Abstract
Purpose
The purpose of study was utilization of sugarcane bagasse, waste product of sugarcane industry, for production of xylooligosaccharides (XOS) and their evaluation of their prebiotic potential.
Methods
The XOS production was carried out in two steps: alkaline extraction of xylan (5, 10 and 15%KOH and NaOH) followed by acid hydrolysis (0.25 and 0.50M H2SO4; 20, 40 and 60 min) of xylan, and quantified using high performance thin layer chromatography (HPTLC). The prebiotic potency of XOS was evaluated for probiotics viz. Lactobacillus brevis, Lactobacillus acidophilus and Lactobacillus viridescens in comparison to standard fructooligosaccharides (FOS).
Results
The chemical compositional analysis indicated that bagasse contain 28.42% hemicellulose out of which 21.46% was estimated to be xylan. Maximum yield of xylan (20.5%) was obtained with 15%NaOH treatment. The best treatments for xylan hydrolysis were found to be 0.25M H2SO4 for 20 and 40 min with concentrations of xylose, xylobiose and xylotriose to be 2.014, 2.106 and 1.228 mg ml− 1, respectively, in 20 min hydrolysis and 2.138, 1.502 and 0.824 mg ml− 1, respectively, in 40 min hydrolysis. XOS were found to be better prebiotics than standard FOS. Pure xylobiose was found to have highest positive effect on growth of all three bacteria tested indicating that effects of XOS were due to presence of xylobiose, xylotriose and XOS with higher degree of polymerization in xylan hydrolysates.
Conclusions
Sugarcane bagasse xylan can be converted into XOS only by controlled acid hydrolysis leading to increased production of XOS which can be used as good prebiotics in drugs and food ingredients after their purification eliminating all the acidic and alkaline residues and also side products.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or limited number of bacteria in colon and thus perk up the health of host [1]. The functional properties of prebiotics include maintenance of gut microbiota and stimulation of intestinal transit, change in colonic microbiota contributing to normal stool consistency leading to prevention of diarrhea and constipation [2], reduction in the level of triglyceride content of blood and liver [3], stimulation of growth of Bifidobacterium [4] and improved nutrient absorption and production of B complex vitamins. All the health benefits together lead to improvement in the immune system [5]. There are numerous prebiotics having diverse origins and chemical properties. Stowell [6] classified the prebiotics based on a set of common criteria. Inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), lactulose and polydextrose were recognized as the established prebiotics whereas isomaltooligosaccharides (IMO), xylooligosaccharides (XOS) and lactitol were categorized as emerging prebiotics. XOS, although classified as emerging prebiotics, may present the same or more desirable properties than the established prebiotics [7].
The XOS occur in very low amounts naturally in most plants. XOS have favorable technological features, including stability at acidic pH, heat resistance, the ability to achieve significant biological effects at low daily doses, low calorie content and non-toxicity [8]. XOS with fewer than four monomer units are relatively new type of non-digestible sugars which have gained a lot of interest recently because they promote the proliferation of bifidobacteria, which are considered as beneficial microorganisms in human intestine [9]. XOS have prebiotic effects when consumed as a part of diet. Four grams of XOS per day for three weeks improves the gut micro biota among people who are above 65 years old [10]. The non-digestible XOS can be incorporated into processed foods and could be promising functional ingredients in nutraceutical products [11]. Their utility is not limited to their potential nutraceutical properties but also for their economic benefits as they provide an opportunity for agro-food industries to produce value added products from wastes and thereby improve the environment through agro-food waste management.
XOS with varying degrees of polymerization can be produced by hydrolysis of xylan polymer chain which is the major constituent of hemicellulose fraction of lignocellulosic materials. Moreover, it will be quite beneficial to isolate them from natural sources like agricultural wastes as it will give dual advantage of production of valuable XOS and utilization of waste materials. Sugarcane bagasse (Saccharum officinarum), the fibrous residue obtained after extracting the juice from sugarcane in the sugar production process, forms an important waste product of sugar milling industry. It is primarily composed of cellulose, hemicellulose and lignin. Hemicellulose fraction is an amorphous polymer with xylan as the major constituent, which is actually a heteropolysaccharide with varying proportions of d-xylose, d-arabinose, d-galactose and d-mannose. These monosaccharides are linked by an assorted combination of ether bonds and weak hydrogen bonds which can be readily hydrolyzed in acid or alkaline conditions. Sugarcane bagasse has 24–29% hemicellulose represented by 1-arabino-(4-O-methyl-d-glucurono)-d-xylan [12]. The advantages of using bagasse as a substrate for production of XOS are that it is available at the site of processing, cheap and its supply in the sugar industry is constant though seasonal. Because of its lower ash content i.e. 1.9% [13], bagasse offers numerous advantages compared with other agro-based residues such as paddy straw (16%), rice straw (14.5%) and wheat straw (9.2%). Therefore, because of the importance of sugarcane bagasse as an industrial waste, there is great interest in production of XOS that offer economic and environmental advantages.
Till date a wide number of raw materials are tested for XOS production including corncobs [14], tobacco stalk [15], rye straw [16], and Bengal gram husk and wheat bran [17]. Sugarcane bagasse has been investigated as raw material for XOS production by enzymatic hydrolysis [18]. In the present study, the production of XOS by dilute acid hydrolysis of sugarcane bagasse xylan was explored followed by the prebiotic effects of XOS for growth of three different species of Lactobacillus as the utilization of bagasse generated XOS by lactobacilli has not been studied in details.
Materials and Methods
Biological Material
Sugarcane bagasse, obtained from local sugar mill, was dried at 60 °C till constant weight and then ground to pass 45-mesh sieve. Fine powdery material obtained was put into plastic bags and stored in desiccator at room temperature until used.
Reference Standards and Chemicals
Xylose, glucose and fructooligosaccharides (FOS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Xylobiose was purchased from TCI chemicals Pvt. Ltd. All other chemicals, reagents and solvents used in the study were of analytical grade and obtained from SD Fine-Chem Limited, Mumbai, India.
Bacterial Cultures
For studying prebiotic potency of XOS, the cultures of three different species of Lactobacillus i.e. Lactobacillus brevis (NCIM 2584), Lactobacillus acidophilus (NCIM 5426), Lactobacillus viridescens (NCIM 2167) were purchased from National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory (NCL), Pune, India.
Instrumentation and Characterization
HPTLC (High performance thin layer chromatography) instrument obtained from M/s CAMAG, Switzerland involved Linomat-5 applicator, Camag–Hamilton Linomat syringe (100 ml), Camag ADC 2 Automatic Developing Chamber, UV lamp with cabinet, TLC scanner 3 with winCATS version 1.4.2.8121 evaluation software. Precoated silica gel (60 F254) TLC aluminum sheets (Catalogue No: 1.05554.0007) were obtained from M/s Merck, Darmstadt, Germany. The absorbance values were recorded on UV–Vis spectrophotometer (Shimadzu UV-1800).
Determination of Chemical Composition of Sugarcane Bagasse
The chemical composition of sugarcane bagasse was determined using detergent system method [19] using Fibra plus FES6 (Pelican Equipments, India). The laboratory analytical procedure (LAP) for standard biomass analysis of National Renewable Energy Laboratory (NREL) was used for complete compositional analysis of bagasse sample [20].
Extraction of Xylan from Sugarcane Bagasse
Sugarcane bagasse was given different alkaline treatments (5, 10 and 15%, w/v of KOH and NaOH) with solid:liquid ratio of 1:10 under steam application (121 °C, 15 psi, 20 min). The extract was separated by vacuum filtration and centrifuged at 5000 rpm for 20 min followed by neutralization (pH 5.0) with glacial acetic acid and precipitation with three volumes of cold ethanol. The xylan precipitated was centrifuged at 8000 rpm for 10 min at 4 °C, dried at 60–70 °C until constant weight, weighed, powdered by grinding and stored at room temperature for further analysis. The recovery (%) of xylan was calculated. Xylan was structurally characterized by FT-IR, 1H and 13C NMR spectra.
Hydrlolysis of Xylan into Xylose and Xylooligosaccharides
Xylan powder (0.2 g) was treated with 10 ml of dilute acid solutions (0.25 and 0.50M H2SO4) under steam application (121 °C, 15 psi) for different time intervals i.e. 20, 40 and 60 min (for each concentration of acid). The reaction mixtures were neutralized by adding calcium carbonate and the supernatants were collected by filtration with Whatman filter paper number 1. The resultant supernatants (xylan hydrolysates) from each treatment were subjected to qualitative and quantitative analysis by high performance thin layer chromatography (HPTLC). Total reducing sugars were also quantified from xylan hydrolysates using DNS method [21] using xylose as standard.
Qualitative and Quantitative Analysis of Xylan Hydrolysates Using High Performance Thin Layer Chromatography (HPTLC)
HPTLC was performed on 10 cm × 10 cm silica gel 60 F254 TLC plates. Appropriately diluted samples were applied to the plates as 8 mm bands, 6 mm apart, 10 mm from the bottom, and at least 15 mm from the edges of the plate. The distance between the tip of the syringe and TLC plate was fixed at 1 mm for sharp application of bands. Nitrogen gas was used for drying the spots. Loaded plates were viewed inside the cabinet under UV light (254 nm) to ensure proper application before development.
The solvent system used for development of the plate was 2-propanol, ethyl acetate, nitromethane and water (6:1:1:2, v/v). The plates were placed gently in developing chamber, previously pre-saturated with the mobile phase (plate preconditioning for 10 min and tank saturation with 10 ml mobile phase for 10 min). Potassium thiocyanate (KSCN, 800 ml) was used for humidity control in the developing chamber.
After development by single ascent, orcinol reagent (0.2% w/v orcinol in methanol:sulfuric acid, 90:10) was sprayed over the dried plates and color development was undertaken after incubating the plates in oven at 105 °C for 3–4 min. The developed TLC plates were scanned using the HPTLC software so as to calculate the concentration of xylose and XOS. The concentrations of xylose and XOS in different hydrolysates were quantified using average peak areas compared with the peak areas of standards and expressed as mg/ml. Xylose and xylobiose were used as standards at the concentrations of 1 mg/ml and linear calibration was achieved for both standards.
Study of Prebiotic Potency of Xylan Hydrolysates
Pure cultures of Lactobacillus brevis, Lactobacillus acidophilus, Lactobacillus viridescens were maintained on MRS (Man Rogosa Sharpe) medium slants and stored at 4 °C. The culture of test bacteria (1%, v/v) was added to MRS broth supplemented with 1% glucose/xylose/xylobiose/fructooloigosaccharides(FOS)/samples containing XOS (produced by hydrlosis of xylan). Serial dilutions (10− 4, 10− 5 and10− 6) of inoculant samples (100 µl) were spread on MRS medium plates. The plates were incubated at 37 °C for 24 h in BOD incubator. Colony forming units (cfu) were enumerated at 24 h post incubation and converted to log value using following formula:
where a is the mean number of bacterial colonies, bn is the dilution factor.
For studying the effects of different compounds and samples containing XOS on bacterial growth, 100 µl of bacterial suspension was used to inoculate modified MRS broth (pH 6.8), containing 1% glucose/xylose/xylobiose/fructooloigosaccharides (FOS)/samples containing XOS. All the inoculants samples were incubated at 37 °C in BOD incubator. Bacterial growth was measured by turbidimetric method by studying absorbance at intervals of 2 h at 600 nm. As control, same amount of bacterial suspension was used to inoculate MRS broth. The growth curves were plotted with absorbance value as function of time interval and the effects of different xylan hydrolysates and pure compounds on the growth of bacteria were studied.
Statistical Analysis
The experimental results are expressed as mean ± SD (standard deviation). Data was analyzed statistically using analysis of variance (ANOVA) appropriate in completely randomized design using CPCS1 and CD (5%) was calculated.
Results and Discussion
Chemical Composition of Sugarcane Bagasse
As determined in our previous study [22], the contents of hemicellulose, cellulose and lignin in sugarcane bagasse estimated by detergent system method were found to be 28.42, 42.11 and 19.20%, respectively. On the basis of compositional analysis using NREL (National Renewable Energy Laboratory) method, the amount of xylan in sugarcane bagasse was found to be 21.46%.
Extraction of Xylan from Sugarcane Bagasse
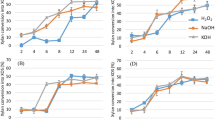
Xylan, a major portion of hemicellulose, was extracted from sugarcane bagasse using different concentrations of potassium hydroxide (KOH) and sodium hydroxide (NaOH). It is reported that physical treatment like milling of barley husk yields only 7.21% xylan [23]. Harsh reaction conditions during extraction or pretreatment lead to partial degradation of xylan and generation of toxic compounds derived from sugar decomposition that could affect the hydrolysis [24]. Therefore, steam treatment was used for extraction of xylan as it has been recorded to be more efficient as compared to other extraction processes [25]. The dried pellet obtained after extraction process was presumed to be xylan since cellulose is neither soluble in sodium hydroxide nor potassium hydroxide [26] and alcohol does not precipitate lignin [27]. The per cent yields of xylan (based on dry matter of initial amount of raw material) obtained with 5, 10 and 15% KOH treatments were found to be 9.5, 16.5 and 17.0, respectively whereas 12.5, 18.5 and 20.5% yields of xylan were obtained with 5, 10 and 15% NaOH treatments, respectively. Taking the xylan content of bagasse sample as 21.4%, the per cent recoveries of xylan were calculated and have been presented in Fig. 1.
It was observed that sodium hydroxide was found to be more effective than potassium hydroxide to ensure higher recovery of xylan. Increasing concentrations of alkali led to increased recovery of xylan. These results were supported by Ruzene et al. [28] who suggested that increasing the concentration of sodium hydroxide or temperature resulted in higher recovery of xylan (49.3% of the total available hemicellulose) from wheat straw and highest extraction yield was possible to achieve with 0.50 mol/l sodium hydroxide. Samanta et al. [14] achieved 83.5% recovery of xylan from corn cobs with 12% sodium hydroxide followed by steam application.
FT-IR spectra of alkali extracted xylan showed characteristic vibrational bands at 3434 (O–H stretching), 2926 (C–H stretching), 1413(C–H deformation), 1248 (C–O–C stretching), 1040 (C–O stretching) [14] and 898 (characteristic of β-xylosidic linkages) [29] confirming the presence of various functional groups in xylan. 1H NMR spectra of xylan showed a large signal at 8.18 ppm due to presence of –OH groups in the xylan structure. Two signals at 3.43 and 1.71 ppm were observed due to aliphatic protons [30]. A large signal at 78 ppm in 13C NMR spectra due to ring carbons of xylan was also observed.
Acid Hydrolysis of Xylan
Xylan can be hydrolyzed to xylose and XOS by chemical methods, direct enzymatic methods or a combination of chemical and enzymatic treatments [31]. Though enzymatic production of XOS is preferred because it requires mild conditions for hydrolysis, however, several hours of incubation are required to generate the XOS through application of specific enzymes [15, 32]. Moreover, enzymatic treatments are expensive and might require special conditions for storage and handling of the enzymes. Therefore, acid hydrolysis was chosen for production of XOS from sugarcane bagasse xylan. The effects of dilute sulfuric acid (0.25 and 0.50 M) on XOS production from bagasse xylan in different hours of treatment (20, 40 and 60 min) were evaluated through estimation of reducing sugars and high performance thin layer chromatography (HPTLC) analysis.
HPTLC plates showing spots of standards and samples hydrolyzed with 0.25M H2SO4 and 0.50M H2SO4 for different time intervals (samples from six different treatments, T1-T6) are shown in Fig. 2. Table 1 shows the results of HPTLC analysis and reducing sugar estimation from different xylan hydrolysates. Xylan was hydrolyzed to a number of oligosaccharides ranging from X1 (xylose) to X3 (xylotriose) and above. From HPTLC plate shown in Fig. 2a, it was evident that increasing the time of hydrolysis with 0.25M H2SO4 enabled higher degradation of xylan into xylose and XOS. XOS with increasing degrees of polymerization appeared as spots with gradually decreasing Rf values [18]. The longer reaction time resulted in further degradation of XOS into xylose. The amount of xylose increased from 2.014 (T1, 20 min) to 2.838 (T2, 40 min) and 3.944 mg ml− 1 (T3, 60 min). Correspondingly, the amounts of xylobiose and xylotriose were found to decrease as the treatment time was increased from 20 to 40 and 60 min. This may be attributed to hydrolysis of xylosidic linkages of xylobiose and xylotriose at increased reaction times to yield the monosaccharide xylose in highest amounts. Treatment with 0.25M H2SO4 for 20 min was found to produce various XOS upto xylohexaose or above as is clear in HPTLC plate whereas in treatment for 40 min, XOS upto four monomers i.e. xylotetraose were formed. Further increasing the reaction time to 60 min reduced the concentration of XOS and higher concentration of xylose was produced. The production of reducing sugars was also found to increase along with time as also reported previously [33].
From Fig. 2b and Table 1, it is clear that in acid hydrolysis with 0.50M H2SO4 (T4–T6), the amounts of xylobiose and xylotriose were decreased with increase in treatment time. Only upto xylotetraose were produced by hydrolysis with 0.50M H2SO4. The spots for XOS were not as clearly separated and visible in this plate as were on HPTLC plate in Fig. 2a. This may be due to increased hydrolysis of oligosaccharides leading to formation of monosaccharides. The formation of xylose and reducing sugars was higher in case of hydrolysis for 20 min (T4) and decreased as the reaction time was decreased. The reason for this may be further hydrolysis of monosaccharide sugars into products such as furfural and hydroxymethylfurfural. The furfural production increases with increasing acidic strength as the stronger acid catalyzed hydrolysis of pentosans into xylose and subsequent cyclodehydration to furfural to greater extent [34].
It was found that the time and concentration of acid played a very critical role in the production of XOS. The acidic hydrolysis treatments using 0.25M H2SO4 for 20 and 40 min (T1 and T2) gave the higher amounts of XOS and hence were considered best among the six treatments employed during the study. Therefore, the experiment revealed that XOS could be produced from the alkali extracted bagasse xylan only by controlled acid hydrolysis.
Prebiotic Potency of Xylan Hydrolysates
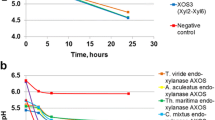
The prebiotic potency of XOS from xylan hydrolysates was assessed by enumerating the colony forming units (cfu) of three probiotic bacteria viz. Lactobacillus brevis, Lactobacillus acidophilus and Lactobacillus viridescens after 24 h of incubation (Table 2). Sugarcane bagasse generated XOS were found to be capable to influence the growth of probiotic organisms positively as compared to xylose, glucose and established prebiotics fructooligosaccharides (FOS) and the growth of bacteria (log cfu/ml) was found to be significant as is clear from the CD values (5%). Pure xylobiose, however, was responsible for growth of bacteria to great extent as compared to all other standard compounds and samples. As compared to xylose, all other compounds and samples were found to have positive effect on growth of Lactobacillus studied. Xylan hydrolysate samples were found to have increased effect on growth of all species of Lactobacillus as compared to standard glucose and fructooligosachharides (FOS). The highest positive effect on growth of all three species of Lactobacillus by pure xylobiose clearly revealed that the samples T1 and T2 served as the most effective prebiotics because of presence of higher concentrations of XOS especially xylobiose in them as compared to other samples. It has been reported that XOS with fewer than four monomer units are important for prebiotic applications because they promote the proliferation of bifidobacteria, which are considered as beneficial microorganisms in human intestine [9].
In a study [14] on in vitro effects of crude XOS from corncob xylan on growth of Enterococcus fecalis, Enterococcus faecium, Lactobacilllus maltromicus and Lactobacillus viridiscens, the influence of FOS on growth stimulation was found to be higher than either XOS or glucose. The authors believed that the differences may be due to crude preparation of XOS compared against pure FOS. On the contrary, in this study, XOS from sugarcane bagasse xylan were found to have higher growth stimulatory effects on Lactobacillus acidophilus, Lactobacillus brevis and Lactobacillus viridescens as compared to glucose and standard FOS. In corroboration with our study, XOS have been reported to be promising oligosaccharides that stimulate increased levels of bifidobacteria to a greater extent than does FOS [35].
Further, growth curves of Lactobacillus acidophilus, Lactobacillus brevis and Lactobacillus viridescens and the effects of XOS, xylose, xylobiose, glucose and fructooligosaccharides (FOS) on growth curves were also studied (Fig. 3) by measuring the absorbances of modified broths. The effects of XOS were studied by taking the xylan hydrolysates from two best treatments (T1 and T2). The growth of three probiotic bacteria viz. Lactobacillus acidophilus, Lactobacillus brevis and Lactobacillus viridescens was found to be stimulated by xylose (X)/xylobiose (X2)/glucose (G)/FOS/XOS. The same effects by different compounds on growth of bacteria were observed as indicated by cfu values. Highest growth increase was caused by xylobiose. XOS (from samples T1 and T2) were found to have more positive effect on growth as compared to glucose and standard FOS. Xylose had less effect on growth but the growth of bacteria was increased as compared to control. These prebiotic effects of XOS enable in the improvement of intestinal functions by increasing the number of healthy gut bacteria.
The enhanced growth of all bacteria indicated that these bacteria possessed carbohydrate degrading enzymes which ferment the oligosaccharides and produce short chain fatty acids which provide metabolic energy for the host [17]. It has been reported that corn cob generated XOS could well support the growth of Lactobacillus brevis, because of their capability to utilize the same as source of energy [36].
To produce food-grade XOS, the autohydrolysis liquors have to be refined by removing both monosachharides and nonsaccharide compounds to obtain a concentrate with XOS content as high as possible. The usual purity of commercial XOS lies in the range of 75–95% [7]. Several methods have been reported for the purification of XOS like solvent precipitation using ethanol, acetone and 2-propanol [37], adsorption in combination with other treatments intending either separation of oligosaccharides [8, 38] or the removal of undesired compounds [39], chromatographic separation [17], ultrafiltration and nanofiltration etc. [7].
Conclusions
Alkali extracted xylan from sugarcane bagasse can be conveniently hydrolyzed using dilute sulfuric acid to produce XOS under controlled conditions as the increased concentration of dilute acid and hydrolysis time could lead to more production of monosaccharides. Therefore, optimization of hydrolysis conditions is necessary and in our study, the best conditions were found to be steam treatment with 0.25M H2SO4 for 20 min; increasing the acid concentration and reaction time led to decrease in production of XOS. The sugarcane bagasse generated XOS were found to be more potential prebiotics for the growth of Lactobacillus acidophilus, Lactobacillus brevis and Lactobacillus viridescens as compared to established prebiotics fructooligosaccharides (FOS). As pure xylobiose was found to increase the growth of probiotic bacteria to great extent as compared to xylan hydrolysates, it is suggested to make attempts for isolation of pure XOS eliminating all the monosaccharide and nonsaccharide compounds produced in the reaction media and their use in prebiotic drugs.
References
Gibson, G.R., Roberfroid, M.B.: Dietary modulation of the human colonic microbiota: introducing the concept of Prebiotics. J. Nutr. 125, 1401–1412 (1995)
Menezes, C.R., Silva, I.S., Pavarina, E.C., Bosscher, A.D., Loo-Van, J., Franck, A.: Inulin and oligofructose as prebiotics in the prevention of intestinal infections and diseases. Nutr. Res. Rev. 19, 216 (2006)
Kaur, N., Gupta, A.K.: Applications of inulin and oligofructose in health and nutrition. J. Biosci. 27, 703–714 (2002)
Losada, M.A., Olleros, T.: Towards a healthier diet for the colon: the influence of fructooligosaccharides and Lactobacilli on intestinal health. Nutri. Res. 22, 1–84 (2002)
Silva, L.P., Nornberg, J.L.: Prebiotics in the nutrition of ruminants. Cienc. Rural. 33, 983–990 (2003)
Stowell, J.: Sweeteners and Sugar Alternatives in Food Technology. Mitchell, H. (ed.) p. 54. Blackwell Publishing Ltd., Oxford (2007)
Aachary, A.A., Prapulla, S.G.: Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties and applications. Comp. Rev. Food Sci. Food Saf. 10, 2–16 (2011)
Vazquez, M.J., Alonso, J.L., Dominguez, H., Parajo, J.C.: Xylooligosaccharides: manufacture and applications. Trends Food Sci. Technol. 11, 387–393 (2000)
Gullon, P., Moura, P., Esteves, M., Girio, F.M., Dominguez, H., Parajo, J.C.: Assessment on the fermentability of xylo-oligosaccharides from rice husks by probiotic bacteria. J. Agri. Food Chem. 56, 7482–7487 (2008)
Chung, Y.C., Hsu, C.K., Ko, C.Y., Chan, Y.C.: Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture and pH value in the elderly. Nutr. Res. 27, 756–761 (2007)
Roberfroid, M., Slavin, J.: Nondigestible oligosaccharides. Crit. Rev. Food Sci. Nutr. 40(6), 461–480 (2000)
Gottschalk, L.M.F., Oliveira, R.A., Bom, E.P.S.: Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem. Eng. J. 51, 72–78 (2010)
Cardona, C.A., Quintero, J.A., Paz, I.C.: Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 101, 4754–4766 (2010)
Samanta, A.K., Senani, S., Kolte, A.P., Sridhar, M., Sampath, K.T., Jayapal, N., Devi, A.: Production and in vitro evaluation of xylooligosaccharides generated from corn cobs. Food Bioprod. Process. 90, 466–474 (2012)
Akpinar, O., Erdogan, K., Bostanci, S.: Enzymatic production of xylooligosaccharides from selected agricultural wastes. Food Bioprod. Process. 87, 145–151 (2009)
Gullon, B., Yanez, R., Alonso, J.L., Parajo, J.C.: Production of oligosaccharides and sugars from rye straw: a kinetic approach. Bioresour. Technol. 101, 6676–6684 (2010)
Madhukumar, M.S., Muralikrishna, G.: Structural characterization and determination of prebiotic activity of purified xylo-oligosaccharides obtained from Bengal gram husk (Cicer arietinum L.) and wheat bran (Triticum aestivum). Food Chem. 118, 215–223 (2010)
Jayapal, N., Samanta, A.K., Kolte, A.P., Senani, S., Sridhar, M., Suresh, K.P., Sampath, K.T.: Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind. Crops Prod. 42, 14–24 (2013)
Goering, H.K., Van-soest, P.J.: Forage fibre analysis. USDA Agricultural Research Service; Agricultural Handbook No. 379 (1970)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, J., Crocker, D.: Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 4, 1–15 (2011)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Kaur, R., Uppal, S.K.: Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym. Sci. 293, 2585–2592 (2015)
Roos, A.A., Persson, T., Krawczyk, H., Zacchi, G., Stalbrand, H.: Extraction of water-soluble hemicelluloses from barley husks. Bioresour. Technol. 100, 763–769 (2009)
Oliva, J.M., Saez, F., Ballesteros, I., Gonzalez, A., Negro, M.J., Manzanares, P., Ballesteros, M.: Effect of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces maxianus. Appl. Microbiol. Biotechnol. 105, 141–154 (2003)
Xu, F., Sun, J., Sun, R., Fowler, P., Baird, M.S.: Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 23, 180–193 (2006)
Strepikheev, A.A., Knunyants, I.L., Nikolaeva, N.S., Mogilevsky, E.M.: The solubility of cellulose in quaternary ammonium bases. Russ. Chem. Bull. 6, 769–771 (1956)
Ni, Y., Hu, Q.: Alcell lignin solubility in ethanol-water mixtures. J. Appl. Polym. Sci. 57, 1441–1446 (1995)
Ruzene, D.S., Silva, P.D., Vicente, A.A., Goncalves, A.R., Teixeira, J.A.: An alternate application to the Portuguese agroindustrial residue: wheat straw. Appl. Biochem. Biotechnol. 147, 85–96 (2008)
Gupta, S., Madan, R.N., Bansal, M.C.: Chemical composition of Pinus caribuca hemicelluloses. Tappi J. 70, 113–114 (1987)
Sun, J.X., Sun, X.F., Sun, R.C., Su, Y.Q.: Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohyd. Polym. 56, 195–204 (2004)
Moura, A., Gullon, P., Dominia, H., Parajo, J.C.: Advances in the manufacturer, purification and application of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 41, 1913–1923 (2006)
Pellerin, P., Gosselin, M., Lepoutre, J., Samain, E., Debeire, P.: Enzymatic production of oligosaccharides from corncobs xylan. Enzyme Microbiol. Technol. 13, 617–621 (1991)
Akpinar, O., Erdogan, K., Bostanci, S.: Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr. Res. 344, 660–666 (2009)
Uppal, S.K., Kaur, R.: Hemicellulosic furfural production from sugarcane bagasse using different acids. Sugar Tech. 13(2), 166–169 (2011)
Tuohy, K.M., Rouzaud, G.C.M., Bruck, W.M., Gibson, G.R.: Modulation of the human gut microflora towards improved health using prebiotics-Assessment of efficacy. Curr. Pharm. Des. 11, 75–90 (2005)
Moura, P., Barata, R., Carvalheiro, F., Girio, F., Loureiro-Dias, M.C., Esteves, M.P.: In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT Food Sci. Technol. 40, 963–972 (2007)
Swennen, K., Courtin, C.M., Van der Bruggen, B., Vandecasteele, C., Delcour, J.A.: Ultrafiltration and ethanol precipitation for isolation of arabino xylooligosaccharides with different structures. Carbohydr. Polym. 62, 283–292 (2005)
Sanz, M.L., Polemis, N., Morales, V., Corzo, N., Drakoularakou, A., Gibson, G.R., Rastall, R.A.: In vitro investigation into the potential prebiotic activity of honey oligosaccharides. J. Agric. Food Chem. 53, 2914–2921 (2005)
Kokubo, I., Ikemizu, S.: Histamine-release inhibitors containing xylooligosaccharides. Japan Patent JP 2004059481 (2004)
Funding
This study was partially supported by Maulana Azad National Fellowship by University Grants Commission, India awarded to Ramandeep Kaur (MANF-2013-14-SIK-PUN-20159).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, R., Uppal, S.K. & Sharma, P. Production of Xylooligosaccharides from Sugarcane Bagasse and Evaluation of Their Prebiotic Potency In Vitro. Waste Biomass Valor 10, 2627–2635 (2019). https://doi.org/10.1007/s12649-018-0266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0266-1