Abstract
A process was developed to fractionate and isolate the hemicellulose B component of corn fiber generated by corn wet milling. The process consisted of pretreatment by soaking in aqueous ammonia followed by enzymatic cellulose hydrolysis, during which the hemicellulose B was solubilized by cleavage into xylo-oligosaccharides and subsequently recovered by precipitation with ethanol. The pretreatment step resulted in high retention of major sugars and improvement of subsequent enzymatic hydrolysis. The recovered hemicellulose B was hydrolyzed by a cocktail of enzymes that consisted of β-glucosidase, pectinase, xylanase, and ferulic acid esterase (FAE). Xylanase alone was ineffective, demonstrating yields of less than 2% of xylose and arabinose. The greatest xylose and arabinose yields, 44% and 53%, respectively, were obtained by the combination of pectinase and FAE. A mass balance accounted for 87% of the initially present glucan, 91% of the xylan, and 90% of the arabinan. The developed process offered a means for production of corn fiber gum as a value-added co-product and C5 sugars, which could be converted to other valuable co-products through fermentation in a corn wet-milling biorefinery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corn is the predominant feedstock used in the production of ethanol in the USA. The Renewable Fuels Association estimated that in 2009, 3.8 billion bushels of corn (90.4 million metric tons) were converted to 10.6 billion gallons of ethanol and 30.5 million metric tons of co-products [1]. Corn-based ethanol is produced from the starch component of the corn kernel, which comprises 70–72% of the kernel on a dry weight basis [2]. Two different processes, wet milling and dry grind, are used in the conversion of corn to ethanol.
Wet milling plants fractionate the corn into its starch, germ, fiber, and protein components. The purified starch component is utilized in fermentation to produce ethanol, while the other components are further processed to a variety of co-products. Dry grind plants, on the other hand, do not fractionate the corn kernel prior to fermentation. This results in a not only greatly reduced capital cost but also fewer co-products. The marketing of co-products is considered critical to the economic viability of ethanol [2]. Typically, co-products from both wet milling and dry grind ethanol plants are marketed as animal feed. However, they are of relatively low monetary value. Because these co-products contain cellulosic material, they hold the potential for conversion into additional ethanol or high value-added products. Utilization of these co-products in such a way adds a revenue stream to the plant, potentially improving the overall cost effectiveness of ethanol production. Corn fiber is a co-product of the corn wet milling process and is composed of the cellulosic components of the corn kernel, namely the pericarp and endosperm fiber. Recently, modifications to the dry grind process have been developed to allow pericarp fiber production there as well [3]. While industrial yields of corn fiber vary, corn fiber production averages 11.5% of the mass of corn processed on a dry basis (db) in corn wet milling facilities.
Wet milling corn fiber is of particular interest because of its high carbohydrate content and its (currently) low value [4]. In addition, unlike many other agricultural residues, corn fiber is already available onsite in ethanol facilities. Thus, its utilization as a feedstock for other processes within the facility avoids the costs of gathering and transportation associated with other agricultural residues such as corn stover. Corn fiber is a lignocellulosic biomass, composed primarily of cellulose, hemicellulose, and lignin. Although the composition of corn fiber varies by source, cellulose and hemicellulose typically account for about 50% of the dry weight, with adherent starch comprising another 10–20%. Thus, corn fiber typically consists of about 70% recoverable sugars.
Because of the complexity and recalcitrance of cellulosic biomass, effective utilization of the non-starch polysaccharides requires pretreatment prior to enzymatic hydrolysis to release fermentable sugars. While a number of pretreatment methods exist, the soaking in aqueous ammonia (SAA) process offers several benefits. SAA is carried out at moderate temperature (typically less than 90 °C), allowing for reduced reactor cost. The SAA process has the advantage of being a very simple batch process. Thus, it is much more easily implemented in a commercial plant and requires less instrumentation and control systems. It does not generate inhibitory compounds that affect yeast growth, demonstrates a high retention of major sugars, a significant degree of lignin removal, and results in a pretreated material that is highly enzymatically digestible [5].
After pretreatment and enzymatic hydrolysis, the resulting sugar stream will contain hexose and pentose sugars, derived from the cellulose and hemicellulose components, respectively. Because Saccharomyces cerevisiae, the organism used in industrial fuel ethanol fermentation, cannot utilize pentose sugars, it may be advantageous to fractionate the cellulose and hemicellulose components from one another to allow separation of the hexose and pentose sugars. The glucose generated may be used for ethanol production by the yeast, whereas the pentose sugars can be used for production of value-added co-products. The isolated hemicellulose fraction itself may be sold as a value-added co-product.
The hemicellulose fraction of corn fiber is an arabinoxylan, typically referred to as corn fiber gum (CFG). It is considered to be one of the most complex and recalcitrant hemicelluloses. It is composed typically of 42–48% xylan and 29–31% arabinan [6]. Industrially, it has several uses. In its native form, CFG may be extracted and used as an emulsifier in the beverage industry [7]. If CFG were hydrolyzed, it could serve as a source of xylose and arabinose for use in a number of high-value product fermentations. Corn fiber hemicellulose is composed of two fractions, hemicellulose A and hemicellulose B. Hemicellulose A typically accounts for less than 10% (w/w) of the total hemicellulose fraction and is discarded in most processes that extract corn fiber hemicellulose [6]. Hemicellulose A is insoluble in water at acidic pH, while hemicellulose B remains soluble under these conditions. Thus, the fractionation of the hemicellulose A component from the total hemicellulose is simple and may be carried out relatively easily.
Corn fiber gum is composed of β-1,4-linked xylose, forming the xylan backbone. This constitutes about half of the CFG mass (db). Typically about 80% of the xylan backbone of CFG is substituted primarily with arabinose. The xylan chains are crosslinked to one another by diferulic bridges [8]. To form the diferulic bridge, two ferulic acid residues (each substituted to a separate xylan chain) form an ester linkage with one another. The cellulose fibers, along with structural proteins, embed within the lattice formed by the crosslinked xylans, thus forming the corn fiber cell wall. Proteins are associated with the hemicellulose, as even purified CFG may contain up to 5% protein [9].
The extraction of hemicellulose from corn fiber is desirable for several reasons. First, the fractionation of hemicellulose allows it to be hydrolyzed separately from the cellulose, yielding separate hexose-rich and pentose-rich streams. This allows greater flexibility, as the pentose sugars can be utilized for any of a number of high-value product fermentations, while the glucose may be added back into ethanol fermentations without the need for modifications to the existing ethanol process. Additionally, if hydrolysis of CFG is not to be performed, the CFG may be sold as a valuable product, for example, for use as a beverage flavor emulsifier.
Due to the complexity of CFG, reported yields of xylose and arabinose are generally much lower than those typical of glucose in cellulose hydrolysis. It has been noted that currently, no commercially available xylanase preparation is able to efficiently hydrolyze CFG [4]. However, it is theorized that a combination of commercially available enzymes may contain the appropriate activities. The objectives of the present work were to develop an enzyme-based process to fractionate corn fiber cellulose and hemicellulose and isolate the hemicellulose fraction, and to investigate the potential applications of commercial enzymes, either individually or in combinations, for hydrolysis of the isolated hemicellulose fraction to produce xylose and arabinose.
Materials and Methods
Chemicals and Corn Fiber
Corn fiber was provided by Archer Daniels Midland (Decatur, IL, USA). All chemicals were reagent grade and purchased from various suppliers. Novo 188 (β-glucosidase) was purchased from Sigma–Aldrich (St. Louis, MO, USA). GC-220 (cellulase), Spezyme Fred (α-amylase), PEKTOZYME Essential (pectinase), and Optidex L-300 (glucoamylase) were provided by Genencor, a Danisco Division (Rochester, NY, USA). Novozymes NS50030 (xylanase), Novozymes NS50012 (multi-enzyme complex), and Novozymes NS50014 (xylanase) were provided by Novozymes (Franklinton, NC, USA). Depol 692 L (ferulic acid esterase) was provided by Biocatalysts Inc. (Wales, UK).
Corn Fiber Starch Removal
To separate the glucose contributions from starch and from cellulose, starch was removed from the corn fiber prior to pretreatment. Corn fiber was mixed with water at 10% dry solids loading, and the pH adjusted to 6.0 by the addition of H2SO4 (either 72% w/w [13.2 M] or 5% w/w [0.92 M] depending on distance from target pH). Spezyme Fred (α-amylase) was added at a loading of 42 μL/g dry corn fiber. The mixture was then heated to 80 °C for 1 h with mechanical stirring and allowed to cool to 55 °C with the aid of a chilled water bath. The mixture was weighed to determine the amount of water lost due to evaporation during heating and that amount was added back using deionized water. The pH was adjusted to 4.5, and Optidex L-300 (glucoamylase) was added at a loading of 50 μL/g dry corn fiber. The mixture was then placed in an orbital shaker maintained at 55 °C and 250 rpm overnight. The solids (destarched corn fiber (DCF)) were recovered by filtering and pressing with cheese cloth. The DCF was then spread in a thin layer in a large dish and dried at 40 °C with occasional stirring to allow even drying. Once dried, the recovered DCF was stored in a sealed container at 4 °C until use. The destarching procedure was carried out one time initially, and this batch of DCF was used as the starting point for all subsequent experiments. The composition of the DCF is shown in Table 1.
Process Batch Sizes
Batches (defined as an iteration of the process beginning with pretreatment through obtaining the precipitated CFG) were conducted with the pretreatment of 7 g dry DCF for the construction of the mass balance, in order to conserve material and allow for all steps to be carried out analytically. Additional batches were conducted beginning with 70 g dry DCF for the purpose of obtaining sufficient dry CFG to carry out subsequent CFG enzymatic hydrolysis experiments. Throughout the discussion, the small batches (7 g) are enumerated 1–3, and the large batches (70 g) lettered A–D.
Soaking in Aqueous Ammonia Pretreatment
SAA pretreatment was carried out by combining either 7 or 70 g DCF and 15% (w/w) NH4OH (7.75 M) at a solid to liquid ratio of 1:11 based on dry DCF in a sealed media jar. The well-mixed slurry was then held at 65 °C for 8 h. At the end of this time, the cap was removed and the open jar left at room temperature (about 25 °C) in a fume hood for 3 h to allow ammonia evaporation. The pretreated biomass was then washed with deionized water to remove soluble lignin and residual ammonia. The pretreated solids were separated from the wash water by centrifugation at 8,000 rpm for 30 min. The supernatant was then decanted, and the pretreated solids were recovered. To allow for analytical recovery of the pretreated material in the small batch, this washing process was carried out a total of three times. In the large batches, the material was washed a total of six times. The recovered solids, i.e. the pretreated destarched corn fiber (PTDCF) were sampled for moisture determination and compositional analysis and stored in a sealed container in the refrigerator until use.
Cellulose Hydrolysis
The PTDCF was combined with water at a solids loading of 5% (db). The pH of the slurry was adjusted to 5 with H2SO4 as described previously. 1 N NaOH was used to correct the pH in the case of overshoot. Novo-188 (β-glucosidase) was added at 0.04 ml/g glucan (30 CBU/g glucan) and GC-220 (cellulase) at 0.35 ml/g glucan (15.75 FPU/g glucan). Hydrolysis was then carried out for 72 h at 55 °C with 250 rpm orbital shaking.
Hemicellulose A Removal
Following hydrolysis, the pH was about 4.5, sufficient to cause the hemicellulose A fraction to precipitate. The hydrolysate was centrifuged (8,000 rpm, 30 min) to remove the residual solids (RS) and precipitated hemicellulose A, and the hydrolysate supernatant (HSN) recovered by decanting. The pH of the HSN was raised to 9 by the addition of 1 N NaOH and then mixed to dissolve any remaining hemicellulose A. The hydrolysate was then passed through a Whatman #1 filter paper to ensure it was free of solids. The pH of the filtrate was reduced to 3 by the addition of 72% (w/w; 13.2 M) H2SO4, precipitating any remaining hemicellulose A, which was removed by filtration.
Hemicellulose B Precipitation
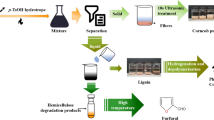
To precipitate the hemicellulose B, seven volumes (i.e. seven times the volume of the HSN used) of cold anhydrous ethanol (stored in the refrigerator prior to use) was slowly added one volume (i.e. the same volume as that of the HSN) at a time to the HSN with stirring. Mixing was continued for 15 min after addition of the final volume. The mixture was allowed to settle for 1 h. After settling, the liquid phase was decanted. The decanted liquid was passed through a pre-weighed Whatman #1 filter paper. After filtration of the ethanol, the filter was dried at 105 °C overnight and then weighed to quantify the mass of hemicellulose lost in the decanted ethanol. A small amount (100 ml) of cold ethanol was added to the precipitate, and mixing was resumed for 15 min. This second ethanol addition removed residual water from the precipitate, reducing its stickiness. The mixture was then passed through a pre-weighed Whatman #1 filter paper. The cake was washed with 100 ml of cold ethanol, dried at 105 °C overnight, and weighed. The resulting powder was the dried hemicellulose B, which was stored in a sealed vial until use. The process developed to fractionate corn fiber gum is shown in Fig. 1. The efficiency of the hemicellulose recovery was determined by dividing the xylan and arabinan content of the recovered CFG by the xylan and arabinan initially present in the pretreated material that was subjected to cellulose hydrolysis.
Process for the extraction and isolation of corn fiber hemicellulose. Solid materials are indicated by a box, liquids are indicated by an oval. All material streams are represented in italic font. Unit operations are in plain text with no symbol (box or oval). Streams marked with asterisk are those utilized in the mass balance
Corn Fiber Gum Enzyme Hydrolysis
The CFG was dissolved in water to a concentration of 35 g CFG/L (dry basis). The pH was adjusted to 5 with 1 N NaOH, and the solution was added to a series of microcentrifuge tubes, at 1 ml solution each. Each tube was dosed with the enzyme(s) called for in the particular experiment at the dosage indicated. The enzymes used in the experiments are listed in Table 2. Hydrolysis was carried out at 50 °C for 72 h. Each experiment was conducted in duplicate, with the averages reported. Initial and final samples were taken and analyzed for xylose and arabinose content via HPLC (described below). The theoretical yield (100% conversion) was determined by dilute acid hydrolysis of the CFG, as described below.
Analytical Procedures
The glucan, xylan, and arabinan content of the DCF, PTDCF, and RS were determined according to the NREL LAP Determination of Structural Carbohydrates and Lignin in Biomass [11]. The composition of the CFG was determined by dilute acid hydrolysis, described by Hanchar et al. [4], with the modification that a sample of synthetic sugars mimicking the expected composition of the CFG was also hydrolyzed under the same conditions to quantify sugar destruction, and the data adjusted accordingly, as recommended in the NREL procedure [11]. In both procedures, monomeric sugars were determined by HPLC. The HPLC was a Shimadzu system utilizing either the Bio-Rad Aminex HPX-87P or Bio-Rad Aminex HPX-87H columns. Standards containing glucose, xylose, and arabinose were analyzed with each set of samples to correct for any bias caused by the differing columns, though none was observed. The flow rate and mobile phase were 0.6 ml/min of nanopure 18 MΩ deionized water (HPX-87P column) or 0.6 ml/min of 5 mM H2SO4 (HPX-87H column), and the system was equipped with an RID detector. All analytical procedures were carried out in duplicate, and the averaged results reported.
Mass Balance
To determine yields throughout the process, mass balances with respect to glucan, xylan, and arabinan were constructed. In cases where data were in the form of glucose, xylose, or arabinose, it was converted to glucan, xylan, or arabinan by multiplying by 0.9 (glucose) or 0.88 (xylose and arabinose) as described by Gulati et al. [12]. The composition of the DCF, PTDCF, and CFG were determined as mentioned above. The composition of the DCF was used to determine the initial mass of glucan, xylan, and arabinan present prior to pretreatment. All percentages reported in the mass balance were calculated as the percentage of the initial mass of the component present in the DCF. After pretreatment, the composition of the pretreated solids was used to determine the amount of glucan, xylan, and arabinan recovered. These were subtracted from the amounts initially present to determine the pretreatment loss, referred to as “PT Loss.” Samples of the PTDCF were taken for moisture determination and compositional analysis. The amounts of glucan, xylan, and arabinan in these samples were accounted for in the mass balance as “Sample Loss.” Also included in this category were losses due to material left in containers after collection, for example the amount of pretreated material stuck to the side of the centrifuge bottle after washing recovery. This amount was very small but included for completeness. Such material losses were calculated by weighing containers prior to use and re-weighing after use to obtain the mass of solids not recovered. Next, the glucan, xylan, and arabinan content of the recovered CFG was subtracted from that of the PTDCF subjected to cellulose hydrolysis, representing the masses of sugars present that were not recovered as CFG. These are accounted for in the category labeled “Pass Through.” The glucan, xylan, and arabinan content of the recovered CFG was then accounted for, as was the glucan, xylan, and arabinan content of the CFG lost during the initial ethanol decanting. These values were summed and included as one value, “CFG.” The masses of each component in each category were then summed to determine the total percentage of the component accounted for in the mass balance, labeled “Sum.”
Statistical Analysis
Presented results represent the average of two trials except when noted otherwise. Standard deviations are also presented where applicable.
Results and Discussion
SAA Pretreatment and Cellulase Hydrolysis
The glucan, xylan, and arabinan contents of the material resulting from SAA pretreatment of DCF are shown in Table 3. The recovery percentages of these components, defined as the percentage of the component present in the washed PTDCF, as compared to the untreated DCF, (i.e. the fraction of each component that was retained) are shown in Table 4. These data confirm very high retention of the available carbohydrate fractions by the SAA treatment, specifically in the case of glucan, where 94% of the initial material was preserved. The figures for xylan and arabinan retentions were 76% and 78%, respectively. The pretreated material was hydrolyzed with cellulase enzymes, releasing, on average, 85.41% of available glucan as glucose. Hydrolysis efficiencies for xylan and arabinan were, as expected, much lower, 8.15% and 16.95%, respectively. This, combined with the observation that only a small amount of residual solids were recovered after hydrolysis, containing very little xylan or arabinan, confirms that the xylan and arabinan were largely degraded into soluble oligomers during the hydrolysis.
Hemicellulose Recovery
The composition of the recovered hemicellulose (CFG) is shown in Table 5. The xylan and arabinan content of the recovered CFG was lower than expected (CFG is composed typically of 42.24–47.52% xylan and 29.04–30.8% arabinan [6]). This is likely due to a mass dilution effect. Because the PTDCF was washed minimally to allow for analytical solids recovery, it is believed that some residual lignin ended up in the recovered CFG. Because of this, the total mass of the material recovered increased, without a subsequent increase in xylan and arabinan content, as lignin contains neither of these compounds. Thus, the mass percentages of xylan and arabinan in the recovered material would appear lower than expected due to dilution by lignin, as observed in Table 5. To provide further evidence of this mass dilution effect, the composition of the CFG recovered from the larger batches was examined. It was found to have the expected xylan and arabinan content, shown in Table 6. It should be noted that while the mass percentages in Table 5 are lower than those in Table 6, the xylan/arabinan ratios are comparable, confirming the mass dilution effect. Next, the amount of material recovered in both sets of experiments was compared on a per volume basis (the mass of material recovered was divided by the volume of HSN from which it was recovered). It was observed that more material was recovered per volume of HSN used in extraction in batches 1–3. The compositions shown in Tables 5 and 6 were used to determine the mass of xylan and arabinan recovered on a per volume basis, shown in Table 7. While a greater mass of CFG was recovered per ml HSN in batches 1–3, the mass of xylan and arabinan therein was comparable to that seen in batches A–D. This provides further support for the mass dilution effect previously mentioned.
The efficiency of the hemicellulose recovery was low, 45 ± 0.03% for xylan and only 28 ± 0.03% for arabinan, on average. These figures were obtained by dividing the masses of xylan and arabinan precipitated by the masses of xylan and arabinan present in the PTDCF that was utilized in cellulose hydrolysis. An example of this calculation, using data from batch 1, is presented below.
After losses due to pretreatment and sampling, 1.71 g of PTDCF (db) was available for further processing. It contained 0.46 g xylan and 0.35 g arabinan (based on the composition provided in Table 3). Treatment of this material ultimately resulted in the precipitation of 0.20 g xylan and 0.09 g arabinan. Dividing these values by the masses of xylan and arabinan initially present in the PTDCF provides recovery efficiencies of 43% for xylan and 26% for arabinan.
The relatively low recoveries observed were likely due to the fact that the solubilities of the xylo-oligosaccharides in ethanol are a function of chain length, i.e., longer chain xylo-oligosaccharides are precipitated more easily, at lower ethanol concentration. The low efficiencies, thus, are likely a result of lower molecular weight xylo-oligosaccharides that remain soluble upon the addition of seven volumes of ethanol. The observation that a precipitate began to form (presumably from the largest xylo-oligosaccharides present) upon addition of the second volume of ethanol supports this theory. It was expected that the arabinan recovery would be lower than that observed for xylan, due to the mechanism of hydrolysis of corn fiber hemicellulose. In order for the xylan backbone to be accessible to enzymes, the substituted species (i.e. arabinose) must first be removed. If the hemicellulose is solubilized by cleavage into xylo-oligosaccharides, it follows that some arabinose must be liberated from the hemicellulose and thus not available for precipitation. This was supported by the observation of a greater hydrolysis yield for arabinose, as mentioned above.
Process Mass Balance
A mass balance was conducted around the process for glucan, xylan, and arabinan. The detailed mass balance is provided in Table 8. The overall mass balance was good, with 86.89% glucan, 90.59% xylan, 89.90% arabinan accounted for. The major sugars compositions of each stream utilized in the mass balance calculation were provided previously in Table 1 (DCF), Table 3 (PTDCF), and Table 5 (CFG).
Hemicellulose Hydrolysis
Commercially available enzymes were used individually and in combinations in an attempt to hydrolyze CFG. Xylanases (N30, N12, N14) were chosen for their expected activity against the xylan backbone. Ferulic acid esterase (D) was chosen to cleave the diferulic bridges cross linking the arabinoxylan, which was expected to improve their digestibility. Pectinase (E) and β-glucosidase (B) were chosen for their possible side activities, which have shown some success in hydrolyzing CFG in previous work [13]. The enzymes were first tested individually at 1× and 10× the manufacturers recommended dosage (see Table 2). The yields, based on total xylan and arabinan available in the hydrolysis, are shown in Table 9. At the 1× dosage level, the highest yields were produced by pectinase, which is not surprising because it was chosen for its wide range of side activities. At the 10× level, the yields achieved with D were slightly higher than with E. The low yields demonstrated by xylanases N30 and N14 were not surprising, as these enzymes were advertised as being relatively pure in their activity (see Table 2). The low yield demonstrated by N12 was more surprising, as this enzyme was advertised as a complex with multiple activities.
To investigate potential synergism between these enzymes, combinations of the enzymes were tested for their ability to hydrolyze CFG. The first set of experiments utilized N30 as the xylanase. The results are shown in Table 10.
At the 1× dosage level, the highest yields were obtained by the combination of all four enzymes. However, the yields in the E + D + B trial were only slightly lower, suggesting that N30 was very ineffective. This is consistent with the results found in the individual trials. This was also seen when N30 was added to E + D and to D + B. It was also observed that B was not very effective at the 1× dosage level, causing very modest increases when added to E + D. However, the combination of D and B exhibited a much higher yield than D alone, suggesting a synergistic effect between the two enzymes.
At the 10× dosage level, the greatest yields were exhibited by E + D + N30, although the increase over E + D was very modest, consistent with the 1× dosage results and those of the individual trial. Interestingly, the addition of B to E + D and to E + D + N30 both caused a decrease in yield of about 5–7%. This effect was not seen in the 1× dosage trials. In the case of N30 + B, yields were actually decreased by the increase in dose. These observations suggest that B is problematic when used in high concentration.
The yields exhibited by N30 were poor, as expected. Thus, xylanases N12 and N14 were investigated as alternatives. The results are shown in Table 11. At the 1× dosage level, the yields obtained by N14 + E + D, N12 + E + D, and N14 + N12 + E + D were very similar, suggesting little difference in the effectiveness of N12 and N14. The same trend was observed at the 10× dosage level. At both dosage levels, the combination of N12 + N14 was ineffective, which is consistent with the individual trials.
The highest yields in the multiple enzyme trials were obtained by the combinations of E + D and E + D + xylanase (little difference was seen between xylanases). In the single enzyme trials, the highest yield was obtained by D, though it was a small increase over E at a significantly greater loading (see Table 2). Thus, E was loaded at 100× dosage and utilized alone and in combinations of E + D and E + D + N30. D and N30 were loaded at the 10× dosage level. The results are shown in Table 12. When E is loaded at the 100× dosage level, the highest yields are observed. The addition of D increased yields over E alone, while the addition of N30 to the mixture was ineffective. Because of the ineffectiveness of the addition of N30, the highest performing combination was considered to be E + D.
Conclusion
A process was developed to fractionate the hemicellulose component of corn fiber. Destarched corn fiber was pretreated by soaking in aqueous ammonia, which retained 94% of the available glucan, 76% of the available xylan, and 78% of the available arabinan. The pretreated material demonstrated a glucan digestibility of 85% when hydrolyzed with cellulase enzymes. Xylan and arabinan digestibilities were low but very little xylan and arabinan remained solid after hydrolysis, suggesting that the majority of the arabinoxylan was solubilized in the form of xylo-oligosaccharides. These were precipitated by the addition of ethanol. The precipitated solids were the corn fiber gum. A mass balance was constructed around the process, which accounted for 87% of glucan, 91% of xylan, and 90% of arabinan. A variety of enzymes were utilized in an attempt to release xylose and arabinose from the recovered corn fiber gum. The greatest xylose and arabinose yields (44.45% and 52.89%, respectively) were achieved when PEKTOZYME Essential, a pectinase, was loaded at 100× manufacturer recommended dosage along with Biocatalysts Depol 692 L, a ferulic acid esterase loaded at 10× MRD.
While it has been demonstrated that the developed process is technically feasible, much work remains in its optimization. A significant amount of the hemicellulose was not recovered by ethanol precipitation, presumably due to low chain length. Thus, a cellulose hydrolysis method should be developed that retains the high glucan digestibility demonstrated above but solubilizes the arabinoxylan by producing longer chain xylo-oligosaccharides. This would allow a greater recovery of xylan and arabinan from a smaller volume of ethanol. Additionally, the volume of the hydrolysate could be reduced by evaporation or ultrafiltration, again reducing the needed amount of ethanol. Finally, the enzymatic hydrolysis of corn fiber gum remains a challenge, and additional work is needed to generate improvements in this step.
Abbreviations
- CFG:
-
Corn fiber gum
- DCF:
-
Destarched corn fiber
- DSW:
-
Destarching water
- HSN:
-
Hydrolysate supernatant
- PT:
-
Passthrough
- PTDCF:
-
Pretreated destarched corn fiber
- RS:
-
Residual solids
- SAA:
-
Soaking in aqueous ammonia
References
RFA (2010) Ethanol Industry Outlook. Available from: http://www.ethanolrfa.org/page/-/objects/pdf/outlook/RFAoutlook2010_fin.pdf
Bothast, R. J., & Schlichler, M. A. (2005). Applied Microbiology and Biotechnology, 67, 19–25.
Dien, B. S., Nagle, N., Hicks, K. B., Singh, V., Moreau, R. A., Tucker, M. P., et al. (2004). Applied Biochemistry and Biotechnology, 133–166, 937–949.
Hanchar, R. J., Teymouri, F., Nielson, C. D., McCalla, D., & Stowers, M. D. (2007). Applied Biochemistry and Biotechnology, 136–140, 313–325.
Drapcho, C. M., Nghiem, N. P., & Walker, T. H. (2008). In L. S. Hager (Ed.), Biofuels Engineering Process Technology (pp. 69–195). New York: McGraw-Hill.
Doner, L. W., & Hicks, K. B. (1997). Cereal Chemistry, 74(2), 176–181.
Yadav, M. P., Johnston, D. B., Hotchkiss, A. T., & Hicks, K. B. (2007). Food Hydrocolloids, 21, 1022–1030.
Saha, B. C. (2003). Journal of Industrial Microbiology & Biotechnology, 30, 279–291.
Yadav, M. P., Fishman, M. L., Chau, H. K., Johnston, D. B., & Hicks, K. B. (2007). Cereal Chemistry, 84(2), 175–180.
Kim, T. H., Taylor, F., & Hicks, K. B. (2008). Bioresource Technology, 99, 5694–5702.
Determination of structural carbohydrates and lignin in biomass (2008) National Renewable Energy Laboratory. Available from: http://www.nrel.gov/biomass/pdfs/42618.pdf
Gulati, M., Kohlmann, K., Ladisch, M. R., Hespell, R., & Bothast, R. J. (1996). Biores. Tech., 58, 253–264.
Dien, B. S., Ximenes, E. A., O’Bryan, P. J., Moniruzzaman, M., Li, X. L., Balan, V., et al. (2008). Biores. Tech., 99, 5216–5225.
Acknowledgements
The authors would like to thank Dr. Tae Hyun Kim (Dept. of Agricultural and Biosystems Engineering, Iowa State University), Dr. Terry Walker (Dept. of Biosystems Engineering, Clemson University), and Ms. Jennifer Thomas, Mr. Gerard Senske, and Mr. John Minutolo (USDA ARS ERRC) for providing their invaluable expertise and assistance throughout the course of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Nghiem, N.P., Montanti, J., Johnston, D.B. et al. Fractionation of Corn Fiber Treated by Soaking in Aqueous Ammonia (SAA) for Isolation of Hemicellulose B and Production of C5 Sugars by Enzyme Hydrolysis. Appl Biochem Biotechnol 164, 1390–1404 (2011). https://doi.org/10.1007/s12010-011-9220-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9220-4