Abstract
In this work, the recycling feasibility of treated calamine processing mine tailing (TCPMT) with glass wastes (GW) for the production of fired bricks is investigated. TCPMT was added into mixtures at a ratio of 0, 10, 20, 30, 40 and 50 % while glass waste was added separately for each formulation at ratios of 0, 5, 10 and 15 % of the dried mass of brick mixtures. The mixes of shale for brick (ShB), which consisted as the reference material, and both wastes were prepared, pressed, dried and fired at previously optimized temperature of 1020 °C. Physical, mechanical, environmental and durability properties of fired bricks were determined. The microstructural properties of fired bricks were investigated by scanning electron microscopy (SEM) and the total porosity was assessed according to ASTM standard. The results show that the increasing substitution proportion of ShB by TCPMT leads to an increase of the porosity and water absorption and to a decrease of flexural strength and density (lighter weight bricks). At the same time, the experiments showed that the addition of glass wastes into the mixtures enhances the mechanical properties of fired bricks. However, when more than 15 % of GW is used, a white scum constituted of a sodium sulphate appears at the fired brick exposed surface. Thus, fired light bricks with suitable physical and mechanical properties could be obtained from mixtures containing up to 30 % TCPMT and 10 % of GW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increasing cost of disposal and environmental regulation laws, it is nowadays a global matter for governments and scientists to find new effective ways to manage the huge amounts of wastes produced unceasingly by worldwide industries and communities. The recycling of industrial and mine wastes is consequently a promising alternative as it allows reducing the high amounts of wastes continuously produced, decreasing its negative environmental impacts and its related disposal costs and finally reducing the greenhouse gas (GHG) emissions. For instance, recycling of mineral wastes as alternative materials in the production of fired bricks may be an alternative solution to respond to these issues. As in the cement industry, brick making processes could absorb high amounts of wastes and allows the immobilization of most of heavy metals in the fired bodies [1, 2]. The feasibility of using multiple wastes in brick manufacture has been widely investigated and reviewed [3–6]. These studies show that the targeted wastes could replace successfully and to a high extent the non-renewable natural resources (clay, sand, aggregates, etc.) extensively consumed in the construction of buildings.
Nowadays, different additives are used in bricks making (glass waste, fly ash, grog, etc.). Amongst these additives, glass waste (GW) has been widely studied in the literature [7–9]. Different sources of glass waste may be distinguished; beverage containers, windows glass, electronic devices, etc. In brick making, waste glass is used particularly for its capacity to produce high amounts of liquid phase during firing leading to simultaneously decrease the firing temperature and improve the mechanical strength of derived bricks [9, 10]. The enhancement of the mechanical strength is due mainly to the formation of glassy phase which tends to fill the porosity (solid weakness zones) and to improve the sintering mechanisms. Several parameters control the efficiency of the sintering mechanism such as chemical composition, particle size distribution, specific surface area, presence of impurities, etc. Therefore, several tests are realized to determine the optimal parameters to obtain good quality bricks.
Another waste, less known, is produced by calamine hydrometallurgical processing plant. This process, based on a sulphuric acid leaching of low oxidized zinc ores, is used to produce zinc oxides. Until now, 0.5 million tons of wastes called calamine processing mine tailings (CPMT) are disposed in the calamine hydrometallurgical processing tailings facility (Marrakech, Morocco) [11]. This typology of wastes, composed mainly of gypsum, was the subject of a recent study [12] and its feasibility to manufacture ceramic products was investigated. The results of this study showed that the use of CPMT solely may provide a potential risk of contamination towards the environment. So, it is unsafe to use it in the current form as ceramic base material. The authors recommended using it as an additive to produce ceramic products. Currently, the amount of landfilled CPMT is estimated at 0.5 million tons and the Company is continuously producing these tailings. Also, glass wastes in Morocco are unfortunately one of the recycled and valorized wastes that attract less attention. More attention has to be given to wastes separation in order to increase the price of the collected glass wastes. Brick making industry was chosen due to high demand on bricks and because Marrakech city is one of the main centers of bricks, pottery and ceramic production in Morocco. Therefore, the current work is focused on the feasibility of using TCPMT and GW as secondary raw materials for the production of fired bricks. The specific objectives aimed in characterizing the raw materials and assessing the effect of the addition of both of wastes on the physical, mechanical, environmental and durability properties of fired bricks.
Materials and Methods
A representative sample of treated calamine processing mine tailings (TCPMT) was supplied by the calamine hydrometallurgical processing plant (Morocco), located at 35 km in the south of Marrakech (Morocco) (Fig. 1). Based on a feasibility study, the company succeeded to extract the maximum possible of the remaining lead in the CPMT using a pilot flotation process. Consequently, a second tailing called treated calamine process mine tailing (TCPMT) is produced and need to be managed. The GW, composed of micronized different color bottles, was supplied by a glass recycling company. The natural shale (ShB) was supplied by a local brick plant and serves as the reference material. The methodology followed in this study to characterize the raw materials, to manufacture and characterize the technological and durability properties of fired bricks is described in Fig. 2.
Raw Materials Characterization
Physical, chemical, mineralogical, thermal and environmental properties of the raw materials were investigated. The particle size distribution was determined using a laser analyzer (Malvern Mastersizer). The specific gravity (Gs) was measured with a helium gas pycnometer (Micromeritics Accupyc 1330). The chemical composition was investigated by X-ray Fluorescence (XRF) and the elemental analysis was performed by Inductively Coupled Plasma (ICP-AES) (Perkin Elmer Optima 3100 RL) after a multi-digestion (HNO3, Br2, HF, HCl). The mineralogical composition was determined by X-Ray diffraction (Bruker AXS Advance D8), using CuKα radiation, and quantified by TOPAS software. The thermal behaviour of raw materials was investigated by thermogravimetric analysis (TGA) (TA Instruments® Q600 SDT). Moreover, the leaching behavior of raw materials was evaluated according to the Toxicity Characteristic Leaching Procedure, TCLP (US Environmental Protection Agency (US EPA) Method 1311). The extraction fluid No. 1 was used to evaluate the mobility of inorganic species. After filtration, the leachates were collected, acidified for preservation and analyzed by ICP-AES.
Fired Bricks Manufacturing
The raw materials were dried, blended and homogenized prior to the production of laboratory fired bricks. A total of twenty-four different ShB/TCPMT/GW mixtures were prepared in order to investigate the effect of the addition of TCPMT and GW on the properties of derived bricks. For each mix containing a proportion of TCPMT, designed as M0, M10, M20, M30, M40 and M50, an amount of 0, 5, 10 and 15 % of GW was separately added (based on dry weight). The tested mixes are reported in Table 1. The dried mixes were blended with 20 wt% of water and 0.02 % of barium carbonate. Barium carbonate is used in order to bond the soluble sulphates and to prevent as possible the white scum formation known as efflorescence phenomenon. Then, the mixes were pressed under 6 MPa, using a hydraulic uniaxial press, to produce laboratory green bricks (100 × 20 × 12 mm3). The green bricks were dried in a non-controlled atmosphere at room temperature for 24 h and then in an electric oven at 60 °C for another 24 h. The dried bricks were then fired in a muffle furnace (Nabertherm©) with a heating rate of 48 °C/h from ambient temperature to an optimal selected temperature of 1020 °C. The bricks were maintained at this temperature for 5 h and then cooled according to the furnace inertia.
Fired Bricks Characterization
The technological properties of fired bricks were investigated to determine the physical, mechanical and durability properties. Five brick samples by mix were tested. The firing shrinkage was measured in accordance with ASTM-C326 [13]. The water absorption, apparent porosity and density were measured using ASTM-C373 [14]. This standard is based on the Archimedes’ method. The flexural strength was investigated using an universal testing machine (Zwick Roell) according to ASTM-C674 [15]. The scanning electron microscopy (SEM) using secondary electron analysis was used to observe the porosity texture on polished sections and backscattered electron (BSE) combined with X-mapping to observe the microstructure and texture of pores on fractured samples. The efflorescence test was assessed using ASTM-C67 [16]. Moreover, the environmental behavior of bricks containing the optimal content in terms of glass and calamine wastes was assessed according to the Toxicity Characteristic Leaching Procedure, TCLP (US Environmental Protection Agency (US EPA) Method 1311). Extraction fluid No. 1 was used to evaluate the mobility of inorganic pollutants. After filtration, the leachate was collected, acidified for preservation and analyzed by ICP-AES.
Results and Discussion
Raw Materials Properties
The physical, chemical and metals leaching characteristics of the raw materials are presented in Table 2. The physical results highlight the presence of high amounts of fine particles in the TCPMT in comparison with the ShB. The GW present a high proportion of particles (92 wt%) in the range 2–63 µm. From a chemical point of view, TCPMT is mainly composed of calcium oxide (CaO) (23.5 wt%), silica (SiO2) (13.4 wt%) and iron oxide (Fe2O3) (13.3 wt%). The fluxing agents (K2O, Na2O) amount is estimated at 4.57 wt% in the ShB, at 1.4 wt% in the TCPMT and at 7.81 wt% in the GW. The low amounts of silica and alumina, compared to ShB for brick, might affect significantly the physical properties of TCPMT based fired bricks. Quartz is known to form the skeleton of ceramic bodies and alumina allows improving raw materials plasticity. The use of GW may improve the fired bricks properties due to its high content in fluxing agents. Relatively high amounts of zinc (2.14 wt%) and lead (0.86 wt%) are observed in the TCPMT sample. When heated to a temperature of 1000 °C, TCPMT present a high loss on ignition of 19.7 %, while ShB material and GW lose less matter, 6.41 and 0.47 wt% respectively. The weight loss in the case of TCPMT is explained by the decomposition of gypsum and carbonates minerals.
The results of the leaching test realized according to TCLP test are presented in Table 2. It is highlighted that the concentrations of only zinc, lead and cadmium leached from TCPMT are above the US-EPA thresholds for granular wastes in landfill [17]. The reference ShB is seen to be in accordance with these limits.
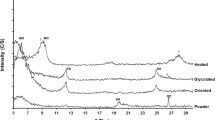
From a mineralogical point of view, it is shown that TCPMT is mainly composed of gypsum (CaSO4.2H2O), quartz (SiO2) and calcite (CaCO3) as major crystalline phases (Fig. 3). The ShB sample is composed mainly of quartz, muscovite (KAl2(AlSi3O10)(OH)2), chlorite, albite (NaAlSi3O8), ankerite (Ca(Fe,Mg,Mn)(CO3)2), calcite (CaCO3) and anatase (TiO2). The mineralogy of GW is not presented in this figure due to its high content in amorphous phase; undetectable by XRD.
The thermal behavior of TCPMT is illustrated in Fig. 4. Four weight loss steps are observed. The double successive weight losses at 148 and 158 °C might be imputed respectively to the decomposition of gypsum (CaSO4, 2H2O) to basanite (CaSO4, 0.5H2O) and then to anhydrite III (CaSO4). The third loss in the range 230–320 °C may be attributed to the iron hydroxides dihydroxylation and to the polymorphic transformation of anhydrite III to the anhydrite II [18]. The last weight loss in the range 640–800 °C can be affected to the carbonate minerals decomposition.
Fired Brick Samples Properties
The main physical and mechanical properties of the laboratory fired bricks are presented in Fig. 5. The substitution of ShB by TCPMT and GW affects significantly the fired bricks properties. The increased addition of TCPMT tends to increase the open porosity and water absorption while the flexural strength and density are affected differently and tend to be reduced. Otherwise, GW improves the flexural strength of fired bricks.
By digging in the details, it is clearly noticed that the increase in the water absorption rate and open porosity of the fired bricks by increasing the proportion of TCPMT can be explained by the decomposition of gypsum and carbonate minerals present in the TCPMT. This decomposition leads to gas release and then to higher values of porosity in the fired brick bodies. The water absorption increased rapidly from 8.73 ± 1.06 to 25.56 ± 1.22 % for the fired bricks produced respectively from the mixes M0 and M50. Moreover, it is noticed that the increased addition of GW leads to higher values of open porosity and water absorption when the proportion of TCPMT is less than 30 %. This could be explained by the increase of open pores amounts induced by the gas flow channels from the interior to the exterior of brick bodies. The movement of gas is affected by the viscosity of the liquid phase formed during firing process. Figure 6 shows some bubbles at the surface of the bricks containing 15 % of GW which are in the form of entrapped gas pockets. However, when more than 40 % of TCPMT is incorporated, the addition of GW leads to different effects and seems to decrease water absorption and apparent porosity.
In relation to weight properties, fired brick densities are observed to decrease from 2.05 ± 0.05 g/cm3 for the reference mix M0 to 1.58 ± 0.02 g/cm3 when 50 % of TCPMT is incorporated. These results are expected since the addition of TCPMT increases the amounts of volatile matter which leads to highly porous bricks. The increasing proportion of GW in brick bodies conducts also to the reduction of density. It was reported that the decrease of bulk density with the increase of heating temperature may be attributed to the pore volume expansion known as bloating phenomenon. This originates from the high pressures of gas such as carbon monoxide and carbon dioxide which are entrapped in closed pores [19]. It was reported that good quality light bricks present densities between 1.5 and 1.8 g cm−3 [20]. SEM observations on polished sections of bricks containing 15 % of GW and 30 % of TCPMT revealed the presence of entrapped pores in the interior structure of these bricks. The diameter of these pores varies in the range 1–100 µm (Fig. 7).
The firing shrinkage increased with increasing the added amount of TCPMT. However, this parameter behavior with the addition of GW does not follow a logic trend and it seems to be affected by the amount of TCPMT added to the brick. As a general rule, glass leads to the increase of firing shrinkage and the enhancement of mechanical strength due to lower values of water absorption and higher bulk density [10]. Such trend of mechanical strength improvement and density increase was effectively observed in bricks containing up to 30 % of TCPMT. After that, increasing GW addition seems to affect significantly the firing shrinkage particularly for bricks containing 15 % of GW. The strange behavior of firing shrinkage with the addition of GW could be related to an unknown effect of TCPMT.
Concerning the flexural strength, there is a strong dependence with the proportion added of TCPMT and GW is clearly visible. The flexural strength is seen to be reduced with the incorporation of TCPMT. The important values of the flexural strength were observed in the reference bricks while the lowest were observed in the mixes containing 50 % of TCPMT. The mechanical strength of ceramic products decreases usually with the increase of porosity. Moreover, this behavior could be attributed to the complex mineralogical transformations occurring during the firing and cooling processes. SEM observations show that some coarse grains in the form of crystals are formed when TCPMT is added to the bricks (Fig. 8). Those crystals were not identified in the reference bricks. The presence of such coarse grains may be explained by their refractory character which inhibited their reaction with the surrounding phases during the heating process. One may observe that these grains are weakly bounded to the surrounding phases resulting in the formation of weak grain bridges. These regions are generally considered as regions of weakness when the ceramic products are stimulated by an external force.
The results showed also that the flexural strength depends significantly of the amounts of GW added. Unlike the TCPMT, the increased addition of GW enhances the flexural strength for all the mixes. As shown in Fig. 9 (same scale SE SEM images), the addition of GW contributes to the formation of high amounts of glassy phase in samples containing 15 % of GW. However, less glassy phase is observed in samples without GW addition. Owing to its low fusion temperature, GW fine particles dissolve in the mixes and contribute to the vitrification and densification mechanisms. Increasing GW addition to 15 % leads to the enhancement of flexural strength by filling the pores with glassy phase.
The durability of fired bricks is an important factor to be controlled in the field of construction materials. The main source of deterioration of building materials is the mineralization of salts [21]. In contact with water, soluble salts contained in fired bricks migrate to the surface and when dried, an unpleasant white deposit appeared. In this study, it was observed that the incorporation of GW affects significantly the mobility of active soluble salts; these salts affect the brick body surface quality. Efflorescence test shows that only in the case where 15 % of GW is added, a white scum on the surface of all fired bricks is formed (Fig. 10). The EDS analysis shows that it is question of a sodium sulfate (Na2SO4) (Fig. 10). The white scum persists even after washing it off. The formation of the sodium sulfate may affect negatively the durability of fired bricks. It is known that this salt is a high damaging salt [22] and its crystallization may cause significant damage to fired bricks.
The results of the TCLP test are compared to the US-EPA thresholds and presented in Table 3. The TCLP test is generally used in the literature to assess the mobility of heavy metals from bricks at the end of their life service; when bricks are considered as construction and demolition wastes. Results show that the mobility of heavy metals and metalloids has significantly been reduced. Pollutants such as As, Ba, Cd, Cr, Mo and Pb are all of them under the regulatory limits. However, Zn fails to meet the requested limits. Further work has to be done to stabilize the bricks when demolished. Moreover, it was observed that the mobility of As had increased after thermal treatment. This behavior has been found by other researchers and found that the increasing release of As may be explained by two facts: (1) the increasing of the firing temperature enhances the oxides crystallinity and reduces the proportion of available sorption sites for As, so it becomes more mobile and (2) the transformation of arsenide minerals into arsenates which are more soluble. However, it is worth mentioning that As is under the requested limits fixed by the US-EPA.
Conclusions
The combined use of treated calamine processing mine tailings (TCPMT) and glass wastes (GW) as alternative materials in fired brick making was investigated in this study. The main conclusions are:
-
The incorporation of TCPMT in the matrix of fired bricks tends to increase both water absorption and open porosity which leads to decrease the flexural strength and apparent density.
-
The addition of glass wastes (GW) leads to a certain extent to the increase of the flexural strength and the decrease of the apparent density of fired bricks containing up to 30 wt% of TCPMT.
-
The use of glass wastes (GW) powder may not present the expected results of enhanced sintering, lower water absorption and increase of bulk density when gypsum rich wastes are used. When both wastes are used, high amounts of gases, released during firing process by TCPMT, may be entrapped in closed pores leading to the decrease of bulk density. This behavior may be attributed to the pore volume expansion known as bloating phenomenon.
-
The efflorescence test shows that the addition of 15 % of GW leads to the formation of sodium sulphate (Na2SO4) on the fired bricks surface. It is recommended to use less amounts of GW in order to prevent the formation of white scum on bricks surface.
-
The TCLP test indicated that that the mobility of pollutants such as As, Ba, Cd, Cr, Mo and Pb was significantly reduced after thermal treatment and are all of them under the regulatory limits. However, the concentration of Zn released from the bricks containing the optimal content in terms of TCPMT and GW was above the acceptable level. This will be the subject of a future follow-up study aiming at the stabilization of Zn in the fired bricks at the demolition level (end of life service). Research shows that the incorporation of adsorbents (magnetite on a zeolite or perlite matrix) in the raw materials before using as brick materials is a promising alternative for the stabilization of oxyanions and other heavy metals.
Based on the results obtained in this study, fired light bricks with suitable physical and mechanical properties may be produced from mixtures containing up to 30 wt% of TCPMT and 10 % of GW. The recycling of TCPMT and GW in brick making is a promising alternative in Marrakech city. However, it is important to reduce the costs related to transportation, processing and commercialization of the valorized products in order to be in a good position in terms of competition.
Abbreviations
- TCPMT:
-
Treated calamine processing mine tailings
- GW:
-
Glass wastes
- ShB:
-
Shales for brick
- GHG:
-
Greenhouse gas
- TCLP:
-
Toxicity characteristic leaching procedure
References
Mymrine, V., et al.: Oily diatomite and galvanic wastes as raw materials for red ceramics fabrication. Constr. Build. Mater. 41, 360–364 (2013)
Huang, S.-C., et al.: Production of lightweight aggregates from mining residues, heavy metal sludge, and incinerator fly ash. J. Hazard. Mater. 144(1–2), 52–58 (2007)
Zhang, L.: Production of bricks from waste materials—a review. Constr. Build. Mater. 47, 643–655 (2013)
Monteiro, S.N., Vieira, C.M.F.: On the production of fired clay bricks from waste materials: a critical update. Constr. Build. Mater. 68, 599–610 (2014)
Muñoz Velasco, P., et al.: Fired clay bricks manufactured by adding wastes as sustainable construction material—a review. Constr. Build. Mater. 63, 97–107 (2014)
Bories, C., et al.: Development of eco-friendly porous fired clay bricks using pore-forming agents: a review. J. Environ. Manag. 143, 186–196 (2014)
Demir, I.: Reuse of waste glass in building brick production. Waste Manag. Res. 27, 572–577 (2009)
Turgut, P.: Limestone dust and glass powder wastes as new brick material. Mater. Struct./Mater. Constr. 41(5), 805–813 (2008)
Tucci, A., et al.: Use of soda-lime scrap-glass as a fluxing agent in a porcelain stoneware tile mix. J. Eur. Ceram. Soc. 24(1), 83–92 (2004)
Dondi, M., et al.: Recycling PC and TV waste glass in clay bricks and roof tiles. Waste Manag. 29(6), 1945–1951 (2009)
Kaddami, A., REMINEX-MANAGEM, Morocco Oral communication 2013
Taha, Y., et al., Manufacturing of ceramic products using calamine hydrometallurgical processing wastes (revision process). J. Clean. Prod. (2016)
ASTM-C326, American Standard and Testing Materials, C326-03 (ASTM). Standard test method for drying and firing shrinkages of ceramic whiteware clays (2003)
ASTM-C373, American Standard and Testing Materials, C373-88 (ASTM). Standard test method for water absorption, bulk density, apparent porosity, and apparent specific gravity of fired whiteware products (1999)
ASTM-C674, American Standard and Testing Materials, C674-88 (ASTM). Standard test methods for flexural properties of ceramic whiteware materials (1999)
ASTM-C67, American Standard and Testing Materials, C 67 (ASTM). Standard test methods for sampling and testing brick and structural clay tile (2003)
US-EPA, Hazardous Waste Characteristics. A user-friendly reference document. October 2009 (2009)
Popescu, M., Simion, A., Matei, V.: Study of thermal behaviour up to 1550° of materials containing calcium sulphate. J. Therm. Anal. 30(2), 297–303 (1985)
Taskiran, M.U., Demirkol, N., Capoglu, A.: A new porcelainised stoneware material based on anorthite. J. Eur. Ceram. Soc. 25(4), 293–300 (2005)
Quijorna, N., et al.: Recycling of Waelz slag and waste foundry sand in red clay bricks. Resour. Conserv. Recycl. 65, 1–10 (2012)
Morillas, H., et al.: Nature and origin of white efflorescence on bricks, artificial stones, and joint mortars of modern houses evaluated by portable Raman spectroscopy and laboratory analyses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 1195–1203 (2015)
Shahidzadeh-Bonn, N., et al., Damage in porous media due to salt crystallization. Phys. Rev. E Stat., Nonlinear, Soft Matter Phys., 81 (2010)
Acknowledgments
This work was financially supported through the International Research Chairs Initiative, a program funded by the International Development Research Centre, Canada (IDRC) and supported by the Canadian Research Chairs Program. The authors thank also the research centre REMINEX-MANAGEM (Morocco), for the great help concerning the valuation of its by-product outcome of Calamine hydrometallurgical process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taha, Y., Benzaazoua, M., Mansori, M. et al. Recycling Feasibility of Glass Wastes and Calamine Processing Tailings in Fired Bricks Making. Waste Biomass Valor 8, 1479–1489 (2017). https://doi.org/10.1007/s12649-016-9657-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9657-3