in Germany. Solid state medium for TM94 consisted of 7.5 g wheat bran, 0.6 g kelp powder, 0.5 g glucose, 0.04 g NaNO3, and 7.5 mL artificial sea water in 250 mL flasks (Wu et al. 2002).
Sulfate content was analyzed with barium chloride-gelatin method (Kawai et al. 1969). The protein concentration was determined by the dye-binding method (Bradford 1976).
Fucoidanase activity
Fucoidanase activity was measured by the dinitrosalicylic acid technique (Miller 1959) to estimate the release of reducing sugars using the following reaction: a mixture consisting of 1 mL substrate solution (1% fucoidan from F. vesiculosus dissolved with 0.1 mol·L−1 citric acid–sodium citric buffer, pH 6.0) and 0.1 mL enzyme solution (crude extract or pure enzyme) was incubated at 50°C for 10 min, using inactivated enzyme solution as blank CK. One unit (IU) of fucoidanase activity is defined as the amount of enzyme that releases 1 μmol fucose per minute under the assay conditions.
Fucosidase activity
Fucosidase activity was measured under the following conditions: the reaction mixture contained 1 mL substrate solution (1% ρ-nitrophenyl-α-l-fucoside dissolved with 0.1mol·L−1 citric acid–sodium citric buffer, pH 6.0) and 0.1 enzyme solution (purified enzyme) was incubated at 40°C for 2 h. One unit (IU) of fucosidase activity is defined as the amount of enzyme that releases 1 μmol ρ-nitrophenyl per minute under the assay conditions.
Amylase assay
The reaction mixture containing 1 mL of 2% (w/v) soluble starch in acetate buffer (pH 5.5) and 1 mL of enzyme solution was incubated with shaking at 40°C for 30 min. The reaction was stopped by placing in boiling water for 5 min and after centrifugation the reducing sugar liberated into the supernatant was measured by the dinitrosalicylic acid method (Miller 1959). One unit (IU) of amylase activity is defined as the amount of enzyme that liberated 1 μmol reducing sugar (as glucose) per minute under the assay conditions.
Extraction, purification, and purity identification of fucoidanase
TM94 was cultured on the solid state medium at 28°C for 24 h for the preparation of fucoidanase. All the following steps were accomplished at 4°C except being indicated specifically. 50 g fermented culture medium was extracted with citric acid–citric sodium buffer (pH 6.0) for 0.5 h. After being filtrated through six layers carbasus, the filtrate was centrifuged at 3,500×g for 20 min, and then ice-cold acetone was added to the supernatant to a final concentration of 66.7% (v/v) with gentle stirring. Insoluble material was obtained by centrifugation at 8,000×g for 0.5 h. The precipitate was dissolved in pH 6.0, 0.1 mol·L−1 citric acid–citric sodium buffer and was centrifuged at 8,000×g for 0.5 h, and the clear solution was collected for use. The enzyme solution was concentrated to about 8 mL by low-temperature vacuum concentration and then loaded on to Sephadex G-100 column (2.5 × 100 cm), which has been balanced with 0.1 mol·L−1 citric acid–citric sodium buffer. The enzyme was eluted at room temperature at the flow rate of 0.33 mL·min−1, fractions was collected at 15 min intervals. The fractions with the highest enzymatic activity were pooled, concentrated by low-temperature vacuum concentration to 10 mL, dialyzed in deionized water, lyophilized, and stored at −18°C.
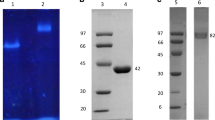
SDS-PAGE was performed to identify the purity of the purified enzyme and calculate the Mw of the proteins in the gel. SDS-PAGE was done with a 7.5% (w/v) polyacrylamide gel containing 20% (w/v) SDS, molecular markers used were myosin (220 kDa), α-2 macroglobulin(170 kDa), β-galactosidase (116 kDa) and transferrin (76 kDa), respectively. The protein was stained by Coomassie bright blue G-250.
Characteristics of the purified enzyme
The purified enzyme was characterized by isoelectrofocusing using cylindrical gel rods (0.5 × 8 cm) containing polyacrylamide gel (6.5% w/v), carrier ampholines (pH range 3–10, Amersham Pharmacia Biotech.) 0.1 mL was added to mixed solution, 0.2 mL 1% TEMED (N,N,N′,N′-tetramethyl-1,2-diaminomethane) and 1 mL 0.5% ammonium peroxydisulfate as gel polymerization regulator and activator, respectively. Protein was prefocalized in 100 V, 5 mA for 2 h, followed by 150–160 V, 4 mA for 2 h, which allowed for a sharp focalization of fucoidanase. The protein was stained by 0.5% amino black.
The pH relative activity of the fucoidanase was determined by detecting the fucoidanase activity over a pH range of 3–8 with addition of acid/base at 50°C. The pH stability of the enzyme was studied by assaying the residual activity after incubating the enzyme for 3 h at room temperature under different pH values. To obtain optimal reactive temperature, a mixture consisting of 1 mL substrate solution (1% fucoidan from F. vesiculosus dissolved with 0.1 mol·L−1 citric acid–sodium citric buffer, pH 6.0) and 0.1 mL enzyme solution was incubated at different temperatures for 10 min. For thermal stability study, 0.1 mL of enzyme solution was incubated at 30–80°C for 1 h, then cooled rapidly in an ice bath for 5 min, and then removed to 25°C. The residual enzyme activity was detected at 50°C for 10 min.
The initial velocity was used to calculate K M, and further to compare substrate specificity between F. vesiculosus fucoidan and Laminaria sp. fucoidan. V max was determined by quantitatively measuring the amount of reducing sugar at different time intervals.
HPGPC-ELSD analysis of hydrolysis product
The reaction mixture contained 50 IU mL−1 enzyme, 100 mg·mL−1 fucoidan and 0.1 mol·L−1 citric acid–citric sodium buffer (pH 6.0) in a final volume of 5 mL at 50°C for 30 min. The reaction was stopped by boiling for 10 min. After centrifugation at 8,000×g for 0.5 h, the supernatant was precipitated with three volume of 95% ethanol at room temperature. After centrifugation at 8,000×g for 0.5 h, the supernatant was freeze dried and used for next experiment.
The hydrolysates of fucoidan by the purified enzyme preparation were determined by HPLC (Waters Alliance, Waters 515 module), using TSK-GEL G4000PWXL column (7.8 × 300 mm, TOSOH Co.). Five microliters of different molecular weight of standard Dextrans (20 mg·mL−1), fucoidan (Sigma), fucoidan enzyme-hydrolyzed sample (20 mg·mL−1) were injected, elution was 0.01 mol·L−1 ammonium acetate aqueous solution at a flow rate of 1 mL·min−1 and detected with evaporative light scattering detector (ELSD, waters 2420 module), whose drift tube temperature was 55°C and nebulizer gas pressure was 30 psi.
Results
The crude enzyme extraction was purified by 66.7% acetone precipitation and Sephadex G-100 gel chromatography (Table 1). The purification fold of fucoidanase activity of 1 mg protein was enhanced from onefold to 26.67-fold while recovery rate was decreased from 100–17.69%. Two protein peaks (E1 and E2) were observed. E1 protein peak showed fucoidanase activities (Fig. 1). The fractions with the highest fucoidanase activity were pooled and concentrated to 10 mL. Fucoidanase-related enzymes such as fucosidase and amylase activity of purified protein were determined, but no related enzyme activity was observed (Table 2).
The isoelectric point of the enzyme was pH 4.4 (Fig. 2) The purified fucoidanase gave a single band on SDS-PAGE gel, which suggested that relatively pure fucoidanase had been obtained. The molecular mass of the fucoidanase was about 180 kDa by SDS-PAGE (Fig. 3). Molecular markers used were myosin (220 kDa), α-2 macroglobulin (170 kDa), β-galactosidase (116 kDa), and transferrin (76 kDa).
The effect of pH on the activity produced from TM94 is shown in Fig. 4. The maximum enzyme activity was at pH 6. The optimum pH of this enzyme was very close to that from marine fungus Fusarium sp. LD8 and Vibrio sp. N-5, while the optimum pH of fucoidanase from hepatopancreas of Patinopecten yessoensis was 5.5. The enzyme displayed stability at pH 6–7; whereas at pH 3.0 and 8.0, an activity loss of about 50% occurred after 4 and 6 h, respectively.
The optimum temperature for maximal activity of the fucoidanase was 50°C at pH 6.0. At 30°C the activity of fucoidanase decreased to 12.5%, while at 80°C to 18.75%. Fifty percent inactivation of the fucoidanase activity occurred at 56°C for 1 h, and total fucoidanase inactivation could be achieved at 100°C for 30 min.
The K M values of the enzyme for F. vesiculosus fucoidan and Laminaria sp. fucoidan determined by Lineweaver–Burk method were 6.56 mg·L−1 and 10.1 mg·mL−1, respectively. At the same time, the V max values for the both substrates were 6.55 and 6.47 mg·min−1·mL−1, respectively.
A HPGPC-ELSD method was used to determine the molecular weight of enzymatic products. The linear range of molecular weight of polysaccharide was 5–270 kDa Table 3), the dextran calibration curve function was Log (Mw) = −0.6231 RT + 9.6 (RT: retention time of dextran, R 2 = 0.9996). Figure 5 showed that there were three peaks in enzymatic products chromatograph. Peak I was the main enzymatic product, the proportion of its peak area was 85%; retention time of peak I was 9.397 and its weight molecular weight was about 5.6 kDa.
Sulfate content of peak I was 4.2% and it was lower than that of fucoidan from F. vesiculosus, which was 11% (from the production instruction and our experiments).
Discussion
Fucoidanase is an induced enzyme, and is produced by fucoidanase-producing organisms in fucoidan or fucoidan-containing medium. Fucoidanase can be found in marine bacteria such as Pseudomonas atlantica, Pseudomonas carrageenovora, Pseudomonas alteromonas, Vibrio sp., Bacillus sp. HTP2, Pseudoalteromonas citrea, (Sakai et al. 2002; Urvantseva et al. 2006); marine invertebrates such as Haliotis sp., sea cucumber (Sakai et al. 2003a,b,c,d), sponges and molluscs (Daniel et al. 1999), and marine fungi D. arenaria TM94 (Wu et al. 2002), Fusarium sp. LD8 (Wu et al. 2004), Aspergillus niger, Penicillium purpurogenum, Mucor sp. (Rodríguez Jasso et al. 2008). Fucoidanase has exo- and endo- enzyme (Kim et al. 2010; Furukawa et al. 1992a,b; Kitamura et al. 1992).
Exo-fucoidanase cleaves off monosaccharides from the end of the polysaccharide chain; endo-fucoidanase cleaves somewhere in the middle of the polysaccharide. The fucoidan from the sporophyll of Undaria pinnatifida was also degraded by fucoidanase from Sphingomonas aucimobilis PF-1 into seven fuco-oligosaccharides LMWF whose molecular weight ranged from 305–3,749 Da. The exact mechanism of these cleavages remains unknown even though the mechanism of cellulases shows a similar pattern (Holtkamp et al. 2009). In our study, 5.6 kDa LMWF produced by TM94 purified fucoidanase was obtained, which suggests that fucoidanase from TM94 have endo-type activity, and can cleave the high molecular weight fucoidan to LMWF. This may be a useful way to produce the low molecular weight fucoidan for clinical use.
Fucoidanase may have different molecular weights in different organisms. It was reported that the molecular masse of fucoidanases E1, E2, and E3 of Vibrio sp. N-5 were 39 kDa, 68 kDa, and 68 kDa, by SDS-PAGE (Furukawa et al. 1992a,b). The molecular weight of TM94 fucoidanase was higher than any of these, while it is close to that of fucoidanase (100–200 kDa) from hepatopancreas of P. yessoensis (Fujikawa et al. 1979).
The first step of the enzyme purification process is precipitation by ammonium sulfate. In our pre-experiments, when fucoidanase was treated by 60% saturated ammonium sulfate precipitation, the activity was almost zero, and recovery rate was 8%, while when the fucoidanase was purified with 66.7% acetone precipitation, the recovery rate was 42.1%. Therefore, acetone precipitation was used for fucoidanase purification. This effect of ammonium sulfate precipitation on fucoidanase may be because of the poor buffer capacity of ammonium sulfate.
Compared with fucoidanase of different origins, the enzyme activity of 1 mg purified fucoidanase from the marine fungus TM94 was about 27-fold by acetone precipitation and Sephadex G-100 chromatography than that of 1 mg crude fucoidanase. Fucoidanase from P. yessoensis was purified about 14 fold with 30–70% saturated ammonium sulfate precipitation with a recovery of 27% (Fujikawa et al. 1979). Fucoidanases E1, E2, and E3 of Vibrio sp. N-5 were purified 29-, 23-, and 51-fold with the recovery of 6.7%, 8.6%, and 10.2%, respectively (Furukawa et al. 1992a,b).
All of the fucoidanases from different microorganism have low enzymatic activity. Fucoidanase activity of TM94 (purified enzyme) was about 2.45 IU·mL−1, while that of A. niger PSH was from 0.0102–0.0138 U·L−1, Mucor sp. and P. purpurogenum GH2 were 0.005 and 0.004 U·L−1. Those results suggested that the enzyme activity may be underestimated because of active proteases (Rodríguez Jasso et al. 2008).
Fucoidanase from D. arenaria TM94 was more sensitive to pH and temperature. The catalytic activity of the fucoidanase of TM94 reached maximum at pH 6.0 which is very close to that of the fucoidanases from Vibrio sp. N-5, while the optimal pH of fucoidanase from hepatopancreas of P. yessoensis was 5.5. Below and above pH 6.0, the enzyme activity decreased rapidly in TM94. At pH 3.0 and 8.0, the enzyme retained 60.8% and 80.5% of the maximum activity at optimum pH, respectively. Compared with that of Vibrio sp. N-5 (37°C) and Flavobacteriaceae SW5 (room temperature), the fucoidanase from TM94 had a relatively higher optimal temperature (50°C). The temperature for loss of half the activity of TM94 fucoidanase was 56°C, while that of bacteria Vibrio sp. N-5 was 65°C (Furukawa et al. 1992a,b).
References
Albuquerque IRL, Queiroz KCS, Alves LG, Santos EA, Leite EL, Rocha HAO (2004) Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz J Med Biol Res 37:167–171. doi:10.1590/S0100-879X- 2004000200002
Bakunina IY, Nedashkovskaya OI, Alekseeva SA, Ivanova EP, Romanenko LA, Gorshkova NM, Sakov VV, Zvyagintseva TN, Mikhailov VV (2002) Degradation of fucoidan by the marine proteo-bacterium Pseudoalteromonas citrea. Microbiology 71:41–47
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chevolot L, Foucault A, Chaubet F, Kervarec N, Sinquin C, Fisher A, Boisson-Vidal C (1999) Further data on the structure of brown seaweed fucans: relationships with anticoagulant activity. Carbohydr Res 319:154–165
Daniel R, Berteau O, Jozefonvicz J, Goasdoue N (1999) Degradation of algal (Ascophyllum nodosum) fucoidan by an enzymatic activity contained in digestive glands of the marine mollusc Pecten maximus. Carbohydr Res 322:291–297
Descamps V, Colin S, Marc L, Jam M, Richard C, Potin P, Barbeyron T, Yvin J, Kloareg B (2006) Isolation and culture of a marine bacterium degrading the sulfated fucans from marine brown algae. Mar Biotechnol 8:27–39
Fujikawa T, Koyabu K, Wada M (1979) Enzymes in hepatopancreas of abalone active on fucoidan(1), crude enzyme and unabsorbed fraction on CM-cellulose. Nippon Nogei Kagakukaishi (In Japanese) 53:87–95
Furukawa S, FujikawaT KD, Ide A (1992a) Production of fucoidan-degrading enzymes, fucoidan- ase, and fucoidan sulfatase by Vibrio sp. N-5. Nippon Suisan Gakkaishi 58:1499–1503
Furukawa S, Fujikawa T, Koga D, Ide A (1992b) Purification and some properties of exo-type fucoidanase from Vibrio sp. N-5. Arch Biotech Biochem 56:1829–1834
Holtkamp AD, Kelly S, Ulber R, Lang S (2009) Fucoidans and fucoidanases—focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol 82:1–11
Kawai Y, Seno N, Anno K (1969) A modified method for chondrosulfatase assay. Anal Biochem 32:314–321
Kim WJ, Koo YK, Jung MK, Moon HR, Kim SM, Synytsya A, Yun-Choi HS, Kim YS, Park JK, Park Y (2010) Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Arch Pharm Res 33:125–131
Kitamura K, Masaru M, Yasui T (1992) Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci Biotechnol Biochem 56:490–494
Maruyama H, Tamauchi H, Hashimoto M, Nakano T (2003) Antitumor activity and immune response of Mekabu fucoidan extracted from sporophyll of Undaria pinnatifida. In Vivo 17:245–249
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mourao PAS (2004) Use of sulfated fucans as anticoagulant and antithrombotic agents: future perspectives. Curr Pharm Des 10:967–981
Nishino T, Nishioka C, Ura H, Nagumo T (1994) Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr Res 255:213–224
Nishino T, Yamauchi T, Horie M, Nagumo T, Suzuki H (2000) Effects of a fucoidan on the activation of plasminogen by u-PA and t-PA. Thromb Res 99:623–634
Rodríguez Jasso RM, Aguilar Gonzalez CN, Pastrana L, Teixeira JA (2008) Identification and evaluation of fungal strains with fucoidan degradation potential. Proceedings of the 10th International Chemical and Biological Engineering Conference-CHEMPOR 2008 E. C. Ferreira and M. Mota. Braga, Portugal, Universidade do Minho, pp 2106–2109
Sakai T, Kimura H, Kato I (2002) A marine strain of flavobacteriaceae utilizes brown seaweed fucoidan. Mar Biotechnol 4:399–405
Sakai T, Ishizuka K, Kato I (2003a) Isolation and characterization of a fucoidan-degrading marine bacterium. Mar Biotechnol 5:409–416
Sakai T, Ishizuka K, Shimanaka K, Ikai K, Kato I (2003b) Structures of oligosaccharides derived from Cladosiphon okamuranus fucoidan by digestion with marine bacterial enzymes. Mar Biotechnol 5:536–544
Sakai T, Kimura H, Kato I (2003c) Purification of sulfated fucoglucuronomannan lyase from bacterial strain of Fucobacter marina and study of appropriate conditions for its enzyme digestion. Mar Biotechnol 5:380–387
Sakai T, Kimura H, Kojima K, SK IK, Kato I (2003d) Marine bacterial sulfated fucoglucurono-mannan (SFGM) lyase digests brown algal SFGM into trisaccharides. Mar Biotechnol 5:70–78
Sakai T, Kawai T, Kato I (2004) Isolation and characterization of a fucoidan-degrading marine bacterial strain and its fucoidanase. Mar Biotechnol 6:335–346
Schaeffer DJ, Krylov VS (2000) Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 45:208–227
Urvantseva A, Bakunina I, Nedashkovskaya O, Kim S, Zvyagintseva T (2006) Distribution of intracellular fucoidan hydrolases among marine bacteria of the family Flavobacteriaceae. Appl Biochem Microbiol 42:484–491
Wang P, Cai J, Qin S, Wu Q, Wu K, Wang R, Xu D (2004) Fermentation of marine bacterium Bacillus sp. H-TP2 for fucoidanase and enzyme properties. Food and Fermentation Industry (In Chinese) 30(3):13–15
Wu K, Wu Q, Yan J, Liu B, Yang B, Zhang J, Qin S, Pan R, Cai J, Meiner M (2002) Production of fucoidanase from the marine fungus Dendryphiella arenaria TM94 by Solid Substrate Fermentation. European Meeting on Marine Biotechnology 5:12–14. Nantes, France
Wu Q, Cai J, Wu K, Qin S (2004) Solid state fermentation of fucoidanase of marine fungus LD8. Mar Sci (In Chinese) 28(6):23–27
Yaphe W, Morgan K (1959) Enzymic hydrolysis of fucoidin by Pseudomonas atlantica and Pseudomonas carrageenovora. Nature 183:761–762
Acknowledgment
This work was supported by funds from Anhui Provincial Nature Science Foundation (No: 03043104), Anhui Provincial Excellent Youth Science and Technology Foundation (No: 04043051), PRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Zhang is co-first author.
Rights and permissions
About this article
Cite this article
Wu, Q., Zhang, M., Wu, K. et al. Purification and characteristics of fucoidanase obtained from Dendryphiella arenaria TM94. J Appl Phycol 23, 197–203 (2011). https://doi.org/10.1007/s10811-010-9588-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9588-5