Abstract
A large volume of biomass residue is disposed daily, and the use of chemical and enzymatic treatments is an alternative to reuse it generating value-added products such as xylooligosaccharides (XOS). Banana peel, guava bagasse, orange bagasse, and restaurant pre-prepare waste were subjected to three treatments for xylan solubilization. Subsequently, xylan was enzymatically hydrolyzed to obtain xylooligosaccharides. The maximum polysaccharide solubilization using alkaline hydrogen peroxide was 90.70% from restaurant residue. Sodium hydroxide solubilized 88.01% of xylan from guava bagasse and 74.20% of xylan from the banana peel, using potassium hydroxide. After enzymatic hydrolysis, the maximum production of XOS was 54.14% with banana peel residue (peroxide solubilized), 59.86% with guava bagasse (sodium hydroxide solubilized), 50.42% for orange bagasse (peroxide solubilized), and 50.80% with restaurant residue (potassium hydroxide solubilized). The results showed that each of the biomass had a different condition of treatment. The best conditions to obtain xylan from banana peel and guava bagasse were using NaOH treatment, and from orange bagasse and restaurant pre-prepare were using KOH. To produce XOS, banana peel and orange bagasse were treated with peroxide, and the guava bagasse and restaurant residue were treated with potassium hydroxide and subsequently submitted to enzymatic hydrolysis for 12 and 48 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A wide variety of residues such as forest and agroindustrial can be a source of lignocellulosic materials. These materials are composed of three major biopolymers, namely, lignin, cellulose, and hemicellulose [1]. Hemicelluloses are smaller molecules (lower degree of polymerization) than celluloses, composed mainly of xylose, arabinose, rhamnose, galactose, and mannose residues. The sugar units from hemicelluloses can be subdivided into components such as pentoses, hexoses, and uronic acids [2]. Among the hemicelluloses, xylan stands out as the most abundant polysaccharide in grass hemicelluloses and, consequently, the one with a higher incidence in agricultural and agroindustrial residues [3]. The structure of isolated xylan present in plants is composed of xylose residues in the main chain, differing by pendant groups and branched residues [4, 5]. Therefore, hemicellulose can be used as feedstock to several industrial interest molecules, applying chemical and enzymatic treatments. However, the hemicellulose solubilization is highly dependent on the chemical used, type of biomass, and its anatomical fraction due to recalcitrance and heterogeneity. Tropical countries produce fruit waste in high amounts, a substrate rich in polysaccharides that can be converted into XOS.
For the production of XOS, the extraction of hemicellulose is necessary to obtain it in isolated form and subsequent hydrolysis for the production of oligomers. The extraction method depends on the final purpose and application of the xylan and may be based on the desired hemicellulose final characteristics. Some important characteristics to consider are the degree of polymerization, degree of branching/substitution, solubility, reactivity, and impurities such as residual lignin [6]. Considering the recovery of hemicellulose based on the polysaccharide or monosaccharide form, the concepts of acid and alkaline treatments can be successfully applied [7]. Acid extraction and its derived processes lead to end products based on monosaccharides and oligosaccharides [8]. Alkaline extraction and its derivative processes lead to end products based on high polymerization macromolecules [2]. Hemicellulose has great potential and applicability in the manufacture of bioplastic [9]. The use of hemicellulose has been highlighted in the health area, as the search for a healthy lifestyle and care with food is increasing. The use of components that stimulate the proper functioning of the body, such as XOS, has several beneficial health effects such as lowering serum cholesterol levels, stimulating bifidobacteria in the gastrointestinal tract, and preventing cavities [10, 11].
XOS have great potential for improving human health and well-being by maintaining intestinal microflora. They can be incorporated into many food products, and their properties can offer a new dimension to the development of functional foods [12]. Because of their many influences on human health, as well as their potential to prevent gastrointestinal problems, XOS are gaining space in the prebiotic market. XOS stand out the advantages of being produced from the lignocellulosic residue, abundant and low-cost material, which are not suitable for human consumption and are available in large quantities. Besides, agricultural by-products are still underutilized and the fact that xylan of these materials can be converted into higher value-added products meets the need for use of this wide variety of residue.
The use of enzymes for XOS production is considered a cleaner form of production as it does not require high temperatures, pressure, or harmful chemicals. XOS recovery is still possible as it does not produce by-products in the process [11]. This enzymatic production, through the action of endoxylanases, allows the low formation of sugar monomers, the absence of by-products generated during hydrolysis, and does not require special equipment or high-temperature conditions. At the same time, this process has the disadvantage of being easily inhibited by compounds present in lignocellulosic biomass, requiring hemicellulose solubilization, in addition to the cost of enzymes [12, 13]. The production of XOS from enzymatic hydrolysis can be done through the action of endoxylanase in the xylan main chain. Hemicellulose extraction from lignocellulosic biomass is a strategy before the enzymatic hydrolysis and the enzyme extract should be free of β-xylosidase, the enzyme that hydrolyzes XOS into xylose, since its presence can reduce the yield of XOS production [10]. Thus, enzymatic hydrolysis of solubilized xylan should be performed by applying the endoxylanase, with little or no activity of the enzyme β-xylosidase, given that the objective is the production of oligomers rather than monosaccharides, such as xylose. In contrast, there is no possibility of controlling the degree of polymerization of the XOS produced, since xylanases with different substrate specificities generate different hydrolysis products [14]. From this, this study aimed to analyze the xylan solubilization from alkali treatments using different agroindustrial lignocellulosic biomasses, which are banana peel, guava, and orange bagasse, and restaurant residue. The solubilized xylan was studied for XOS production applying enzymatic hydrolysis using a purified endoxylanase of Aspergillus versicolor.
Materials and Methods
Agroindustrial and Food Residue
Biomasses used were banana peel, guava bagasse, orange bagasse, and restaurant residue. Residues of guava were gently provided by the company Predilecta (Matão, SP – Brazil) and the orange bagasse by Selial company (Rio Claro, SP – Brazil). The banana peel was collected in the local market. Restaurant residue was collected at the university restaurant and was composed of pre-prepare residues, such as onion and garlic peel, vegetable peels, and leaves in general. Residues were dried at 60 °C until moisture was lower than 10%, ground in a knife mill, and the material that passed through a 20 mesh (0.84 mm) sieve was selected [7].
Biomass Chemical Composition
The biomasses were chemically characterized to determine cellulose/glucan, lignin, and hemicellulose content. Approximately 300 mg of dry biomass were hydrolyzed with 3 mL of 72% sulfuric acid at 30 °C for 1 h. To the reaction was added 84 mL of distilled water and it was autoclaved at 121 °C for 1 h. The hydrolysate was filtered with a porous plate crucible. The liquid fraction was collected to determine soluble lignin by spectrophotometer at 215 and 280 nm and sugar quantification by HPLC (Waters – Massachusetts, EUA—Alliance system with Refractive index Waters IR 2414). The solid residue (retained on the crucible) was dried at 105 °C to determine insoluble lignin content [15]. Monosaccharide concentration determined in HPLC was used to calculate the anhydrous sugars, converting glucose released in glucan/cellulose present in the biomass, with a hydration factor of 0.9. For xylose and arabinose, the factor was 0.88, and for acetic acid was 0.72. The hemicellulose content was reported as a sum of xylan, arabinan, and acetic acid. Experiments were performed in triplicate.
Hemicellulose Solubilization
Previously to the solubilization, the biomass was washed with 0.2% (m/v) ethylenediaminetetraacetic acid (EDTA) for 1 h at 90 °C to remove metal cations such as iron and manganese ions. These ions promote the decomposition of hydrogen peroxide reducing its performance [6, 16]. The xylan solubilization was evaluated with and without washing. The biomasses were subjected to xylan solubilization using three reaction solutions: alkaline hydrogen peroxide (pH 11.6), sodium hydroxide (NaOH), and potassium hydroxide (KOH). Samples containing 10 g of biomass were treated with alkaline peroxide (6%, m/v) in a reaction volume of 200 mL, with pH adjusted to 11.6 with 6 mol/L NaOH solution, and shaken at 120 rpm at room temperature. The solubilization with sodium hydroxide (NaOH) and potassium hydroxide (KOH) occurred in a thermostated bath at 70 °C and was shaken at 70 rpm [17]. After 4 h, the liquid was filtered and the insoluble fraction was discarded. The supernatant liquid was adjusted to pH 6 with 6 mol/L HCl and then concentrated to about one-third of its volume under air circulation at 45 °C. Hemicellulose was precipitated by adding 3 volumes of 95% ethanol. Subsequently, the process was repeated with 70% (v/v) ethanol until a clear liquid fraction, and the material was dried at 45 °C. The yields were calculated based on the sum of the biomass anhydroarabinan and anhydroxylan contents, from their initial chemical characterization [6, 16].
Enzymatic Hydrolysis of Xylan
Enzymatic hydrolysis was performed with the solubilized hemicellulose applying endoxylanase produced by Aspergillus versicolor grown in wheat bran and purified by ion-exchange chromatography and gel filtration techniques. The purified enzyme showed 1689 UI/mL and presented 19 kDa by SDS-PAGE and gel filtration [18]. Enzymatic hydrolysis was performed by varying the parameter of reaction time to evaluate the best conditions for XOS production. The hydrolysis was conducted in 50-mL centrifuge tubes containing 5 mL of 50 mmol/L sodium phosphate buffer (pH 6) at 55 °C in a water bath with 120 rpm agitation. The reaction was conducted with A. versicolor purified endoxylanase applying 30 IU/g [11]. The enzymatic hydrolysis analyzed parameters such as reaction time and xylan solubilization treatment (2, 4, 6, 8, 12, 24, and 48 h, NaOH, KOH, and H2O2). At time intervals the tubes were collected to monitor the XOS content, heated in boiling water for 5 min to stop the reaction. The hydrolysate was filtered with a 0.22-µm syringe filter before HPLC injection for XOS quantification.
Xylooligosaccharides, Monosaccharides, and Acetic Acid Quantification
XOS production was determined by high-performance liquid chromatography (HPLC—WATERS equipment) under the following conditions: Aminex HPX-87C Bio-Rad column (300 × 7.8 mm) at the temperature of 80 °C; eluent: ultrapure water with flow 0.6 mL/min; injection volume of 20 μL. The XOS concentration was determined in a detector of refractive index (Waters 2414). Solutions of xylose (Sigma), xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) were used as standards. Total XOS and xylose content was calculated relative to the amount of xylan in the material.
Pentoses, hexoses, and acid acetic resulting from the liquid fraction of chemical characterization were quantified by HPLC. An Aminex column Bio-Rad HPX-87 H (300 × 7.8 mm) was used with mobile phase 0.05 mol/L H2SO4, in a flow of 0.4 mL/min, oven temperature of 65 °C and RID detector.
Statistical Analysis
Statistical treatment was carried out for some of the results obtained through the analysis of variance (ANOVA) with a single criterion using the BioEstat software (version 5.3).
Results and Discussion
Characterization of Biomasses
The moisture content was 88.37% for banana peel, 39.06% for guava bagasse, 79.43% for orange bagasse, and 99.67% for restaurant residue (Table 1). The ash content, inorganic material, was 13.22% (dry basis) for banana peel, 1.14% for guava bagasse, 4.50% for orange bagasse, and 12.89% for restaurant residue. The total extractives content (in ethanol and water) was 62.97% for banana peel, 27.39% for guava bagasse, 48.69% for orange bagasse, and 48.49% for restaurant residue. The result of lignin content was 30.28% (dry basis) for banana peel, 31.94% for guava bagasse, 21.45% for orange bagasse, and 12.10% for restaurant residue.
The amount of total hemicellulose in the biomass was calculated with the sum of xylan contents, arabinan, and acetyl groups. The sum of the components resulted in the average of hemicellulose, which was 38.42, 28.33, 31.04, and 20.58% (dry basis) for banana peel, guava bagasse, orange bagasse, and restaurant residue, successively. The glucan/cellulose (anhydroglucose) content was 29.28, 29.93, 24.42, and 50.20% for banana peel, guava bagasse, orange bagasse, and restaurant residue (Table 1).
The values found for the chemical composition of banana peel and guava bagasse were similar to those found in the literature. For banana peel, ash was reported as 11.47%, moisture as 89.47% [19]. For guava, bagasse ash was reported as 1.36%, hemicellulose as 37.20%, and moisture content as 53% of the biomass [20]. The results of orange bagasse chemical characterization found in the literature were obtained from bagasse with pectin or oil extraction, which may explain the difference from the results obtained in this study. It was found in the literature that orange bagasse contains 2.90% of lignin, 26.45% of hemicellulose [21], other study reported 12.40% cellulose, 8.90% lignin, and 7.50% of hemicellulose [22]. For restaurant residue, it was reported 10.78, 23.26, 33.62, 4.86, 56.58, and 91.78% of ash, lignin, cellulose, hemicellulose, extractives, and moisture, successively [15]. The restaurant residue has its heterogeneity as a characteristic, which may explain the difference in the results found in the literature to those obtained in this study.
Hemicellulose Solubilization
The biomass hemicellulose was solubilized using different chemical agents and submitted to the enzymatic hydrolysis for XOS production, using Aspergillus versicolor endoxylanase purified [11]. The hemicellulose solubilization was evaluated by washing with EDTA and without washing. The EDTA can remove ions that could interfere in the hydrogen peroxide action. Alkaline hydrogen peroxide is an effective oxidizing agent for breaking/removing lignin and hemicellulose, capable of increasing cellulose accessibility, isolating, and bleaching fibers. In addition, a combination of alkaline reagents and oxidants such as peroxide is effective for pretreatment and may result in the high conversion of lignocellulosic biomass into sugars, by the further enzymatic depolymerization of cell wall polysaccharides [23,24,25]. The washing step with EDTA is used to avoid hydrogen peroxide degradation [6, 16, 26]. In the present study, the washing step influenced orange bagasse and restaurant residue increasing hemicellulose solubilization. Biomasses can have other components such as pectin in banana peel [27], and extractives and oil in guava bagasse [28], which can influence the final hemicellulose solubilization. Moreover, the washing step can contribute to remove components such as extractives, reducing ethanol washing in the hemicellulose clarification step.
The alkaline hydrogen peroxide treatment highest yields of solubilization were using orange bagasse and restaurant residue, while using sodium hydroxide treatment, guava bagasse and restaurant residue washed with EDTA were the biomasses with higher solubilization yield. For the potassium hydroxide treatment, the highest yields obtained were using the restaurant residue, in both washed and not washed forms (Table 2). The maximum hemicellulose solubilization yields were 74.20% (dry basis) for the banana peel (washed with EDTA and treated with NaOH), 88.01% for the guava bagasse (not washed and treated with NaOH), 80.81% for the orange bagasse (washed with EDTA and treated with KOH), and 91.74% for the restaurant residue (not washed and treated with H2O2).
The hemicellulose solubilization with NaOH and KOH resulted in a high yield for all the biomass in a general comparison with alkaline hydrogen peroxide. However, the clarification of the liquid fraction after hemicellulose precipitation required 8 to 10 washes with 70% (v/v) ethanol for NaOH and KOH treatments. Alkaline hydrogen peroxide treatment required 4 ethanol washes to obtain a clear liquid fraction. In this ethanol wash, occurs salts and lignin-derived compounds removal, collaborate to a better quality of the hemicellulose [17, 29].
The hemicellulose solubilization yields obtained in the present study were higher than those found in the literature for different biomass. A yield of 33.3% was reported using 12% NaOH at 80 °C for 4 h of reaction, and 21.4% yield using 2% KOH at 75 °C for 3 h of reaction applied to sweet sorghum bagasse [30]. Sorghum biomass separated in the stalk, leaf, and bagasse resulted in different yields for hemicellulose solubilization, requiring 10% of alkaline hydrogen peroxide to achieve a high solubilization yield. Treatment with KOH resulted in 96% of hemicellulose solubilization from sorghum stalk, indicating heterogeneity among biomass fractions [17]. For pigeon pea stalk, the recovery of xylan for treatment with 12% NaOH, at room temperature and overnight incubation was 12.63% and with 12% KOH, at room temperature and overnight incubation it was 12.35% [31]. For sugarcane bagasse, using H2O2 in alkaline medium (6%, m/v, 60 °C, 16 h), the solubilization yield was 60.8%, and with 1 mol/L NaOH at 40 °C and 18 h, it was 62.10% [16, 32]. The characteristics of each biomass, especially chemical composition, influence the optimal hemicellulose solubilization conditions and the final purpose of the solubilization process. For hemicellulose to be converted into new products, after their separation from the other components, it must be in specific and desirable quality conditions, regardless of the extraction method.
Enzymatic Hydrolysis for XOS Production Using Washed Biomass
Xylanases are the most important enzymes to perform xylan hydrolysis and are responsible to reduce the degree of polymerization of the xylan chain by hydrolyzing the β-1,4 glycosidic bonds [33] The main enzymes responsible for the hydrolysis of xylan chains are endo-1,4-β-xylanases and β-xylosidases [34]. Endo-β-1,4-D-xylanases (EC 3.2.1.8) act on the xylan main chain, forming xylooligosaccharides, while β-xylosidases hydrolyze xylobiose and short-chain xylooligomers, releasing xylose units [33]. To maximize XOS production, it is necessary to apply purified endoxylanase in order to avoid xylose production.
The solubilized xylan was used as a substrate for enzymatic hydrolysis with endoxylanase produced by A. versicolor in order to produce XOS. As expected, no xylose content was detected in any of the samples, which proves the effectiveness of xylan extraction by alkaline treatment, the absence of β-xylosidases, and the efficient purification of xylanase. The predominant oligomers generated were xylobiose (X2), xylotriose (X3), and xylotetraose (X4) (Tables 3 and 4).
Xylooligosaccharides were produced by enzymatic hydrolysis with a degree of polymerization from X2 to X4. X2 was produced in a low amount with all the biomass, even with a long reaction time (Table 3 and 4). The X3 was produced mainly after 6–8 h of reaction, increasing constantly after this period of time. The highest XOS concentration was obtained after 12–24 h of an enzymatic reaction, producing up to 10.45 g/L. Hemicellulose from banana pseudostem required 24 h to produce 11 g/L of XOS, with a predominance of X4, X5, and X6, via enzymatic hydrolysis using endoxylanase of A versicolor [11]. A strategy to increase the concentration of XOS is to perform enzymatic hydrolysis with high substrate loading (hemicellulose concentration). Using 10% of hemicellulose as a substrate for enzymatic hydrolysis it was possible to reach 22.5 g/L of XOS [11]. In this study, with a highly concentrated substrate, the production of X4, X5, and X6 was also predominant.
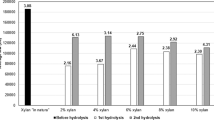
The biomass with the highest generation of total XOS was guava bagasse, showing potassium hydroxide as the best treatment and presenting maximum result with NaOH treatment (60.82% and 48 h). Enzymatic hydrolysis presented a better performance after xylan solubilization with KOH treatment for banana peel and restaurant residue, reaching an average of 50% conversion after 48 h. The orange bagasse also showed an average of 50% conversion, although after the alkaline hydrogen peroxide treatment (Fig. 1).
The conversion of xylan into XOS using all of the biomasses presented in the present study were similar to higher than those reported in the literature. A yield of 14.7% of XOS was reported for sugarcane bagasse hemicellulose (treated for 3 h at 35 °C using 24% KOH including 1% (m/v) NaBH4) after 48–72-h reaction using 60 UI/g of xylanase from Aspergillus fumigatus M51 (35.6 U/mL). The enzymatic hydrolysis performed with 500 UI/g of xylanase allowed to reduce the reaction time to 3 h [35]. A yield of 42.96% using residual hemicelluloses of the dissolving pulp was obtained, with 120 IU/g enzyme loading after 8 h of reaction [36]. A study using xylanase from Aspergillus niger (22.7 U total enzyme activity) and powdered corn as biomass at 55 °C for 24 h, showed the highest yield of 62% XOS [37], similar to those obtained in the present study.
Pectin can be previously removed from biomass such as orange bagasse and banana peel. A sequence of acid, alkaline, and enzymatic treatment was applied to banana peel for xylose and XOS production. In this process, 65% of hemicellulose was depolymerized, of which 33% was into XOS with a degree of polymerization from 2 to 6 [27]. Besides scarce publications about xylooligosaccharides production from orange bagasse and restaurant residue, it is possible to predict that the chemical composition can influence hemicellulose solubilization. In this scenery, a biorefinery process with the objective of recovering oil, antioxidants, and pectin could be appropriately related to hemicellulose valorization. Fermentable sugar production such as glucose could contribute in this biorefinery associated to hemicellulose and XOS production [38, 39].
Mass balance for Hemicellulose and XOS Production
Food and agroindustrial waste are inexpensive and abundant raw materials rich in hemicellulose, which can be converted into XOS. Among several lignocellulosic materials, a specific condition needs to be determined for each material as its recalcitrance and heterogeneity can respond differently to each type of treatment and conversion process available. A scale-up process should consider which chemical to use and the parameters associated: one or two-stage, heating, shaking, washing, precipitation/filtration/centrifugation, concentration, purification, and reaction time, among others. For hemicellulose solubilization, alkaline and alkaline oxidant chemicals are effective. Moreover, each type of biomass/waste can respond differently in terms of hemicellulose solubilization yield and quality.
The present study showed that each of the food and agro-industrial waste could result in better hemicellulose solubilization following a specific treatment, e.g., alkaline or alkaline hydrogen peroxide (Fig. 2). From banana peel 285.08 kg of hemicellulose can be solubilized using NaOH; from guava bagasse, 127.17 kg of hemicellulose can be solubilized using H2O2; from orange bagasse 250.83 kg of hemicellulose can be solubilized using KOH; from restaurant pre-prepare waste 186.66 kg of hemicellulose can be solubilized using KOH. The study was performed with endoxylanase purified from A. versicolor, applying 30 UI/g of the substrate during 12 h to 48 h, depending on the previous treatment. For each ton of biomass (dry basis) submitted to hemicellulose solubilization and further enzymatic hydrolysis can be produced: 150.01 kg of XOS in the hydrolysis of hemicellulose solubilized with KOH from banana peel; 66.13 kg of XOS in the hydrolysis of hemicellulose solubilized with H2O2 from guava bagasse; 125.42 kg of XOS in the hydrolysis of hemicellulose solubilized with KOH from orange bagasse; 93.33 kg of XOS in the hydrolysis of hemicellulose solubilized with KOH from restaurant pre-prepare waste.
The choice about the chemical used for hemicellulose solubilization takes into consideration the effectiveness in the hemicellulose solubilization and the further enzymatic hydrolysis yield. The use of NaOH, KOH, or H2O2 also needs to consider the chemical cost, technology cost to the recovery of the chemicals, and environmentally friendly process [17, 39].
Conclusion
Biomass waste study is important to generate knowledge about the biomass and the technologies that can be employed in order to reuse, rather than dispose of them. In the present study, as the goal was XOS production, different alkali pretreatments and alkaline hydrogen peroxide were evaluated for hemicellulose solubilization. For each of the biomass, a specific treatment was identified as with the best result, e.g., the banana peel with KOH or NaOH, guava bagasse with H2O2, orange bagasse with KOH, and restaurant pre-prepare waste with KOH or NaOH. Mass balance is an important tool to evaluate the overall process for XOS production, taking into account the hemicellulose content in the raw material, hemicellulose solubilization, and the XOS production by enzymatic hydrolysis. This indicates that treatments need to be studied to optimize the reuse, depending on the compound that is intended to be produced and the source of biomass. The important aspect of the fruit waste is that the bioactive molecules solubilized/obtained from them can be added to the final processed-fruit industrial product (like juice, sweet). This XOS incorporation into the fruit derivatives will bring special nutraceutical property, and probably higher consumer acceptance.
References
Melati RB, Shimizu FL, Oliveira G et al (2019) Key factors affecting the recalcitrance and conversion process of biomass. Bioenergy Res 12:1–20. https://doi.org/10.1007/s12155-018-9941-0
Brienzo M, Carvalho AFA, de Figueiredo FC, de Oliva-Neto P (2016) Sugarcane bagasse hemicellulose properties, extraction technologies and xylooligosaccharides production. In: Riley GL (ed) Food Waste. pp 155–188
Gírio FM, Fonseca C, Carvalheiro F et al (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101(13):4775–4800. https://doi.org/10.1016/j.biortech.2010.01.088
Madeira JV, Contesini FJ, Calzado F, et al (2017) Agro-industrial residues and microbial enzymes: an overview on the eco-friendly bioconversion into high value-added products. In: Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications. Elsevier Inc., pp 475–511
Álvarez C, González A, Negro MJ et al (2017) Optimized use of hemicellulose within a biorefinery for processing high value-added xylooligosaccharides. Ind Crop Prod 99:41–48. https://doi.org/10.1016/j.indcrop.2017.01.034
Alves RC, Melati RB, Casagrande GM et al (2021) Sieving process selects sugarcane bagasse with lower recalcitrance to xylan solubilization. J Chem Technol 96:327–334. https://doi.org/10.1002/JCTB.6541
Fernandes ÉS, Bueno D, Pagnocca FC, Brienzo M (2020) Minor Biomass Particle Size for an Efficient Cellulose Accessibility and Enzymatic Hydrolysis. ChemistrySelect 5:7627–7631. https://doi.org/10.1002/SLCT.202001008
Forsan CF, de Freitas C, Masarin F, Brienzo M (2021) Xylooligosaccharide production from sugarcane bagasse and leaf using Aspergillus versicolor endoxylanase and diluted acid. Biomass Convers. https://doi.org/10.1007/s13399-021-01403-2
Abe MM, Branciforti MC, Brienzo M (2021) Biodegradation of hemicellulose-cellulose-starch-based bioplastics and microbial polyesters. Recycling 6. https://doi.org/10.3390/RECYCLING6010022
de Freitas C, Carmona E, Brienzo M (2019) Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact Carbohyd Diet Fibre 18:100184. https://doi.org/10.1016/J.BCDF.2019.100184
de Freitas C, Terrone CC, Masarin F et al (2021) In vitro study of the effect of xylooligosaccharides obtained from banana pseudostem xylan by enzymatic hydrolysis on probiotic bacteria. Biocatal Agric Biotechnol 33:101973. https://doi.org/10.1016/J.BCAB.2021.101973
Aachary AA, Prapulla SG (2011) Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr Rev Food Sci Food Saf 10:2–16. https://doi.org/10.1111/j.1541-4337.2010.00135.x
Qing Q, Li H, Kumar R, Wyman CE (2013) Xylooligosaccharides production, quantification, and characterization in context of lignocellulosic biomass pretreatment. In: Wyman CE (ed) Aqueous pretreatment of plant biomass for biological chemical conversion to fuels and chemicals. Wiley Blackwell, Oxford
Akpinar O, Erdogan K, Bakir U, Yilmaz L (2010) Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT-Food Sci Technol 43:119–125. https://doi.org/10.1016/j.lwt.2009.06.025
Pereira BS, Castrisana RN, de Freitas C et al (2021) Chemical composition determines the bioenergy potential of food waste from pre- and post-production. J Mater Cycles Waste Manag 23(4 23):1365–1373. https://doi.org/10.1007/S10163-021-01215-6
Brienzo M, Siqueira AF, Milagres AMF (2009) Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem Eng J 46:199–204. https://doi.org/10.1016/j.bej.2009.05.012
Martins RP, Schmatz AA, de Freita LA et al (2021) Solubilization of hemicellulose and fermentable sugars from bagasse, stalks, and leaves of sweet sorghum. Ind Crop Prod 170:113813. https://doi.org/10.1016/J.INDCROP.2021.113813
Cano Carmona E, Brochette-Braga MR, Aparecida Pizzirani-Kleiner A, Jorge A (1998) Purification and biochemical characterization of an endoxylanase from Aspergillus versicolor. FEMS Microbiol Lett 166:311–315. https://doi.org/10.1111/j.1574-6968.1998.tb13906.x
Gondim JAM, Moura MDFV, Dantas AS et al (2005) Centesimal composition and minerals in peels of fruits. Food Sci Technol 25:825–827
Silva NCR (2014) Using banana peels as biosorbent for the adsorption of lead (II) in aqueous solution. 2014 45 f. Completion of course work (Bachelor of Environmental Engineering) - Federal Technological University of Paraná. Campo Mourão, 2014
Orozco RS, Hernández PB, Morales GR et al (2014) Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. Bioresources 9:1873–1885
Mantovan J, Giraldo GAG, Marin BM, Kishima JOF, Mali S (2020) Valorization of orange bagasse through one-step physical and chemical combined processes to obtain a cellulose-rich material. Sci Food Agric 101:2362–2370. https://doi.org/10.1002/jsfa.10859
Bragatto J, Segato F, Squina FM (2013) Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind Crop Prod 51:123–129. https://doi.org/10.1016/j.indcrop.2013.08.062
Teixeira LC, Linden JC, Schroeder HA (2000) Simultaneous Saccharification and Cofermentation of Peracetic Acid-Pretreated Biomass. Appl Biochem Biotechnol 84–86:111–128. https://doi.org/10.1385/abab:84-86:1-9:111
Monte JR, Brienzo M, Milagres AMF (2011) Utilization of pineapple stem juice to enhance enzyme-hydrolytic efficiency for sugarcane bagasse after an optimized pre-treatment with alkaline peroxide. Appl Energy 88:403–408. https://doi.org/10.1016/j.apenergy.2010.08.009
Melati RB, Sass DC, Pagnocca FC, Brienzo M (2021) Anatomic influence of sugarcane biomass on xylan solubilization. Ind Crop Prod 164:113357. https://doi.org/10.1016/J.INDCROP.2021.113357
Pereira MAF, Monteiro CRM, Pereira GN et al (2021) Deconstruction of banana peel for carbohydrate fractionation. Bioprocess Biosyst Eng 44:297–306. https://doi.org/10.1007/s00449-020-02442-1
Iha OK, Martins GBC, Ehlert E et al (2018) Extraction and characterization of passion fruit and guava oils from industrial residual seeds and their application as biofuels. J Braz Chem Soc 29:2089–2095. https://doi.org/10.21577/0103-5053.20180083
de Figueiredo FC, Carvalho AFA, Brienzo M et al (2017) Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresour Technol 228.https://doi.org/10.1016/j.biortech.2016.12.097
Wei L, Yan T, Wu Y et al (2018) Optimization of alkaline extraction of hemicellulose from sweet sorghum bagasse and its direct application for the production of acidic xylooligosaccharides by Bacillus subtilis strain MR44. PLoS ONE 13:1–15. https://doi.org/10.1371/journal.pone.0195616
Samanta AK, Jayapal N, Kolte AP et al (2013) Application of pigeon pea (Cajanus cajan) stalks as raw material for xylooligosaccharides production. Appl Biochem Biotechnol 169:2392–2404. https://doi.org/10.1007/s12010-013-0151-0
Xu F, Sun JX, Liu CF, Sun RC (2006) Comparative study of alkali- and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr Res 341:253–261. https://doi.org/10.1016/j.carres.2005.10.019
Brienzo M, Arantes V, Milagres AMF (2008) Enzymology of the thermophilic ascomycetous fungus Thermoascus aurantiacus. Fungal Biol Rev 22:120–130
Goswani GK, Rawat S (2015) Microbial Xylanase and their Applications. Int J Cur Res Acad Revi 3:436–450
Carvalho AFA, De Oliva Neto P, De Almeida PZ et al (2015) Screening of xylanolytic Aspergillus fumigatus for prebiotic xylooligosaccharide production using bagasse. Food Technol Biotechnol 53:428–435. https://doi.org/10.17113/ftb.53.04.15.4160
Wang Y, Cao X, Zhang R et al (2018) Evaluation of xylooligosaccharide production from residual hemicelluloses of dissolving pulp by acid and enzymatic hydrolysis. RSC Adv 8:35211–35217. https://doi.org/10.1039/c8ra07140c
Aragon C, Ruiz-Matute A, Corzo N et al (2018) Production of Xylo-oligosaccharides (XOS) by controlled hydrolysis of Xylan using immobilized Xylanase from Aspergillus niger with improved properties. Integr Food Nutr Metab 5:1–9. https://doi.org/10.15761/ifnm.1000225
Li H, Xiong L, Chen X et al (2019) Enhanced enzymatic hydrolysis of wheat straw via a combination of alkaline hydrogen peroxide and lithium chloride/N, N-dimethylacetamide pretreatment. Ind Crop Prods 137:332–338. https://doi.org/10.1016/J.INDCROP.2019.05.027
Li H, Chen X, Xiong L et al (2019) Stepwise enzymatic hydrolysis of alkaline oxidation treated sugarcane bagasse for the co-production of functional xylo-oligosaccharides and fermentable sugars. Bioresour Technol 275:345–351. https://doi.org/10.1016/J.BIORTECH.2018.12.063
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES—Finance Code 001), and support of the São Paulo Research Foundation (FAPESP, process 2017/22401–8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, B.S., de Freitas, C., Contiero, J. et al. Enzymatic Production of Xylooligosaccharides from Xylan Solubilized from Food and Agroindustrial Waste. Bioenerg. Res. 15, 1195–1203 (2022). https://doi.org/10.1007/s12155-021-10373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10373-2