Abstract
We have numerically studied structure and dynamics in Mg2SiO4 liquid. Eight models at pressure up to 40 GPa and at a temperature of 3500 K have been constructed by molecular dynamics simulation. The structural characteristics such as short-, intermediate-range order (denoted as SRO, IRO, respectively) structure and network structure are analyzed via the radial distribution functions, coordination units, bond angle, bond length as well as the number of corner-, edge- and face-sharing bonds and characteristics of all type OTy linkages. To clarify the dynamical properties, the models at different pressures are relaxed in NVE ensemble for a long time. The diffusivity of the individual atomic species is determined through the dependence of the mean-squared displacement on number of MD steps. Under compression, the local environment around Si and Mg atoms as well as the IRO structure in Mg2SiO4 changes significantly. The degree of polymerization of the Si–O network increases with pressure. The Si–O bonds are broken; the Mg atoms tend to incorporate into the Si–O network via both bridging oxygen (BO) and non-bridging oxygen (NBO) to form the –Si–O–Mg– network in Mg2SiO4 liquid. In the considered pressure range, the Mg atom always diffuses faster than the O atom and the O atom diffuses faster than the Si atom. The diffusivity of Si, Mg and O atom decreases with increase in pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium silicate (MgO–SiO2) is one of the major planetary materials. Thus, the understanding of its properties under high temperature and pressure conditions is important to understand the interior structure of Earth. Furthermore, magnesium silicate is also an important material in many high technology applications such as porous ceramic membranes for catalytic reactors, biomedical glass and refractory brick. Hence, the knowledge of structure and properties of MgO–SiO2 in both glass and liquid is necessary to our understanding about the physical and thermal evolution of the Earth as well as controlling the process of material fabrication technology. Therefore, MgO–SiO2 system has been widely studied by different experiments and simulation methods during the last several years [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. However, experiment is usually difficult to do due to the high melting temperature of MgO–SiO2 (2163 K ± 25 K [7, 13]). Available experimental results are usually limited to the ambient or low pressure and temperature. Using neutron and X-ray diffraction data with a reverse Monter-Carlo modeling [11,12,13], authors indicated that the mean coordination number (CN) of Mg is 4.5 ± 0.1 and Mg–O bond length (BL) is about 1.99 ± 0.01 Å and 2.21 ± 0.01 Å in MgSiO3 glass. For Mg2SiO4 glass, each Si atom links to 4 oxygen to form SiO4 tetrahedron with the mean Si–O BL of approximately 1.60–1.64 Å. X-ray diffraction study [13] showed that in MgSiO3 glass, there are two Mg–O BLs at 2.50 Å and four Mg–O BLs at 2.08 Å in the MgO6 distorted octahedra. Moreover, the mean BL of Mg–O in MgO4 tetrahedra is 2.04 Å. The Mg–O average CN is 4.5 [9, 14]. However, this value is 5.1 in ref [7, 15] and 5.0 in ref [10]. In Mg2SiO4 at 300 K and 0 GPa [10], the Si–O average CN is 4.1, the Si–O and Mg–O BLs are 1.63 and 2.0 Å, respectively.
The nuclear magnetic resonance experiment [16] found that Mg atom is dominant sixfold coordinated and minor fivefold coordinated in both MgSiO3 and Mg2SiO4 systems. Other studies [3, 17] showed that structure of MgSiO3 consists of MgOx polyhedra (x = 4, 5, 6) which are linked to the SiO4 polyhedra by corner-sharing bonds. In situ diffraction studies of magnesium silicate liquid [3] demonstrated the Mg–O BLs in MgO4 polyhedra are shorter than the one in other MgOy polyhedra (y = 5, 6). The high proportion of MgO4 units (68.80%) indicated that the role of Mg can act as a network former in magnesium silicate. Computer simulation can give insight the microstructure and dynamics of multi-component oxide systems as MgO–SiO2 system. Recently, many simulations of multi-component oxide systems have been focused on investigation of TOx structural units as well as distribution of linkages between adjacent TOx [18,19,20,21,22,23]. The structure of MgSiO3 glass consists of the SiO4 tetrahedra network which connects to each other via one, two and three bridging oxygens (BO) [18]. The existence of free oxygen (FO) atoms may be the cause of the inhomogeneous of Mg distribution in MgSiO3 glass [19]. Using MDS, Omar et al. [7] indicated that in Mg2SiO4 melts, the CN of Si is mainly four and changes slightly under compression; meanwhile, the CN of Mg increases from five to seven at 24 GPa. The Si–O–Si bond angle decreases as increasing pressure and the IRO structure depend on pressure. Using ab initio MDS for MgSiO3 melt [24], authors showed the CN of Si significantly increases; meanwhile, the CN of Mg and O almost unchanged with pressure. At ultra-high pressure to 464.7 GPa, the mean CN of Mg, Si and O is about 8.3, 7.3 and 9.9, respectively. In the work [17], the results illustrated the Mg–O BL is about 1.92–1.96 Å. Meanwhile, in Ref [25] the authors indicated the Mg–O BL is about 2.0 Å. The proportion of MgO4 is dominant (67.4%). Fraction of MgO5 and MgO6 is 30.2% and 4.7%, respectively. In other study [26], the results reported that the mean Mg–O BL is about 2.07 Å and MgO6 units is distorted. For Mg2SiO4 forsterite, by first principles simulation, Ghosh et al. [27] showed that at ambient pressure, the Si–O and Mg–O BL are 1.63 and 1.98 Å, respectively. The O–O, Si–Si, Mg–Si and Mg–Mg distances are 2.68, 3.00, 3.19 and 2.92 Å, respectively. The works [4, 28] also studied the local environment of oxygen atoms. The number of FO increases with pressure. In Mg2SiO4 liquid at 3000 K, the work [4] showed that the Mg–O and Si–O BL are 1.97 and 1.63 Å, respectively. The Si–O and Mg–O average CN are 4.1 and 5.1, respectively. The viscosity and diffusivity of species of atoms in MgSiO3 and Mg2SiO4 are also indicated in woks [4, 8]. In addition, the structural and chemical local orders as well as degree of polymerization of the Si–O network in silicate glasses and melts can be evaluated by the Qn species. In fact, the mixture of Qn species (n = 1–4) relates directly to different glass properties and to different silicate melt behaviors [9, 10, 13].
This paper serves as the basic knowledge about microstructure and dynamics of Mg2SiO4 liquid under compression at atomic level. Furthermore, the structural organization, network structure and degree of polymerization of the silica network in Mg2SiO4 liquid are also clarified in detail in this work.
2 Calculation method
The models of Mg2SiO4 liquid (3500 K, 0–40 GPa) have been constructed by MDS using periodic boundary condition. The Oganov potential was used in this work [28,29,30,31,32]. The Verlet algorithm was applied to integrate Newton’s equation of motion with the MD step of \(\Delta t\) = 0.5 fs. The MD programs were written in C language and executed on Linux operating system. The MD initial configuration is generated by randomly placing all atoms in a simulation box with density of 3.47 g/cm3 [33,34,35]. Next, the model is heated up to 6000 K to remove the effect of remembering initial configuration. Then, the model is cooled down to the temperature of 3500 K at ambient pressure with the cooling rate of about (2.5)1012 K/s. After that, it relaxed in NPT ensemble (constant pressure and temperature) for a long time (over 106 MD steps) to get the equilibrium sample. From this model, we continue compressing to desired pressure from 5 to 40 GPa and at 3500 K. Finally, these models are relaxed for a long time to reach the equilibrium at constant temperature and pressure for 106–107 MD steps without any disturbance. By this way, we obtained eight models for Mg2SiO4 system. The density of Mg2SiO4 liquid at ambient pressure is in good agreement with calculated result and experiment in works [33,34,35]. The structural properties of each model are calculated by averaging over 1000 configurations during the last 105 MD steps. To study dynamical properties, the obtained models are relaxed in NVE ensemble (constant volume and energy). The dependence of mean-squared displacement of atoms on MD steps allows to calculate the diffusion coefficient of Si, Mg, O atom via Einstein equation \(D = \lim_{t \to \infty } \frac{{r^{2} \left( t \right)}}{6t}\) where <r(t)2> is mean-squared displacement over time t.
3 Results and discussion
3.1 Structural properties
The SRO structure in Mg2SiO4 liquid under compression is clarified via analyzing the pair radial distribution functions (PRDF) of Si–O (gSiO), Mg–O (gMgO) and O–O (gOO), distribution of TOn coordination units (T = Si, Mg), distribution of O–T–O bond angle and T–O BL in TOn coordination units. The results of the gSiO and gMgO at different pressures are shown in Fig. 1. The position of the first peak of gSiO and gMgO indicates the Si–O and Mg–O BL, respectively. It can be seen that at 0 GPa, the Si–O, Mg–O and O–O BLs are 1.58, 1.90 and 2.66 Å, respectively. This result is compared with previous experiment and simulation [4, 7, 9, 10, 12, 14, 15, 28] (see Table 1).
The position of the first peak of gSiO and gMgO tends to shift to right with pressure. Meanwhile, the position of the first peak of gOO displaces strongly to left under compression. This reveals that the average Si–O and Mg–O BLs increase, but the average O–O BLs decrease as the pressure increases. The BLs of Si–O, Mg–O and O–O are 1.60, 1.92 and 2.54 Å, respectively, at 40 GPa. The elongation of the Si–O and Mg–O BL under compression will be explained in detail in the next section. To calculate the CN, we have used the cut-off distance rSi–O = 2.4 Å and rMg–O = 3.02 Å chosen as first minimum after the first peak of the Si–O and Mg–O PRDF, respectively. The mean CN of Mg, Si in Mg2SiO4 liquid (3500 K, 0 GPa) is about 4.4 and 4.1 (see Table 1). It can be seen that the CN of Si is in agreement with works [4, 10]. The CN of Mg is consistent with work [9, 12, 14, 28], but the CN of Mg is lower than the one in the work [7, 10, 15]. We clarify the local environment around Si and Mg atoms. The results are shown in Table 2.
As can be seen that at ambient pressure, most of Si atoms are surrounded by 4 O atoms to form SiO4 coordination unit (basic structural unit). Most of Mg atoms are surrounded by 3, 4 and 5 O atoms to form MgO3, MgO4 and MgO5 coordination units, respectively. The fraction of SiO4, MgO3, MgO4 and MgO5 is 92.2, 14.2, 39.9 and 32.9% in Mg2SiO4 liquid at ambient pressure. This fraction decreases as increase in pressure. At higher pressure (40 GPa), most of Si atoms are coordinated by 5 and 6 O atoms, most of Mg atoms are coordinated by 6, 7 and 8 O atoms. The fraction of SiO5, SiO6, MgO6, MgO7 and MgO8 coordination units is 46.9, 42.3, 24.7, 41.5 and 24.3%, respectively. This result indicates that the local environment around Si and Mg atoms in Mg2SiO4 depends significantly on pressure. According to above analysis, the structure of Mg2SiO4 liquid is built by SiOx (x = 4–6) and MgOy (y = 3–8) coordination units. These units can be linked to each other through oxygen atoms to form the network structure of Mg2SiO4 liquid. By using visualization technique, the SiOx (x = 4–6) and MgOy (y = 3–8) network structures in Mg2SiO4 liquid are shown in Fig. 2. This result again confirms that the local environment around Si and Mg atoms in Mg2SiO4 liquid changes significantly with pressure. Now, we continue clarify the topology of SiOx and MgOy coordination units under compression via analyzing distribution of T–O–T bond angles and T–O BLs (T is Si, Mg) that shown in Fig. 3. Figure 3a shows that at ambient pressure, the O–Si–O bond angle distribution in SiO4 units has a peak at 105°. There is the main peak and a shoulder peak at about at 90° and 160°, respectively, in the distribution of O–Si–O bond angle in SiO5 units. For SiO6 units, it has two peaks including the main first peak at 85° and the second small peak at 165°. The O-Si–O bond angle in SiO5, SiO6 units is always larger than the one in the SiO4 units. When pressure compresses to 40 GPa, the distribution of bond angle and bond length for each SiOx units (x = 4, 5, 6) is almost unchanged with pressures. This indicates the shape of every SiO4 or SiO5 or SiO6 units is identical under compression. Regarding to the distribution of Si–O BL, Fig. 3b indicates that the distribution in SiO5 and SiO6 units at ambient pressure is similar to the one at high pressure. However, there is a slight shift to the left of the distribution in SiO4 units as pressure increases. It means that the size of SiO5 and SiO6 units is independent on pressure, but the size of SiO4 is distorted slightly under compression. The results also show that the Si–O BL in SiO4 unit is shorter than the one in SiO5 unit, and the Si–O BL in SiO6 is the longest. Thus, the elongation of the Si–O BL under compression can be explained as following: when the pressure increases the Si–O coordination number rises to 5, 6. Besides, the shape and size of each SiO5 or SiO6 unit are identical and not dependent on pressure. Accordingly, the O–O bond distance decreases, the Coulomb repulsion between O and O goes up. Hence, this leads to elongation of the Si–O BL. Figure 3c and d shows the distribution of Mg–O–Mg bond angles and Mg–O BLs in MgOx units (x = 3–8). The results show that the shape and size of MgOx units are slightly dependent on pressures. The MgOx units are easily more distorted than SiOx units under compression. Furthermore, most of MgOx units are MgO3, MgO4, MgO5 units at low pressure and are MgO6, MgO7 and MgO8 at high pressure. Besides, the Mg–O BL in MgOx units with x = 3–5 is always shorter than the one in MgOx units with x = 6–8. Namely, the Mg–O BL in MgO3, MgO4 and MgO5 is about 1.90 ± 0.02 Å and in MgO6, MgO7 and MgO8 is about 1.96 ± 0.02 Å. This leads to the average Mg–O BL slightly increases as pressure increases.
The IRO structure relates to the radial distribution function of Si–Si (gSi–Si), Si–Mg (gSi–Mg) and Mg–Mg (gMg–Mg) as well as the distribution of the linkages between two adjacent TOn units. Figure 4 shows that the position of the first peak of all the PRDF shifts to left as increasing pressure. Namely, the first peak of the gSi–Si, gSi–Mg and gMg–Mg locates at 3.08, 3.24 and 3.28 Å at ambient pressure and 3.04, 2.92 and 2.78 Å at 40 GPa at high pressure, respectively. Thus, the gSi–Si, gSi–Mg and gMg–Mg are significantly dependent on pressure. It means that the IRO structure changes significantly under compression. Furthermore, we found that the adjacent TOn units can be connected by one, two and three sharing oxygen to form the corner-, edge- and face-sharing bonds, respectively (denoted as Csb, Esb and Fsb, respectively). Thus, we calculated the average number of Csb, Esb and Fsb per one unit at various pressures shown in Table 3. The result shows that there is only Csb between the adjacent SiOx units. The fraction of Esb and Fsb is almost zero. But two adjacent MgOy units or between two MgOy and SiOx units have all Csb, Esb and Fsb. It is clear that at 0 GPa, the Si atom has 0.75 average Csb with other Si atoms. The Si atom has 1.33 average Csb and 0.21 average Esb with Mg atoms. The Mg atom has 2.26 average Csb, 0.74 average Esb and 0.11 average Fsb with other Mg atoms. The average number of all types of links per one structural unit TOn increases as increasing pressure and in considered pressure range, Nc > Ne > Nf.
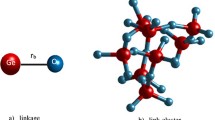
The change of IRO structure in Mg2SiO4 liquid is clarified through investigating distribution of oxygen atoms forming types of OTm linkages in Mg2SiO4 liquid (see Fig. 5) as well as the percentage of OTm coordination units (T = Si, Mg) under compression (see Table 4).
The structure of Mg2SiO4 units consists of TOn units which are connected to each other via oxygen atoms to form network structure of system. The network structure of Mg2SiO4 liquid is visualized in Fig. 6. It can be seen that the oxygen atoms can be linked to Si and Mg atoms to form OTm linkages. The fraction (%) of OTm linkages is described in Table 4, and characteristics of all type of linkages liquid are also shown in detail in Fig. 5. It indicates that at ambient pressure, most of OTm linkages are OT3 (45.5%), OT4 (31.65%) and OT2 (13.94%). For OT3 linkages, most of them are Si2–O–Mg and Si–O–Mg2; the fraction of OSi3 and OMg3 is very small, at nearly 5%. For OT4 linkages, the proportion of Si–O–Mg3 accounts for the highest. The fraction of OTm linkages (m = 2 ÷ 4) decreases, while the fraction of OTm linkages (m = 5 ÷ 8) increases as increasing pressures. At 40 GPa, most of OTm linkages are OT5 (42.72%), OT6 (24.51%) and OT4 (23.25%). Most of oxygen link to one Si and four Mg to form Si–O–Mg4 linkage or link to two Si and three Mg to form Si2–O–Mg3 linkage in OT5 linkages. The Si–O–Mg5 or Si2–O–Mg4 linkages are mainly in OT6.
The above analysis indicates that the existence of Mg atoms in SiO2 leads to the Si–O network broken to form most of –Si–O–Mg– network in Mg2SiO4 liquid. The Mg atoms tend to incorporate into the Si–O network via both BO and NBO forming most of Si2–O–Mg, Si–O–Mg2, Si–O–Mg3, Si–O–Mg4, Si2–O–Mg3, Si2–O–Mg4 and Si–O–Mg5 linkages. Figure 7 represents the distribution of BO, NBO and FO in Mg2SiO4 liquid as a function of pressure, where the BO and NBO denote the O atoms link to at least two Si atoms and only one Si atom, respectively. FO means the O atoms do not link to any Si atoms. It illustrates that there is existence of FO in Mg2SiO4 and Mg atoms tend to link with these FO forming O–Mgy (y = 3–6). The fraction of BO, NBO and FO is 18.7%, 63.4% and 17.9%, respectively, at 0 GPa. The fraction of BO increases, while the figures for NBO and FO have an opposite trend with increase in pressure. At 40 GPa, the proportion of BO, NBO and FO is 38.5%, 50.4% and 11.1%, respectively. Under compression, the Si–O bonds are broken leading to incorporation of Mg atoms into Si–O subnet to forming linkages that O atoms are linked to both Mg and Si atoms at high pressure. We continue to analyze the distribution of BO in SiOx units to clarify degree of polymerization of the Si–O network in Mg2SiO4 liquid. The results are shown in Table 5. For SiO4 units, there are 112 Si4-0 units (isolated SiO4 units), 247 Si4-1, 198 Si4-2, 98 Si4-3 units and 4 Si4-4 units at ambient pressure. However, the number of Si4-n drops sharply due to the fraction of SiO4 units decreases rapidly under compression. Note that Si4-n means the SiO4 units with n bridging oxygens. The results also illustrate that most of the SiO4 units have 1, 2 or 3 BO. For SiO5 and SiO6 units, it is clear that the number of Si5-0 and Si6-0 (isolated SiO5 and SiO6 units) is small. In the considered pressure range, most of SiO5 units have 2, 3 or 4 BO (87 Si5-2, 126 Si5-3 and 103 Si5-4 at 40 GPa), while most of SiO6 units have 3, 4 or 5 BO (54 Si6-3, 92 Si6-4 and 46 Si6-5 at 40 GPa).
The number of Si5-n and Si6-n increases strongly with pressure. It is easy to understand because the fraction of SiO5 and SiO6 units increases under compression and they account for the highest proportion at 40 GPa. The analysis from Fig. 7 and Table 4 indicates that the fraction of BO increase with pressure. Therefore, the degree of polymerization of the Si–O network increases under compression. We found that SiOx subnets tend to incorporate together to form larger networks that replace isolated subnets and small subnets. This problem is clarified via analysis size distribution of SiOx subnets at various pressures, shown in Table 6.
To calculate size distribution of SiOx subnets, we do the following: in the set of SiOx units, all atoms are denoted from 1 to n. If two Si atoms have least one O atom, they will belong to the same subnet and have the same label and the O atoms that link with the Si atom will have the same label with it. Finally, the atoms with the same label will belong to one subnet. The result indicates that the network structure of SiOx units in the model comprises large subnets, many small subnets and several subnets of isolated SiOx units. At 0 GPa, it consists of the largest subnets of 424 atoms, three subnets with size from 99 to 126 atoms and many small subnets from several atoms to several dozens of atoms. There are 113 isolated SiOx units including 109 isolated SiO5-units and 4 isolated SiO4-units. At 40 GPa, the largest subnet has 3193 atoms. There are 4 small subnets with 11–21 atoms and 8 isolated SiO6- and SiO7-units. The size of the largest subnet increases with pressure. The number of small subnets and isolated SiOx units decrease. It means that the polymerization of silica network increases under compression.
3.2 Dynamical properties
To clarify the dynamical properties, the models at different pressures are relaxed in NVE ensemble (constant volume and energy) for a long time. The diffusivity of the individual atomic species is determined through calculating the dependence of the mean-squared displacement \(r^{2} \left( t \right)\) on the number of MD steps. The diffusion coefficient D of Mg, O and Si is determined by Einstein relation: \(D = \lim_{t \to \infty } \frac{{r^{2} \left( t \right)}}{6t}\). The results are shown in Figs. 8 and 9. We find that the dependence of mean-squared displacement is linear function with the number of MD steps. Under compression, the mean-squared displacement of individual atomic species decreases. Therefore, the diffusivity of Mg, Si and O atom decreases with increase in pressure because the polymerization of silica network increases under compression. At 0 GPa, the diffusion coefficient of Si, O and Mg atom is DSi = 0.346·10−4, DO = 0.474·10−4 and DMg = 1.042·10−4 cm2/s, respectively. The diffusion coefficient of Si, O and Mg atom goes down with pressure. At 40 GPa, DSi = 0.115·10−4, DO = 0.161·10−4 and DMg = 0.215·10−4 cm2/s. In considered pressure, it shows that DMg > DSi > DO. It is suitable with the previous simulated results [7, 28]. Thus, the Mg atom diffuses the fastest, and the O atom diffuses faster than the Si atom in Mg2SiO4 liquid.
4 Conclusions
In this paper, we have presented the studied results for Mg2SiO4 system at 3500 K and in the pressure range from 0 to 40 GPa by using MD simulation method. The studied results serve as the basic knowledge about microstructure and dynamics of Mg2SiO4 liquid at atomic level under compression. Namely, the structure of Mg2SiO4 liquid consists of MgOy and SiOx units (x = 4–6, y = 3–8) which are connected to each other via the corner-, edge- and face-sharing bonds to form network structure of system. The average number of all types of bonds per one structural unit TOn increases with increase in pressure and in the considered pressure range, Nc > Ne > Nf. Under compression, the local environment around Si and Mg atoms as well as the intermediate-range order structure in Mg2SiO4 liquid changes significantly. The existence of Mg atoms in SiO2 leads to the Si–O network broken to form most of –Si–O–Mg– network in Mg2SiO4 liquid. The Mg atoms tend to incorporate into the Si–O network via both BO and NBO forming most of Si2–O–Mg, Si–O–Mg2, Si–O–Mg3, Si–O–Mg4, Si2–O–Mg3, Si2–O–Mg4 and Si–O–Mg5 linkages. The network structure of SiOx units in the model comprises large subnets, many small subnets and several subnets of isolated SiOx units. The size of the largest subnet increases with pressure. The number of small subnets and isolated SiOx units decrease. It means that the polymerization of silica network increases under compression. In considered pressure range, we always have DMg > DSi > DO. The diffusivity of Mg, Si and O atom decreases with increase in pressure due to increase in the polymerization of silica network under compression.
References
N Tomioka and T Okuchi Sci. Rep. 7 17351 (2017)
S Kohara, J Akola, H Morita, K Suzuya, J K R Weber, M C Wilding and C J Benmore, Proc. Natl. Acad. Sci. USA 108 14780-1475 (2011)
M C Wilding, C J Benmore and J K R Weber J. Mater. Sci. 43 4707–4713 (2008)
N P de Koker, L Stixrude and B B Karki Geochim. Cosmochim. Acta 72 1427–1441 (2009)
T Sakai, H Dekura and N Hirao Sci. Rep. 6 22652 (2016)
S H Guan, X J Zhang and Z P Liu J. Phys. Chem. C 120 25110–25116 (2016)
O Adjaoud, G S Neumann and S Jahn Chem Geo. 256 185–192 (2008)
B Cochain, C Sanloup, C Leroy and Y Kono Geophys. Res. Lett. 44 818–826 (2017)
P S Salmon, G S Moodya, Y Ishiib, K J Pizzeya, A Polidoria, M Salannec, A Zeidlera, M Buscemia, H E Fischerd, C L Bulle, S Klotzf, R Weberg, C J Benmoreg and S G MacLeodi J. Non Cryst. Solids X 3 100024 (2019) https://doi.org/10.1016/j.nocx.2019.100024
S Kohara, K Suzuya, K Takeuchi, C K Loong, M Grimsditch, J K R Weber, J A Tangeman and T S Key Science 303 1649–1652 (2004)
T Taniguchi, M Okuno and T Matsumoto J. Non Cryst. Solids 211 56–63 (1997)
L Cormier and G J Cuello Phys. Rev. B 83 224204 (2011)
M C Wilding, C J Benmore, J A Tangeman and S Sampath Europhys. Lett. 67 212–218 (2004)
M C Wilding, C J Benmore, J A Tangeman and S Sampath J. Chem. Geol. 213 281–291 (2004)
M Guignard and L Cormier Chem. Geol. 256 111–118 (2008)
C J Benmore, E Soignard, M Guthrie, S A Amin, J K R Weber, K McKiernan, M C Wilding and J L Yarger J. Non Cryst. Solids 357 2632 (2011)
C C Lin, S F Chen, L G Liu and C C Li J. Non Cryst. Solids 353 413–425 (2007)
D B Ghosh, B B Karki and L Stixrude Am. Mineral. 99 1304–1314 (2014)
Y Matsui and K Kawamura Nature 285 648–649 (1980)
P Peiderer, J Horbach and K Binder Chem. Geol. 229 186–197 (2006)
N V Hong, N V Yen, M T Lan and P K Hung Can. J. Phys. 92 1573–1580 (2014)
K Zheng, Z Zhang, F Yang and S Sridhar ISIJ Int. 52 342–349 (2012)
V V Hoang Physica B 400 278–286 (2007)
G Zhao, H F Mu, X M Tan, D H Wang and C L Yang J. Non Cryst. Solids 385 169–174 (2014)
J D Kubicki and A C Lasaga Phys. Chem. Miner. 17 661–673 (1991)
K Shimoda and M Okuno J. Phys. Condens. Matter 18 6531–6544 (2006)
D B Ghosh and B B Karki Phys. Chem. Miner. 41 163–171 (2014)
F J Spera, M S Ghiorso and D Nevins Geochim. Cosmochim. Acta 75 1272–1296 (2011)
Z J Liu, C R Zhang, X W Sun, J B Hu, T Song and Y D Chu Phys. Scr. 83 045602 (2011)
D Nevins, F J Spera and M S Ghiorso Am. Minearal. 94 975–980 (2009)
A M Goryaeva, P Carrez and P Cordier Phys. Chem Miner. 42 793–803 (2015)
L T San, N V Hong, T Iitaka and P K Hung Eur. Phys. J. B 89 73 (2016)
J L Mosenfelder, P D Asimow and T J Ahrens J. Geophys. Res. 112 B06208 (2007)
D J Lacks, D B Rear and J A V Orman Geochim. Cosmochim. Acta 71 1312–1323 (2007)
J Xin, L Gan, L Jiao and C Lai ISIJ Int. 57 1340–1349 (2017)
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under project number 103.05-2018.37.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mai, L.T., Nguyen, N.T. Structural and dynamical investigation of Mg2SiO4 liquid. Indian J Phys 95, 1723–1733 (2021). https://doi.org/10.1007/s12648-020-01839-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-020-01839-8