Abstract
In nuclear medicine practices, unsealed radio-pharmaceuticals are administered to the patients for diagnosis (imaging) and/or treatment of various types of cancer, neurological disorders, cardiac disease, etc. Therefore, to ensure the safety and efficacy of the diagnosis and therapy procedures, it is absolutely essential that the activity of administered radiopharmaceuticals measured using radionuclide calibrators are accurate and reproducible. Bhabha Atomic Research Centre (BARC)—India’s Designated Institute (DI) for ionizing radiation metrology—has been conducting national quality audit programmes among nuclear medicine centers for providing traceability of measurements as well as check the accuracy and consistency of radionuclide calibrators since 1981. In this paper, we present results of the audit carried out during 2020–2021 for traceable 131I activity measurements with radionuclide calibrators in which 200 nuclear medicine centers participated. We also present a comparison of results of all the audits conducted during past four decades. The results of latest audit are very encouraging with 93% of the radionuclide calibrators compliant within the acceptable window of ± 10%. During the initial years of the audit, only a few nuclear medicine centers participated in the audit program and also the fraction of compliant radionuclide calibrators was significantly lower. However, once the audit program was made mandatory by the regulator body, not only did the number of participants increased but also the fraction of compliant radionuclide calibrators increased drastically. This implies that India’s nuclear medicine centres have ensured safe and efficient dose delivery to the patient for diagnosis and/or therapy of various diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bhabha Atomic Research Centre (BARC) is the Designated Institute (DI) for ionizing radiation metrology in the country [1, 2]. In collaboration with International Atomic Energy Agency (IAEA) and World Health Organization (WHO), BARC is also recognized as the secondary standards dosimetry laboratory (SSDL) for India. As a DI for ionizing radiation metrology, BARC designs, develops, establishes, maintains various national standards for physical quantities of ionizing radiation. Equivalence of these standards as shown in part A of Fig. 1 is established with other national metrology institutes (NMIs) around the world by participating in the international intercomparison programmes organized by Bureau International des Poids et Mesures (BIPM), Asia Pacific Metrology Programme (APMP) and IAEA [3,4,5,6,7,8]. In addition, BARC has the responsibility to ensure the traceability of end user measurements in the country. End users are a wide spectrum of users from industry, health care, agriculture, defence, research & development organizations etc. End user measurement traceability to national standards as shown in part B of Fig. 1, is established by: (i) disseminating ionizing radiation standards, (ii) providing calibration services and (iii) conducting quality audit programmes. Measurement traceability for the end user is provided through the secondary standards which in turn are calibrated against the primary/national standards. The primary standards maintained as shown in Fig. 2 for activity measurements are 4π β(PC)-γ, 4π β(LS)-γ, 4π β(PS)-γ coincidence systems. The secondary standards for activity measurements are re-entrant well-type 4π gamma ionization chamber and High Purity Germanium (HPGe) gamma spectrometer Fig. 3.
In nuclear medicine, the radiopharmaceutical is administered to the patient either intravenously or orally. Radiation dose delivered with sealed sources in the other modalities of therapy procedures such as external beam therapy, brachytherapy is precise and accurate while in nuclear medicine procedures dose delivered to the patient is difficult to measure directly. This is mainly due to the fact that the radiation source as radiopharmaceutical is situated internally in the patient’s body and variation in the biological distribution of the radiopharmaceutical between patient to patient depending upon individual metabolism. Hence, it is essential to ensure that clinically prescribed activity is administered as accurately as possible [9]. Clinically, a minimum level of administered activity, required for diagnostic/therapeutic outcome, is governed by a delicate balance between its effectiveness and an overall safety of the medical procedure. If the administered activity is higher than the prescribed value, the overdose received by the patient can unnecessarily affect normal organs [10, 11]. On the other hand, if administered activity is lower than the prescribed value, poor imaging quality obtained can lead to poor diagnosis, and in the case of therapy, the treatment could be inefficient. Thus, accurate measurement of radioactivity in the nuclear medicine centres (NMCs) plays a pivotal role in the diagnostic or therapeutic procedures.
Worldwide the applications of nuclear medicine are continuously growing and to ensure the optimal uses of radiopharmaceuticals, International Atomic Energy Agency (IAEA) has come up with technical report on quality assurance for radioactivity measurement in nuclear medicine [11]. In India, the Atomic Energy Regulatory Board (AERB), through its Safety Code for Nuclear Medicine Facilities, has made mandatory that NMCs should measure the activity of radiopharmaceuticals before administering to patients and ensure a regular calibration of radionuclide calibrators, also known as “dose calibrators” [12, 13]. A photograph of radionuclide calibrators at BARC is shown in Fig. 4, consists of a well-type ionization chamber (gas-filled detector) and an electrometer. Radionuclide calibrators measure the total activity present in radiopharmaceutical and are calibrated to ensure the accuracy and consistency of measurements that are traceable to the national and international standards [14].
In India, as DI of ionizing radiation BARC has been ensuring the traceability of radioactivity measurements in the field nuclear medicine by conducting nationwide quality audit programme (QAP) biannually. QAPs ensure the calibration and performance of radionuclide calibrators so that the activity of the radiopharmaceutical measured before administering to patients for diagnostic/therapeutic applications is compliant within ± 10% of the prescribed activity [12, 15, 16]. On the global scenario, QAPs are carried out in different countries by their respective NMI/DI, viz. Brazil, Cuba, Republic of South Korea, Switzerland, UK, Czech Republic and India etc., to ensure the traceability of radioactivity measurements using radionuclide calibrators [15,16,17,18,19,20,21,22,23,24,25,26,27].
Since 1981, BARC has been conducting national audit programme of 131I activity measurements. Though, 99mTc, 18F, 131I, etc. are extensively used in the country, However, owing to short half-lives of 99mTc and 18F, a national audit using them is challenging for a vast country like India, which causes logistical difficulties in timely distribution of these radioisotope standards. Thus, 131I was specifically chosen for the QAP due to its convenient half-life of 8 days.
In this paper, we discuss the methodology adopted for national audit, results of recently conducted 131I audit in 2020–2021, and comparisons of all the audit results since 1981. The results of latest audit show that 93% of the radionuclide calibrators in the country are compliant with the acceptable window of ± 10% and the radionuclide calibrators are traceable to the national standards. A detailed analysis of comparison of all the QAPs conducted since 1981 show that the audit program has contributed in increasing the number of compliant radionuclide calibrators, and consequently, an effective diagnosis/treatment of the patients.
2 Methodology of the Audit Programme
The audit protocol involves a test source in injection glass vial similar to the clinical geometry, calibrated by the NMI/DI against the national standard and then shipped to the participating hospitals. The sources are measured in radionuclide calibrator by the participants as per their usual procedures or protocol given and the results are reported to the NMI/DI for further evaluation. This format of audit has an advantage of having a complete control of source preparation as well as calibration by the NMI/DI which ensures consistency, accuracy and harmonization of procedures at all stages.
In India, BARC conducts biannually the national audit of activity measurements with 131I for radionuclide calibrators in collaboration with various stakeholders, as depicted in Fig. 5. The modus operandi for conducting the national audit program has two components: (i) procedures followed at BARC, and (ii) protocol followed by the participating NMCs.
The details of the procedures implemented at BARC for national audit is as follows:
-
(i)
A specially designed consent form is sent to all nuclear medicine centres in the country, for their consent to participate in the audit programme.
The consent form which apart from the usual details of the NMC, seeks two specific queries. First, the details of AERB authorization to ensure that NMC has the permission to procure and second, the customer number of NMC given by Board of Radiation and Isotope (BRIT) to ensure hassle free delivery of 131I standard to the NMC. In the national audit of 2020–2021, this consent form was sent to 237 NMCs across the country.
-
(ii)
For 2020–2021 audit, 200 NMCs agreed to participate, as listed in Table 1. Based on NMC’s consent for participation, glass vials similar to the clinical geometry like injection glass vials (IG) containing radioactive 131I solution (1 ml) of nominal activity of 100 MBq were procured from BRIT, Mumbai. The 131I standard IG vial distributed to the participants for measurements is shown in Fig. 6. Each of these IG vial is checked for source integrity, and surface contamination, and labelled with unique identification number. Source integrity is ensured by checking the sealing of the vials. The surface contamination is examined by collecting the swipe samples from the surfaces of the vials and analyze them using the end window Geiger Muller (GM) counting setup. Unique identification number allotted to each IG vial facilitates the tracking/retrieval of the source.
-
(iii)
The radioactivity in each vial is determined by carrying out measurements with the secondary standard, i.e., high-pressure re-entrant gamma ionization chamber (GIC), maintained in the DI laboratory of ionizing radiation. The sensitivity coefficient of GIC is traceable to the primary standards 4π β (PC)-γ and 4π β (LS)-γ coincidence counting systems, maintained by the laboratory, whose international equivalence [7] has been well established by participating in international intercomparison organized by BIPM and APMP.
-
(iv)
A set of six measurements on three different days for each 131I source were recorded and for every measurement the source was removed and replaced in the GIC. Each activity measurement is an average of 20 instantaneous current measurements made at 5 s interval. The standard deviation of these 20 current measurements represents the repeatability and was ± 0.03% while the standard deviation of the six measurements acquired on three different days represents the reproducibility and was ± 0.2%. A typical detailed uncertainty budget due to various components in the 131I activity by GIC is presented in Table 2. The largest uncertainty component is from the calibration of the GIC to the primary standards maintained in the laboratory. The other components like source positioning, source volume, background current, half-life etc., contribution is trivial to the combined standard uncertainty. The combined standard uncertainty (uc) is 1.15% for coverage factor of k = 1.
-
(v)
The standardized radioactive solution (1 ml) of 131I in IG vial was sent to each participant as a blind sample, i.e., activity not disclosed, for measurements at the NMC. Before dispatching, the source integrity and surface contamination were thoroughly checked once again.
-
(vi)
Detailed protocol for performing the measurements at the NMCs and an excel spreadsheet was sent for maintaining uniformity in measurements as well as reporting of the measurement results.
-
(vii)
The results received from NMCs were all checked for calculation of mean, standard deviation, decay correction and % deviation from reference value (BARC value) was determined.
The activity measurement protocol followed by NMCs in the national audit program:
-
(i)
Source received is checked for integrity.
-
(ii)
Background reading of the radionuclide calibrator is noted before and after measurement of the source.
-
(iii)
Activity of the source received at NMCs is measured using radionuclide calibrator with the manufacturer specified calibration number. Measurements are carried out on two different days to demonstrate their reproducibility. Each day measurements are carried out ten times at 2-min interval which indicates the repeatability of measurements. Each time the source is removed and replaced in the well of radionuclide calibrator.
-
(iv)
The source number, manufacturer and model of radionuclide calibrator, background observations, date and time of measurement, mean activity corrected to reference date and time, standard deviation and long-lived reference source used are reported to BARC within the time frame given to them. However, daily quality check procedures and constancy checks with long-lived reference source are not part of the measurement protocol.
3 Results and Discussion
The 19th national audit of 131I activity measurements was organized during Nov 2020–April 2021. As shown in Table 1, out of 237 invited NMCs, 200 NMCs accepted to participate in this audit. Thus, a participating fraction of 84.4% is considered to be very good, especially when the participating NMCs are spread across the country. The entire audit was scheduled into five batches. Most interestingly, a total of 394 results were reported from 200 NMCs which implies that many NMCs have more than one radionuclide calibrators which reflects the increasing demand for nuclear medicine procedures in India. In the present audit from the data, it is observed that 122 NMCs have more than one radionuclide calibrators.
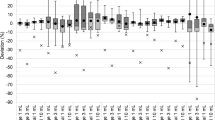
In the present audit results, the performance statistics of radionuclide calibrators were analyzed using two methods: calculation by percentage difference and by z-scores [28]. The obtained results are presented in Tables 3 and 4, respectively. The results of calculation by percentage difference show that fraction of radionuclide calibrators having deviation from BARC activity within 0 to ± 5% and ± 5 to ± 10% are 73% and 20%, respectively. The % deviation of each radionuclide calibrator of the NMCs is shown in Fig. 7. In Fig. 7 for the convenience of plotting for those radionuclide calibrators whose deviation was > ± 15% were scaled down to ± 15%. The results by the z-score test indicate that 82% of the results are acceptable with z ≤ 2, 11% are acceptable with a warning with 2 < z < 3, while 7% are non-acceptable with z ≥ 3. From both analyses, it is clear that 93% of the results received from the NMCs have demonstrated compliance within the acceptable limits of ± 10%.
In order to comprehend how the national audits starting from 1st in 1981 to recent 19th in 2021 fared in the country, all the statistics of participating NMCs and their performances are presented in Figs. 8 and 9, respectively. In Fig. 8, we show the bar chart indicating the number of NMCs invited for the national audit, number of NMCs participated, NMCs with one radionuclide calibrator, and NMCs with more than one radionuclide calibrator. As shown in the inset of Fig. 8, only ~ 22% NMCs participated in the first national audit (i.e., in 1981), which with certain ups and down ascended to ~ 84% in 2021. Also, most of the NMCs in the country had only one radionuclide calibrator until 2001. However, rapid increase in number of NMCs having more than one radionuclide calibrators is seen only after 2010, which escalated to a whopping 61% (of the total NMCs participated in the national audit) in 2021. The reasons attributed to this could be manifold, e.g., during 1980’s the field of nuclear medicine was not very mature, country had a limited resource and only a few NMCs were setup in the country, national audit was voluntary in nature and also probably the awareness about the audit program was less, etc. However, during the first decade of the twenty-first century, AERB implemented web-based e-Licensing of Radiation Applications (eLORA) for obtaining the permission to procure and handle radioactive material by NMCs. In addition, the national audit of radio-pharmaceuticals also became mandatory. This has encouraged NMCs to participate in the audit program, which is evident from an increasing trend after the year 2010. This is also supported by the data of Table 2 and data of inset of Fig. 8, which clearly show that the percentage of NMCs having more than 1 radionuclide calibrator is continuously on rise.
Figure 9 presents the performance analyses of the radionuclide calibrators for all the 19 national audits based on percentage difference and by Z-scores. The bar chart shows total number of radionuclide calibrators verified in the audit program, number of radionuclide calibrators having deviation < ± 10% (i.e., compliance of acceptable limits) and those non-compliant , i.e., deviation > ± 10%. A largest number of radionuclide calibrators (i.e., > 450) were verified during 2018 national audit. However, in 2021, despite of being conducted during COVID pandemic period, 394 radionuclide calibrators were verified. It is evident from the inset of Fig. 9 that in the first national audit, only 58% of radionuclide calibrators demonstrated compliance of acceptable limits and remaining 42% were non-complaint. After each audit program, the non-compliant NMCs were recommended to go for a recalibration of the radionuclide calibrators and to participate in next new audit program. This helped in steady increase of compliant radionuclide calibrators, which soared to 93% in 2021. As a result, non-compliant radionuclide calibrators also reduced considerably, and remained only 7% in 2021. This can be attributed to the success of national audit program carried by BARC. This is seen as a very good result from the perspective of the country as not only the application of ionizing radiation in medicine is increasing but also the focus on quality control is increasing. This implies that the national audit program has contributed in a definitive manner for accurate and efficient dose delivery to the patient for nuclear medicine diagnostic and therapeutic applications in the country.
Now we briefly summarize the results on similar audits conducted by other countries. Globally, NMIs of many countries have regularly been conducting such an audit program to improve the performance of radionuclide calibrators. For example, in Czech Republic, only 80% of the NMCs were compliant within ± 10% in the audit conducted in 1991, which by 2002 increased to 100% participants [14]. Similarly, 95% of the participating hospitals in the UK's audit exercise with 99mTc were within the acceptable limits of ± 5% from the reference value [14]. In Cuba, a considerable increase in the acceptable results within ± 10% was observed in the audits conducted in 2000 and 2002 and the same is maintained in further audit exercises [14]. In the first audit by Brazil, only 62.5% complied but in the 1999s audit 72.7% conformed the regulatory limits of ± 10% [29]. Later, with increased participation of NMCs, the Brazilian NMI expanded its audit program by conceiving regional laboratories to cater to the geographical vastness of the country [30]. Similar to Brazil, India is also a vast country, and therefore, BARC conducts audit program using 131I only, owing to its reasonable half-life of 8 days that favors the logistics of conducting the national audit. In order to cater to the NMCs requirements of audit for short-lived isotopes such as 99mTc and 18F and other diagnostic/therapeutic radioisotopes, it would be appropriate to create several zonal laboratories in the country, which are traceable to BARC. Audits of radionuclide calibrators with short-lived isotopes such as 99mTc and 18F and other therapeutic radioisotopes used at NMC would ensure the correctness of the calibration number of the radionuclide calibrator for each isotope and subsequently ensures safe and effective use of the various radiopharmaceuticals for diagnostic/therapeutic outcomes.
4 Conclusions
In this paper, we have presented one of the rigorous efforts of BARC, as DI, toward the measurement traceability, dissemination of ionizing radiation standards to various NMCs for safe and effective use of radiopharmaceuticals in the country. Best diagnostic (imaging) and therapeutic outcomes of nuclear medicine procedures depend upon how accurately the activity of unsealed radiopharmaceuticals are administered to the patient. This requires regular calibrations of radionuclide calibrators, which is achieved by carrying out biannual national quality audit program of 131I activity measurements over four decades since 1981. So far, 19 national audit programs have been conducted. In the first national audit only ~ 22% NMCs participated, which rocketed to ~ 84% in 19th audit program (i.e., in 2021). The percentage of radionuclide calibrators demonstrating regulatory compliance, escalated from 58% in the first audit to that of 93% in 19th audit. Quality audit programmes ensure continuous improvement which is an important aspect of the quality assurance program and is being achieved as demonstrated by the results of 131I audit.
References
D. K. Aswal, Metrology for Inclusive Growth of India, Springer, 2020, 1–37.
D. K. Aswal, Quality Infrastructure of India and Its Importance for Inclusive National Growth. MAPAN-J. Metrol. Soc India, 35 (2020) 139–150.
D.B. Kulkarni, Leena Joseph, R. Anuradha, M.S. Kulkarni and B.S. Tomar, Standardization of 68Ge–68Ga Using 4πβ(LS)-γ Coincidence Counting System for Activity Measurement. Appl. Radiat. Isot., 123 (2017) 6–10.
A. Yunoki, Y. Sato, L. Joseph, A. Ravindra, D.B. Kulkarni, M. Yuan, K.B. Lee, J.M. Lee, A. Agusbudman, T.-S. Park, P. da Cruz, C. da Silva, A. Iwahara, M. Zhang, J.C. Liang, H.R. Liu, M.J. van Staden, J. Lubbe, M.W. van Rooy, B.R.S. Simpson, Paukkachane, N. Sastri, T. Soodprasert, P. Marsoem, H. Holnisar and G. Wurdiyanto, Report of APMP Comparison of the Activity Measurements of Fe-59 (APMP.RI(II)-K2.Fe-59). Metrologia, 57, 1A (Tech. Suppl.) (2020) 06002.
C. Michotte, G. Ratel, S. Courte, P. Arenillas, C. Balpardo, L. Joseph, R. Anuradha, D.B. Kulkarni, R. Galea, K. Moore, A. Stroak, M. Zhang, J. Liang and H. Liu, Update of the BIPM Comparison BIPM.RI(II)-K1.Co-60 of Activity Measurements of the Radionuclide 60Co to Include the 2011 Result of the CNEA (Argentina), the 2012 Results of the BARC (India) and the NRC (Canada), and the 2014 Result of the NIM (China). Metrologia, 54, Tech. Suppl. (2017) 06002.
B.E. Zimmerman and S. Palm, Results of an International Comparison of 57Co. Applied Radiation and Isotopes, 68 (2010) 1217–1220.
C. Michotte, G. Ratel, S. Courte, A. Yunoki, Y. Unno, R. Fitzgerald, L. Pibida, C. Frechou, C. Bobin and C. Thiam, BIPM Comparison BIPMRI(II)-K1I-131 of Activity Measurements of the Radionuclide 131I for the NMIJ (Japan), the NIST (USA) and the LNE-LNHB (France), with Linked Results for the APMPRI(II)-K2I-131 Comparison. Metrologia, 51(1A) (2014) 1–25.
C. Michotte, S. Courte, M. Nonis, K. Kossert, J. Marganiec, Gałązka, Update of the BIPM Comparison BIPM.RI(II)-K1.Mn-54 of Activity Measurements of the Radionuclide 54Mn to Include the 2017 Result of the PTB (Germany) and the Linked Results from the CCRI(II)-K2.Mn-54 Comparison. Metrologia, 57 (2020) Tech. Suppl. 06005.
S. Mattsson, L. Johansson, B. Nosslin, T. Smith and D.M. Taylor, Dosimetry for Radiopharmaceuticals. Radiat. Protect. Dosim, 79 (1998) 343–345.
A. Iwahara, A.E. de Oliveira, L. Tauhata, C.J. da Silva and R.T. Lopes, Inter-comparison of 131 I and 99m Tc Activity Measurements in Brazilian Nuclear Medicine Services. Appl. Radiat. Isot., 54 (2001) 489–496.
Quality assurance for radioactivity measurement in nuclear medicine. — Vienna : International Atomic Energy Agency, 2006. p.; 24 cm. — (Technical reports series, ISSN 0074–1914 ; no. 454).
Safety code for nuclear medicine facilities, AERB Safety Code No. AERB/SC/MED-4 (Rev. 1), (2001).
International basic safety standards for protection against ionizing radiation and for safety of radiation sources. Basic Safety Series 115(1996).
B.E. Zimmerman and S. Judge, Traceability in Nuclear Medicine. Metrologia, 44 (2007) S127–S132.
B.E. Zimmerman and J.T. Cessna, Experimental Determinations of Commercial ‘dose calibrator’ Settings for Nuclides Used in Nuclear Medicine. Appl. Radiat. Isot., 52 (2000) 615–619.
L. Joseph, R. Anuradha and D.B. Kulkarni, Quality Audit Programme for 99mTc and 131I Radioactivity Measurements with Radionuclide Calibrators. Appl. Radiat. Isot., 66 (2008) 994–997.
M.d. C. de F. Fragoso, A. M. de Albuquerque, M. L. de Oliveira, F. F. de Lima, F. C. P. Barreto and R. de A. Lima, Comparison of the Activity Measurements in Nuclear Medicine Services in the Brazilian Northeast Region. Appl. Radiat. Isot. 82 (2013) 36–44.
J.A. dos Santos, A. Iwahara, A.E. de Oliveira, M.A. da Silva, C.J. da Silva, L. Tauhata and R.T. Lopes, National Intercomparison Program for Radiopharmaceutical Activity Measurements. Appl. Radiat. Isot., 60 (2004) 523–7.
L. Joseph, R. Anuradha, R. Nathuram, V.V. Shaha and M.C. Abani, National Intercomparison of 131I Radioactivity Measurements in NMCS in India. Appl. Radiat. Isot., 59 (2003) 359–362.
G.Y. Kim, H.K. Lee, H.K. Jeong and M.J. Woods, Comparison of Radioactivity Measurements with Radionuclide Calibrators in the Republic of Korea. Appl. Radiat. Isot. 63 (2005) 201–205.
V. Olsovcova, Activity Measurements with Radionuclide Calibrators in the Czech Republic. Appl. Radiat. Isot., 60 (2004) 535–538.
P. Oropesa, A.T. Hernandez, R. Serra and C. Varela, Comparisons of Activity Measurements with Radionuclide Calibrators—A Tool for Quality Assessment and Improvement in Nuclear Medicine. Appl. Radiat. Isot., 63 (2005) 493–503.
P. Oropesa, A.T. Hernandez, R. Serra, E. Martinez and C. Varela, Comparisons of Activity Measurements with Radionuclide Calibrators. Appl. Radiat. Isot., 59 (2003) 383–387.
M.J. Woods, J.D. Keightley, M. Ciocanel and Intercomparisons of 67Ga and 123I: Assays in UK Hospitals. Appl. Radiat. Isot., 49(1998) (1996) 1449–1452.
C. Wastiel, J.F. Valley, A.B. Delaloye, M. Leresche, R. Linder, M. Sassowsky and F.O. Bochud, Intercomparison of Activity Measurements for Beta-Emitters in Swiss Nnuclear Medicine Laboratories. J. Nucl. Med. Technol., 33 (2005) 238–42.
B.E. Zimmerman, C. Herbst, J.P. Norenberg and M.J. Woods, International Guidance on the Establishment of Quality Assurance Programmes for Radioactivity Measurement in Nuclear Medicine. Appl. Radiat. Isot., 64 (2006) 1142–1146.
Veronika Olˇsovcova, A. Iwahara, Pilar Oropesa, Leena Joseph, Anuradha Ravindra, Mostafa Ghafoori, Hye-KyungSon , MariaSahagia , SelmaTastan , Zimmerman. B., National Comparison of 131I Measurement Among Nuclear Medicine Clinics of Eight Countries, Appl. Radiat. Isot., 68 (2010) 1371–1377.
ISO 13528, International Organization for Standardization, 2015. Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison.
A. Iwahara, A.E. de Oliveira, L. Tauhata, C.J. da Silva, C.P.G. da Silva, A.M.S. Braghirolli and R.T. Lopes, Performance of Dose Calibrators in Brazilian Hospitals for Activity Measurements. Appl. Radiat. Isot., 56 (2002) 361–367.
A.E. Oliveira, A. Iwahara, C.J. Silva, P.A.L. Cruz, R. Poledna, R.L. Silva, A.S. Laranjeira, J.U. Delgado, L. Tauhata, J.S. Loureiro, B.C. Toledo, A.M.S. Braghirolli, E.A.L. Andrade, J.L. Silva, H.O.K. Hernandes, E.S. Valente, H.M. Dalle, V.M. Almeida, T.G. Silva, M.C.F. Fragoso, M.L. Oliveira, E.S.S. Nascimento, E.M. Oliveira, R. Herrerias, A.A. Souza, E. Bambalas and W.A. Bruzinga, Traceability from Governmental Producers of Radiopharmaceuticals in Measuring 18F in Brazil. Appl. Radiat. Isot., 109 (2016) 236–241.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravindra, A., Kulkarni, D.B., Sharma, R. et al. National Audit for Traceable 131I Activity Measurements with Radionuclide Calibrators Among Nuclear Medicine Centers in India. MAPAN 39, 49–61 (2024). https://doi.org/10.1007/s12647-023-00645-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-023-00645-x