Abstract
The bulk 3-component xAg2O–40TeO2–(60 − x)V2O5 oxide glasses with different silver oxide contents of x = 0, 10, 20, 30, 40 and 50 mol% were prepared using the standard press-melt quenching method. The effect of the high DC electric field on the DC conductivity of the mentioned glasses was investigated using the gap-type electrode configuration. At the regime of low DC electric fields, the conduction of these samples was ohmic, whereas at high electric fields regime, present bulk samples show nonlinear behavior (i.e., non-ohmic conduction). The current–voltage characteristics show increasing deviation from Ohm’s law with increasing current density, implying the non-ohmic behavior in close coincidence with Poole–Frenkel effect (PFE) which occurs at electrical fields nearly above 104 (V/cm); so, samples show the switching phenomena at a threshold voltage (Vth) from resistive state to conductive state. Based upon PFE, the lowering factor of potential barrier (βPF) was determined for the understudied samples. Also, results show that generally the threshold voltage in which switching phenomenon occurs increases by increasing the electrode distance and decreases by increasing the sample temperature, but Vth shows two distinct regions of 0 ≤ x ≤ 20 and 20 < x ≤ 50, in which the highest value of Vth is devoted to the case of x = 20 mol% with respect to other glasses; this result implies the structural change and then probable formation of crystallites in the amorphous matrix to be hard for the mentioned case, which is in good agreement with the previously reported mechanical aspects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, tellurium oxide-based glasses have attracted a great interest due to their unique properties, such as high refractive index [1], high dielectric constants [2, 3] and high glass-forming ability [4, 5]. Also, these glasses have relatively high chemical stability, suitable mechanical strength [6, 7] and low melting point [8,9,10] beside their resistance against moisture for long times [9, 11], stability against devitrification and non-toxic feature [12]. Tellurium oxide (TeO2) as a conditional glass former can readily form glass with a modifier such as an alkali metal oxide or alkaline earth metal oxide and also transition metal oxides (TMOs) [13]. The basic structure of pure TeO2 and tellurium oxide-based glasses consists of TeO4 trigonal bipyramid (tbp). Also, glassy materials containing V2O5 have been proposed as cathode materials for lithium solid-state batteries [14,15,16,17,18,19]. Besides that, glasses containing noble metal oxides have been the subject of various investigations due to their potential as optical fiber lasers, amplifiers, scintillating glasses [20,21,22] and acousto-optical devices [23] and so forth. In some of the previous works, physical, electrical, optical and thermal properties of the different glasses like MoO3–TeO2–V2O5 [24], Sb2O3–TeO2–V2O5 [25,26,27], Sb–TeO2–V2O5 [9, 10, 28,29,30,31,32] and Ag2O–TeO2–V2O5 [33,34,35,36] have been studied. In the study of oxide glasses, one of the interested scientific fields is the high electric field phenomena and its mechanism, as will be discussed later. At electrical fields ~ > 103 V/cm, switching (high resistive state to high conduction state) or negative resistance phenomena were observed at a threshold voltage (Vth). Negative resistance characteristic means that under special conditions, the current will decrease with an increase in voltage [37]. Negative resistance and electrical switching effects in materials can find applications in a variety of areas such as power control and information storage. The first reference to a switching in chalcogenide glasses occurred ~ 57 years ago (Ovshinsky, 1959), but a later paper by the same author (Ovshinsky, 1968) properly marked the in which its subject attracted of serious interests in solid-state electronics. The theoretical models and factors affecting switching and memory phenomena in chalcogenide glass are normally classified into “threshold” (or monostable) and “memory” (or bistable) devices. The threshold switching refers to the case in which continuous electrical power is required to maintain the highly conducting ON state; and memory switching refers to the case in which both ON and OFF states can be maintained without electrical power [38]. Different mechanisms have been proposed to explain the phenomenon of electrical switching in oxide and chalcogenide glasses. These include electronic, space charge, electrothermal and thermal mechanisms. It is now established more or less beyond doubt that in general, threshold switching is electronic in origin whereas memory switching is of thermal origin. Electrothermal mechanism becomes relevant while examining the influence of temperature on the various parameters that determine the switching behavior [39].
In the thermal mechanism, the transition from insulating to a conducting state is caused mainly by a joule heating effect, which is responsible for the increase in the temperature with the subsequent reversible phase transition. The electronic mechanism, developed for switching in thin films, assumes no heating of samples and results from space charges localized at the electrodes. The electrothermal mechanism, as a hybrid of two other mechanisms, is associated with the formation of both hot conductive channels in the material and space charges at electrodes [30]. The application of a high enough electric field causes Joule heating of the medium, which leads to crystallization of the glass into fine filaments. This results in excess carrier concentration in the current path [39]. In the family of oxide glass, vanadium-based glasses are usually found to exhibit electrical switching and high electrical conductivity. The investigation of the conductivity and switching behavior of these glasses can help to understand the mechanism of conduction and hence to select the suitable materials for device applications (e.g., memory switching and gas sensor). In our previous works, the optical [33], mechanical [34], some thermal properties [36] and also the low-field DC conductivity [35] of Ag2O–TeO2–V2O5 glasses have been studied. In the present work, the high-field conduction of the same glasses was investigated for the first time to complete their previously reported data. In our past work [35], for Ag2O–TeO2–V2O5 samples, the low-field DC electrical conductivity measurements were done within the temperature range of 150–380 K [34], and as mentioned before, the purpose of the present investigation is to study the high field electrical behavior of Ag2O–TeO2–V2O5 and extract some physical parameters such as threshold voltage and lowering factor of potential barrier, to more complete about the features of these glassy systems.
2 Experimental details
2.1 Preparation of bulk glasses and structural information
Glasses were prepared by the conventional melt quenching method. Bulk glass systems of xAg2O–40TeO2–(60 − x)V2O5 with x = 0, 10, 20, 30, 40 and 50 mol% (hereafter termed as TVAgx) were prepared by mixing appropriate weights of raw TeO2, V2O5 and Ag2O materials; in this process, the precisely weighted and well-mixed starting raw powders were melted employing an electric furnace. It should be mentioned that during the melting process, regular stirring of the melt is mandatory to achieve the homogeneous melt; later, the melt was poured on to a steel block and pressed by another block. XRD patterns of TVAgx samples have been reported in our recent works [33,34,35,36], confirming their non-crystalline nature except for TVAg50. More details of the glass preparation process, structural characterization, glass density and some physical, thermal, optical and mechanical properties were described in our recent works [33,34,35,36].
2.2 Current–voltage (I–V) characteristics
Carefully, surface-polished bulk samples were used for electrical measurements, and electrical measurements were done with gap-type electrode configuration, using a high-voltage power supply (Molian Toos, 0–5 kV, Iran) and sensitive multimeters (TTi, 196, High-resolution computing multimeter, England). For each sample, the I–V characteristic was measured to verify the ohmic properties of the contacts. Also, the effect of different factors such as temperature and electrode distance on the I–V characteristic was investigated.
3 Results and discussion
3.1 Switching behavior and temperature dependence of threshold voltage (V th(T))
In this section, results of typical measurements on the I–V characteristics are shown in Fig. 1(a, b) for 2 different molar percentages of x = 10 and 20 mol% with a certain electrode distance at different ambient temperatures. All obtained data of the mentioned samples and other studied samples are tabulated and summarized in Tables 1 and 2.
From Fig. 1(a, b),the increase in electrical conduction of glasses with increasing the temperature can be observed, which reveals the semiconducting nature of these samples. Also, during the experiments, the influence of Ag2O content on the I–V characteristics was evident. The obtained results show the increase in resistivity with the increase in Ag2O content up to x = 20 mol% and then decrease for the content of silver oxide above 20 mol%, showing the mixed polaronic-ionic origin of conduction (see Ref. [35]); this result is in agreement with the results of low-field DC conductivities reported in Ref. [35]. In other words, there is two region for Vth: 0 ≤ x ≤ 20 and 20 < x ≤ 50, meaning a maximum at x = 20 mol% (i.e., substitution of V2O5 with Ag2O and changing of the conduction mechanism from polaronic to mixed polaronic–ionic). As mentioned, it has been reported such behavior change in low DC electric field experiments [35], which has been interpreted upon the change in the conduction mechanism at x = 20. Also, such behavior change has been reported in optical band gap [33], elastic velocities and moduli [34] and thermal stabilities [36], introducing x = 20 as a critical ratio of V2O5/Ag2O; so, it is reasonable for a glass with higher thermal stability to have higher Vth, as discussed before. Besides that, it is evident from Fig. 1(a, b) that the samples exhibit an ohmic behavior in the high-resistance OFF state, and then deviate from ohmic behavior to reach the threshold voltage at which the sample shows a current-controlled negative resistance or switching behavior, which eventually leads to a low-resistance ON state. The samples are found to revert back to their high-resistance OFF state, when the current is reduced below the holding current, indicating a threshold-type electrical switching behavior. In other words, at low electric fields, the conduction of these samples was ohmic, while at high electric fields, bulk samples show nonlinear behavior (non-ohmic conduction). The current–voltage characteristics show increasing deviation from Ohm’s law with increasing current density.
Actually, the results of studying the I–V curves show that the increase in temperature decreases the effect of the electric field to a certain extent. In other words, the electrical conductivity of the samples is influenced by both the temperature and the electric field.
Variation in threshold voltage of samples, with respect to temperature, is shown in Fig. 2. The threshold switching voltage data, obtained at different temperatures, are presented in Table 2. The results show that the threshold switching voltage of the glasses decreases with the increase in temperature as shown in Figs. 1(a, b) and 2, which is a common feature exhibited by other ternary glasses such as Sb–TeO2–V2O5 [30], MoO3–TeO2–V2O5 [40,41,42], P2O5–Li2MoO4–Li2O, P2O5–Na2MoO4–Na2O [43] and CuO–ZnO–P2O5 [37].
For the present TVAgx glasses, Vth has its highest value for TVAg20 with respect to other studied glasses; this result insists the structural change and then probable formation of crystallites in the amorphous matrix (along with the hot current filament) to be hard for TVAg20, which is in excellent agreement with the data of glass transition temperature and thermal stability of this glass composition, which can be followed from Ref. [36] and also from its mechanical and elastic moduli from Ref. [34].
3.2 Switching behavior and electrode distance dependence of threshold voltage (V th(L))
The typical I–V characteristics of TVAg10 and TVAg20 systems for different electrode distances at the room temperature are shown in Fig. 3(a, b). For each sample, at a constant temperature, the increase in Vth with the increase in the electrode distance is evident. The increase in electrode distance increases the resistivity of the material between the electrodes and also increases the Vth (see Fig. 4 and Table 1, which report the complete data for all understudied samples). Also, it should be mentioned that (similar to what before-stated explanations for the case of different ambient temperatures) the highest value of Vth is devoted to TVAg20 with respect to other glasses; this result implies the structural change and then probable formation of crystallites in the amorphous matrix (along with the hot current filament) to be hard for TVAg20, which is in excellent agreement with the data of glass transition temperature and thermal stability of this glass composition, which can be followed from Ref. [36] and also from its mechanical and elastic moduli from Ref. [34].
3.3 Poole–Frenkel effect (PFE) and determination of lowering factor of the potential barrier
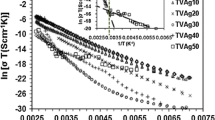
Curves of Tln(I) versus E1/2 were plotted at different temperatures for different samples. Figure 5a shows the Tln(I) − E1/2 characteristics at different temperatures for TVAg20 sample. Also, Fig. 5b shows the fitting of the linear region of Fig. 5a at the temperature of 299 K. This review has been done at different temperatures for understudied samples, but not shown in this article, and only the results of these studies are mentioned in Table 2. These figures show linear behavior at fields exceeding (103–104) V/cm as a bulk effect, based upon the Poole–Frenkel effect. (E and I are the electric field and the electric current, respectively.)
The Poole–Frenkel effect is applicable if the trap center is neutral with the captured carrier (e−, h+), as only then an attractive (Coulomb) interaction is working when the charged carrier escapes from the charged trap. The general mechanism of the PFE is sketched in Fig. 6. The barrier e0Φtn = |Ec − Etn| for an electron to escape from its trap at energy level Etn into the conduction band is equal in both directions in this simplified linear model for the field-free state (E = 0). This is changed by the presence of an electric field E ≠ 0. In forward direction (in our case, the direction of the drift of the electrons), the barrier is diminished by ΔϕPF compared to the field-free state by the applied and/or internal electric field E with the appropriate sign. A field with the opposite sign enlarges the barrier in the same direction by about the same value. Usually, the Coulomb interaction between the leaving carrier and remaining charged trap is used [44].
Schematic sketch of the Poole–Frenkel effect without and with the electric field, E, [44]
We suppose that the material contains neutral impurity centers so that the potential energy of an electron or hole at distance x from these centers in the direction of electric field E is reduced relative to the state where the electric field is absent, namely:
The maximum of the potential barrier occurs at \(x_{m} = \frac{{\beta_{{\text{PF}}} }}{{2\,E^{1/2} }}\), where \(\beta_{{\text{PF}}} = \left( {\frac{e}{\pi \,k}} \right)^{1/2}\) is the lowering factor.
Therefore, the probability of the thermal hopping P and current I become [41, 42, 45,46,47]:
An almost linear relation is obtained when the electric field is about 103–104 V/cm. As temperature increases for each sample, the field strength required for the onset of non-ohmic behavior is reduced due to increasing conduction with an increase in temperature (see Fig. 1a, b). Therefore, we can state that the Poole–Frenkel effect is thermal excitation caused by an electric field [46]. As the threshold voltage for the onset of negative resistance shows a decreasing trend with the increase in temperature and with the decrease in electrode distance (see Tables 1, 2), electrical behavior is interpreted by an electrothermal model. Details of the proposed model and further discussions are presented in Sect. 1.
Also, comparing the I − V curves of each sample [Fig. 1(a, b)] at different temperatures, one can conclude that the present samples have a semiconducting nature (decreasing resistance with increasing temperature). Based on the results of the measurements presented in Fig. 5(a, b), one can determine the lowering factor βPF of these glasses using the slope of the linear part of the Tln(I) versus E1/2 curves. At different temperatures, for the understudied amorphous samples, we have employed the least-squares technique to determine the lowering factor βPF. According to the above discussions, βPF was determined for each sample (in SI units) and is listed in Table 2.
Results show that lowering factor of potential barrier of TVAgx samples in different temperatures increases with the increase in temperature for understudied samples except for TVAg50 at the temperature of 343 k. This behavior probably is due to the crystalline nature of TVAg50 sample, which is obtained from XRD [33]. From the regression analysis, the R2 values were at the range of 0.863–1 for TVAgx (x = 0–50 mol%) (R is the correlation coefficient) as listed in Table 2. This analysis demonstrates a satisfactory fit to the data.
4 Conclusions
From the present work, the following aspects can be mentioned for xAg2O–40TeO2–(60 − x)V2O5 glasses:
-
1.
The increase in electrical conduction of all glasses with increasing the temperature can be observed, which reveals the semiconducting nature of these samples. Also, the obtained results show the increasing resistivity with increasing Ag2O content for 0 ≤ x ≤ 20 mol%.
-
2.
Threshold voltage depends on different parameters such as ambient (sample) temperature and electrode distance. Results show that the threshold voltage at switching phenomenon increases and decreases with the increase in electrode distance and sample temperature for understudied samples, respectively.
-
3.
As the threshold voltage for the onset of negative resistance shows a decreasing trend with the increase in temperature and with the decrease in electrode distance, electrical behavior is interpreted by an electrothermal model.
-
4.
The highest value of Vth is devoted to the case of x = 20 mol% with respect to other glasses; this result implies the structural change and then probable formation of crystallites in the amorphous matrix (along with the hot current filament) to be hard for the mentioned case.
-
5.
The electrical conductivity of the samples is influenced by both the temperature and the electric field.
-
6.
The high electrical field experiments showed that at high field, I–V characteristic become non-ohmic as a result of lowering the potential barriers (Poole–Frenkel effect). Also, upon the Poole–Frenkel effect, lowering factor was determined for each sample (\(\beta_{{\text{PF}}}\)). For each sample, the \(\beta_{{\text{PF}}}\) value increases with the increase in temperature except for TVAg50 at the temperature of 343 k. This behavior probably is due to the crystalline nature of TVAg50 sample.
References
D Souri In book: Tellurite Glass Smart Materials, Springer: ISBN: 978-3-319-76568-6, https://doi.org/10.1007/978-3-319-76568-6_5, 67–105 (2018)
H M Moawad, H Jain, R El-Mallawany, T Ramadan and M El-Sharbiny J. Am. Ceram. Soc. 85 2655 (2002)
S E Ibrahim, Y S Rammah, I Z Hager and R El-Mallawany J. Non-Cryst. Solids 498 443
R El-Mallawany, Tellurite Glass Smart Materials, Springer: ISBN: 978-3-319-76568-6, https://doi.org/10.1007/978-3-319-76568-6_5, 1–17 (2018)
I Z Hager and R El-Mallawany J. Mater Sci. 45 897 (2010)
I Z Hager, R El-Mallawany and A Bulou Physica B 406 972 (2011)
G Lakshminarayana, H Yang and J Qiu J. Alloys. Compd. 475 569 (2009)
M A Sidkey and M S Gaafar Physica B 348 46 (2004)
D Souri, H Zaliani, E Mirdawoodi and M Zendehzaban Measurement 82 19 (2016)
D Souri Physica B: Condensed Matter 456 185 (2015)
R El-Mallawany Mater. Chem. Phys. 53 93 (1998)
Y Gandhi, N K Mohan and N Veeraiah J.Non-Cryst. Solids 357 1193 (2011)
J C S Moraes, J A Nardi, S M Sidel, B G Mantovani, K Yukimitu, V C S Reynoso, L F Malmonge, N Ghofraniha, G Ruocco, L H C Andrade and S M Lima J. Non-Cryst. Solids 356 2146 (2010)
A Kaur, A Khanna, C Pesquera, F Gonzalez and V Sathe J. Non-Cryst. Solids 356 864 (2010)
Y B Saddeek, I S Syahia, W Dobrowolski, L Kilanski, N Romcevic and M Arciszewska Optoelectron. Adv Mat. 3 559 (2009)
I S Yahia, Y B Saddeek, G B Sakr, W Knoff, T Story, N Romcevic and W Dobrowolski, J. Magn. Magn. Mater. 321 4039 (2009)
V Rajendran, N Palanivelu, B K Chaudhuri and K Goswami J. Non-Cryst. Solids 320 195 (2003)
I Ardelean, C Marcus and R C Lucacel Optoelectron. Adv Mat. 9 836 (2007)
A G Kalampounias, G N Papatheodorou and S N Yannopoulos J. Phys. Chem. Solids 67 725 (2006)
S Gupta and A Mansingh Philos. Mag. B 78 (3) 265 (1998.
M J Weber J. Non-Cryst. Solids 42 189 (1980)
M Oomen Advanced Materials 3 403 (1991)
A Abd El-Moneim Mater. Chem. Phys. 52 36 (1998)
D Souri Measurement 44 1904 (2011)
D Souri and Z Torkashvand J. Electron. Mater. 46 (4) 2158 (2017)
D Souri and Y Shahmoradi J. Therm. Anal. Calorim. 129 (1) 601 (2017)
D Souri J.Mater. Sci. 47 (2) 625 (2012)
D Souri J. Non-Cryst. Solids 470 112 (2017)
D Souri, Z Siahkali and M Moradi J. Electron. Mater. 45 (1) 307 (2016)
D Souri, R Ghasemi and M Shiravand J.Mater. Sci. 50 (6) 2554 (2015)
D Souri, P Azizpour and H Zaliani J. Electron. Mater. 43 (9) 3672 (2014)
D Souri, M Mohammadi and H Zaliani Electron. Mater. Lett.10 (6) 1103 (2014)
D Souri and Z E Tahan Appl. Phys. B: Lasers and Optics 119 273 (2015)
D Souri, F Honarvar and Z E Tahan J. Alloys. Compd 699 601 (2017)
D Souri, Z E Tahan and S A Salehizadeh Indian J. Phys. 90 407 (2016)
D Souri J. Non-Cryst. Solids 475 (2017) 136
M Mirzayi and M H Hekmatshoar Physica B 405 4505 (2010)
R A H El-Mallawany Tellurite Glasses Hand book: physical properties and data, CRC Press, Second edition, ISBN: 9781439849835, Springer, 532 pages (2012)
R Rajesh and J Philip Semicond. Sci. Technol. 18 133 (2003)
H Hirashima, Y Watanabe and T Yoshida J. Non-Cryst. Solids 95(96) 825 (1987)
M Elahi and D Souri Indian J. Pure. Appl. Phys. 40 620 (2002)
D Souri and M Elahi Czechoslovak. J Phys 56(4) 419 (2006)
D Souri, M Elahi and M S Yazdanpanah Cent. Eur. J. Phys. 6(2) 306 (2008)
H Schroeder J. Appl. Phys. 117 215103 (2015)
M A Sidkey, R Elmallawany, R I Nakhla and A Abdelmoneim J. Non-Cryst. Solids 215 75 (1997)
A K Jonscher, In: P.G. Lecomber, J. Mort (Eds.) Electronic and structural properties of amorphous semiconductors (Academic Press, London) p. 329 (1973)
I G Austin and M Sayer J. Phys. C 7 905 (1974)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souri, D., Esmaeili Tahan, Z. & Hakimyfard, A. Negative-resistance behavior of bulk Ag2O–TeO2–V2O5 oxide glasses. Indian J Phys 93, 1293–1299 (2019). https://doi.org/10.1007/s12648-019-01395-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-019-01395-w