Abstract
It is now well recognized that a bidirectional relationship between gut microbiota and the brain, referred to as the gut-brain axis, plays a prominent role in maintaining homeostasis and that a disruption in this axis can result in neuroinflammatory response and neurological disorders such as Parkinson’s disease (PD). The protective action of probiotics such as Bifidobacterium animalis ssp. lactis Bb12 and Lactobacillus rhamnosus GG in various animal models of PD has been reported. Therefore, in this study, we used an inflammatory model of PD to assess the effects of a combination of these two probiotics (Microbiot®) on motor behavior as well as on the response of microglia, including microglia morphology, to gain a better understanding of their mechanism of action. Microbiot® (300 µL) was administered orally once daily for 15 days in a lipopolysaccharide-induced PD model using male Wistar rats. Although LPS-induced motor asymmetry in cylinder test was not affected by Microbiot®, impairment of motor coordination in the narrow-beam test was significantly reduced by this probiotic. Moreover, Microbiot® treatment reduced microglial activation suggesting an anti-inflammatory effect. While further mechanistic investigation of Microbiot® in neurodegenerative diseases is warranted, our results support the potential utility of probiotics in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD), associated with loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), is the second most common progressive neurodegenerative disorder following Alzheimer’s disease (Aarsland et al. 2021). Main symptoms include motor deficits characterized by akinesia, rigidity, resting tremor, and postural instability, as well as non-motor symptoms such as emotional changes (e.g., depression, apathy, and anxiety), sleep perturbations (e.g., insomnia/hypersomnia), autonomic dysfunction (e.g., bladder disturbances, orthostatic hypotension, sweating), sensory symptoms (e.g., pain, visual and olfactory deficits), and gastrointestinal symptoms (e.g., constipation, nausea) as well as cognitive deficits (e.g., mild to severe memory impairment) (Gonzalez-Latapi et al. 2021; Tizabi et al. 2021).
Neuronal degeneration in PD is associated with several cellular and molecular events including accumulation of misfolded proteins via mutations in several genes including alpha-synuclein and GBA (a gene that encodes for the lysosomal enzyme glucocerebrosidase), as well as oxidative stress and neuroinflammation (Dorszewska et al. 2021; Harms et al. 2021; Smith and Schapira 2022). Hence, it is believed that genetics and immune dysregulation are primary suspects in the etiology of PD (Dorszewska et al. 2021; Harms et al. 2021; Smith and Schapira 2022).
In recent years, a crucial role for gut microbiota in immune regulation and the pathogenesis of PD has been suggested (Rani and Mondal 2021; Klann et al. 2021; Nuzum et al. 2022; Shannon 2022). Indeed, overexpression of pro-inflammatory bacteria has been observed in the gut of PD patients (Shannon 2022). It is now believed that the gut microbiota is not only critical in maintaining general homeostasis but also in neurogenesis, and that its disruption or dysbiosis is at least partially responsible for progression of neuropsychiatric and neurodegenerative diseases including PD (Mitrea et al. 2022). Hence, it is being suggested that probiotic formulations may be used as a novel therapeutic intervention in depression, and bipolar disorder as well as in PD (Tizabi et al. 2021; McGuinness et al. 2022; Mitrea et al. 2022; Zhang et al. 2022).
Several in vitro and in vivo studies also support the potential use of probiotics in management of motor as well as non-motor symptoms of PD (Getachew and Tizabi 2019; Getachew et al. 2019a, b, 2020; Tizabi et al. 2021; Nuzum et al. 2022). Specifically, in vivo studies have consistently shown that Lactobacillus and Bifidobacterium probiotics can attenuate the negative effects of the toxic substances in experimental PD models (Klann et al. 2021; Cuevas-Carbonell et al. 2022). However, there exists a significant gap in our understanding of the specific effects of probiotics on markers of neurodegeneration as well as the behavioral consequences of such treatments in PD models. Thus, this study was undertaken to provide a detailed assessment of probiotics on markers of neuroinflammation, and neurodegeneration as well as motor impairments in an inflammatory model of PD (Tan et al. 2021).
Materials and Methods
Subjects
Adult male Wistar rats (n = 38), purchased from Harlan in Mexico by the Universidad Autónoma de Yucatán (UADY) and raised in our facilities, were housed singly in polycarbonate cages (length, 38 cm; width, 24 cm; height, 20 cm), in a temperature-controlled room and 12-h light/dark cycle (lights on at 7 AM), with free access to food and water. All animal procedures were carried out in accordance with the Mexican Council for Care and Use of Laboratory Animals and the Norma Oficial Mexicana NOM-062-ZOO-1999. All the protocols were approved by the Animal Care Committees of UADY.
Unilateral Intra-striatal Lesion
We employed a well-established inflammatory model of PD which has also been used by us in previous studies and consists of intracranial (i.c.) injection of lipopolysaccharide (LPS) into the striatum (Parra et al. 2020).
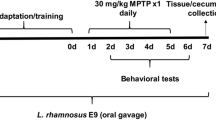
The rats were randomly divided to receive unilateral intra-striatal injection of LPS as a toxic treatment or isotonic saline solution (ISS) as a vehicle control. Each animal was anesthetized with a combination of ketamine (115.2 mg/ml) and xylazine (20 mg/ml) (85:16 mg/kg; i.p.) and placed in a stereotaxic apparatus (Stoelting Co., Wood Dale, IL, USA) for the administration of 2 μL of vehicle or 2 μL of LPS (16 μg/μl, i.c.) (Escherichia coli O26:B6; Sigma Aldrich, St. Louis, MO, USA). LPS was dissolved in ISS. Using an infusion pump, neurotoxin or vehicle was injected into the left dorsolateral striatum at 0.2 μL/min. The stereotaxic coordinates were AP + 0.7 mm from Bregma, ML + 3.4 mm from midline, DV1 −4.8 mm, and DV2 −5.2 mm below dura, according to Paxinos and Watson’s Stereotaxic Atlas 2007 (Fig. 1). After surgery, animals were given saline subcutaneously and were closely monitored until recovery.
Timeline scheme of the experimental design. Treatment with probiotic or vehicle was carried out for 14 days prior and on the day of stereotaxic surgery. The i.c. injection was performed according to coordinates of Paxinos and Watson (2007). The animals were sacrificed 14 days post-surgery, during which no probiotic was administered. The brains were processed for immunohistochemistry as detailed in the methods
Probiotic Treatment
Rats were divided into five groups. Group 1 received vehicle injection (isotonic saline = ISS) in the striatum and vehicle treatment (sunflower oil + vitamin E) instead of probiotics. This group (n = 6) was designated as (ISS + SOE). Group 2 received vehicle injection in the striatum and probiotic (P) treatment. This group (n = 6) was designated as (ISS + P). Group 3 received LPS injection in the striatum and vehicle instead of probiotic. This group (n = 9) was designated as (LPS + SOE). Group 4 received LPS injection in the striatum and probiotic (P). This group (n = 11) was designated as (LPS + P). Group 5 received LPS injection in the striatum only. This group (n = 6) was designated as LPS and served as control for other LPS treatment groups (Fig. 1).
The commercial probiotic mixture used in the study Microbiot® contained live Lactobacillus rhamnosus GG and Bifidobacterium animalis lactis (BB-12). Probiotics (1 × 109 CFU each probiotic) or 300 μl of vehicle (sunflower oil + vitamin E [1.6 mM v/v]) were given orally, daily for 15 days, including on the day of surgery, where the animals received intracranial injections of ISS or LPS. The rats were sacrificed 14 days after the i.c. injection. No probiotic treatment was administered during this period.
Cylinder Test
In this test, each rat was placed in a transparent Plexiglas cylinder (30 cm high and 20 cm wide) and its activity was video-recorded for 5 min. A minimum of eight wall contacts was considered as a normal activity. Values below the mean cutoff score represented overall asymmetry, which has been correlated with the degree of striatal DA damage (Schallert et al. 2000; Schallert and Tillerson 2000; Tillerson et al. 2001). This test was performed 3 times. First, at 18 days prior to i.c. injection to establish baseline; second, 2 h prior to i.c. injection (i.e., following 15 days of probiotic treatment); and third, at 14 days post-injection and 2 h before the sacrifice. The 2-week period was chosen because it is the time required to manifest motor impairment following LPS injection (Chang et al. 1999; Miyanishi et al. 2019; Parra et al. 2020). Scores as a percentage of use of ipsilateral or contralateral were calculated as described previously (Parra-Paz et al. 2021).
Beam Test
The wooden beam, 100 cm long and 2 cm wide, was suspended 100 cm above the ground by wooden supports at both ends. The “start platform” 20 cm long and 4 cm wide was placed at one end, and a dark box 25 cm long and 4 cm wide was placed at the other end. Initially, all rats were trained in this task at days 17 and 16 prior to i.c. injection. During the training sessions, the rats learned to travel the beam in the least time. Following the training, the rats were tested on 4 different occasions. Once at 15 days prior to i.c. injection (i.e., prior to probiotic administration to establish baseline), once at 8 days prior to i.c. injection, once at 7 days post injection, and the last time at 13 days after the i.c. injection (Fig. 2). In each test, the rat was placed as far away from the start line and allowed to cross the beam according to its motor abilities. Each test was video recorded with a Canon Vixia Hf R800 camera at 1080p resolution and 60 frames per second (fps) for analysis.
The following variables described below were measured: (1) latency, (2) mean speed, (3) number of steps, (4) cadence, (5) motor function (e.g., characterization of step error, described in more detail below), (6) support and (7) balance time, (8) cycle step, (9) the speed of balance, (10) length of strides in hindlimb, (11) length of strides in forelimb, and (12) impression positions (Hamers et al. 2006; Koopmans et al. 2007; Hsieh et al. 2011). The videos were analyzed with the Adobe Premier Pro-2019 and FIJI programs. Parameters 1, 3, 5, 6, and 7 were evaluated frame by frame with the Adobe Premiere Pro software; variables 10, 11, and 12 with the FIJI software and the rest of the variables (2, 4, 8, and 9) were derived from the initial motor analysis. The values obtained for parameters were the average of 5 effective crossings.
Briefly, latency was defined as the time it takes the rat to cross the starting line. Mean speed as the ratio between the length of the beam and the latency (100 cm/time to cross in seconds). Number of steps as the number of times in which the experimental subject places the posterior contralateral limb on the beam. Cadence is the number of steps/second. Support time was defined as the duration in milliseconds (ms) when the contralateral hind limb is in contact with the beam in one step cycle. Balance time as the time (ms) in which the contralateral hindlimb is not in contact with the beam. Step cycle as the time it takes for the contralateral hindlimb to take a step (equals the sum of the time of support and balance). The balance speed is the relation between the stride length and the balance time (cm/s). Stride length as distance between two continuous touches of the hindlimb and forelimb. The impression position is the relative positions of fore- and hind-paws (Hamers et al. 2006; Koopmans et al. 2007; Baker 2013; Batka et al. 2014; Boix et al. 2018).
The percentage of errors was calculated as the number of step errors in relation to 100% of steps. Motor deficit was scored (1–5) using Brailowsky’s et al. scale (1986) (Brailowsky et al. 1986; Bueno-Nava et al. 2010; Avila-Luna et al. 2018), according to the following types of step errors: (1) if the intact paw was placed inside the beam, it was scored as “zero”; if the paw was correctly placed on the beam and all 4 toes protruded from it, it was scored as “1”; if the paw slid off once, it was scored as “2”; if the paw slid off twice, it was scored as “3”; if the paw slid off the beam 3 or more times, it was scored as “4.” If the hindlimbs slid off the beam, it was scored as “5.” The characterization of the step errors and their percentage of such errors was performed by detail frame-by-frame analysis of performance on the beam test (Fig. 2).
Various gait variables in rodents have a statistical relationship with speed, implying that cadence, support time, swing time, step cycle, stride length, swing speedl and the impression position depend on the speed (Clarke & Parker; 1986; Batka et al. 2014; Aceves et al. 2020). In order to avoid any statistical error due to this correlation, each value for each beam crossing of the above parameters was multiplied by a normalization factor (F) according to the equation F = (Mean speed of each beam crossing)/(Average of the mean speed) as detailed by Boix et al (2018).
Immunohistochemistry
For immunohistochemical studies, animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), perfused intracardially with 4% paraformaldehyde in PBS buffer (pH 7.4). The brains were removed and kept in a paraformaldehyde buffer for 72 h. Coronal Sects. (60 μm thick) were obtained by a microtome Leica SM 2010R and stored in PBS buffer (pH 7.4) for immunohistochemical assessments. Immunostaining was carried out in free-floating brain sections with standard avidin–biotin immunohistochemical protocol as detailed previously (Parra et al. 2020). Briefly, the sections were incubated overnight with specific primary antibody, a mouse monoclonal anti-tyrosine hydroxylase (TH) antiserum (Chemicon, USA) diluted 1:2000, or a rabbit polyclonal anti-ionized calcium–binding adaptor molecule-1 (Iba-1) (Wako Pure Chemical Industries, Ltd. Osaka, Japan) diluted 1:1000, in PBS solutions containing normal goat serum. After careful washing, the sections were incubated first with the secondary biotinylated antisera, diluted 1:500 (Vector) at room temperature and then with HRP-streptavidin (1:5000, Thermo Fisher Scientific) and finally incubated with diaminobenzidine (1 mg/2 mL; DAB) complex for 10 min. After washing, two slices from each experimental subject were mounted on gelatin-coated slides, air-dried, and dehydrated in ascending concentrations of ethanol, cleared with xylene, and cover slipped. Controls were performed to confirm the specificity of the primary and secondary antibodies. Images were obtained and captured using an optical microscope (Zeiss® Axiolab 5).
Quantification of TH-positive neurons (TH+) in the SNpc region (reflecting dopaminergic neurons) and Iba-1-positive cells (Iba-1+, reflecting microglial cells) was performed in the dorsolateral striatum and SNpc using FIJI software as detailed previously (Mendieta et al. 2016; Parra et al. 2020). For quantification of TH-positive neurons, the entire extent of the SNpc was counted under a light microscope using a 10 × objective. For quantification of Iba-1+ cells in dorsolateral striatum and entire SNpc, also a light microscope using a 20 × objective was used. Total microglial cells and microglial subtype cells were semi-automatically sorted out into five morphological subtypes (ramified, primed, activated, amoeboid, and non-microglial macrophage-like cells) as detailed by Torres-Platas et al. (2014), Lee et al. (2018), and Parra et al. (2020). Briefly, ramified microglia was identified as a small and rounded cell body (~ 20–25 μm2) with processes in the form of tree branches and containing a number of nodes (bifurcation points of the processes); primed microglia as an elongated cell body (~ 50–70 μm2) which is larger than the ramified phenotype but has thicker and shorter processes; activated microglia as a large oval or spindle shape cell body (~ 50–70 μm2) with processes not so extensive and coarse; amoeboid microglia as not having a fully rounded cell body with two or less processes; and non-microglial macrophage cells as completely rounded cells without protoplasmic expansions. Microgliosis was defined as a significant increase in Iba-1 + cells compared to the ISS group.
Statistical Analysis
For immunochemistry, statistical analyses were performed using one-way analysis of variance (ANOVA) for more than two mean comparisons. Student’s t test was used for two mean comparisons. For behavioral analyses, also one-way ANOVA was used. All ANOVAs were followed by a Tukey post hoc test to determine specific group differences. Statistical significance was set at p < 0.05. GraphPad Prism software version 8.0 for Windows was used for all analyses. Data are presented as mean ± standard error of the mean (SEM).
For the cylinder test and the morphometry of the microglial subtypes, GraphPad Prism 8 software was used. Here, the Kolmogorov-Smirnoff test was performed to check the normal distribution of the data. If the data adjusted to normal distribution, an ANOVA and a Tukey post hoc test were performed. If a normal distribution was not evident, a non-parametric data test was performed. Specifically, for the cylinder test and the morphometry, a two-way ANOVA, and for body mass and gait parameters, a repeated measure analysis was applied. For the number of TH+ and Iba-1+ cells, we used a one-way ANOVA followed by Tukey post hoc test.
Results
Figure 3 depicts the effects of probiotics on motor asymmetry in an LPS-induced parkinsonian model. Animals with intrastriatal LPS injection or LPS plus P showed a motor asymmetry, with the exception of LPS + SOE (F8,286 = 5.446, p < 0.05). As seen, 15 days of pre-treatment with the probiotic treatment did not modify the motor asymmetry induced by LPS as assessed 14 post-surgery days (p > 0.05). There was also no effect on baseline measures with probiotics prior to LPS injection.
Effects of probiotics on motor asymmetry in an LPS-induced parkinsonian model. Pre-treatment with the probiotics for 15 days did not modify the motor asymmetry induced by LPS. The motor behavior was assessed 14 days after the surgery with no probiotic treatment. Examples of the types of forelimb scores in the cylinder test, a ipsilateral, b contralateral, c forelimb use percentage, were assessed at 3 time points as detailed in the methods. The vertical dotted line indicates the day of the stereotaxic surgery. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). Statistical analysis was performed with a two-way ANOVA and a post-Tukey test. Values are expressed as the mean ± standard error of the mean (SEM). *p < 0.05 vs ISS + SOE
Figure 4 depicts the effects of probiotics on growth, speed, and strides in an LPS-induced parkinsonian model. Here also, 15 days of pre-treatment with the probiotic did not modify the impairments in growth, speed, and strides induced by LPS as assessed 14 days after intrastriatal injection of LPS. Probiotics did not affect the baseline measures in these parameters prior to LPS injection (growth: F20,165 = 1.634, p > 0.05; speed: F12,99 = 1.25, p > 0.05; strides: F12,99 = 2.787, p > 0.05). Latency, number of steps, cadence, speed balance, and the impression positions were also not affected by the probiotic treatment (data not shown).
Effects of probiotics on growth, speed, and strides in an LPS-induced parkinsonian model. Probiotic treatment did not modify the normal growth, the weight (a), the speed (b), the number of strides (c), or the motor coordination of the animals in the beam test. The vertical dotted line indicates the day of stereotaxic surgery. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). Statistical analysis for (a) was performed using two-way ANOVA and a post-Tukey, p < 0.05. Statistical analyses for (b) and (c) were performed using a mixed effects model for repeated measures and a post-Tukey test. Values are expressed as the mean ± SEM
Figure 5 depicts the effects of probiotics on motor function in an LPS-induced parkinsonian model. Although pre-treatment with probiotic did not modify percentage of step errors (F12,99 = 7.082, p > 0.05) (A), or Brailovsky’s motor deficit (F12,99 = 8.067, p > 0.05) (B) at any time point, it did cause a significant increase in avoidance of type 3 error at 7 days after LPS injection (LPS + SOE vs LPS + P, F45,396 = 2.811, p < 0.05) (C), this beneficial effect was absent at 13 days post LPS injection. Table D shows the comparison between groups for each type of error in Brailovsky’s motor test.
Effects of probiotics on motor function in an LPS-induced parkinsonian model. Probiotic treatment reduced the motor impairments caused by LPS but did not modify the percentage of errors (a) or the Brailovsky’s motor deficit (b). However, the increase in type 3 errors (c, d) were reduced by probiotic treatment. The vertical dotted line (a, b) indicates the day of stereotaxic surgery. ISS, isotonic saline solution; psd, post surgery day; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). Statistical analyses for (a) and (b) were performed using a mixed effects model for repeated measures and a post-Tukey test. Analysis for (c) was performed by two-way ANOVA and a post-Tukey. *p < 0.05, **p < 0.01, ***p < 0.001. Red symbol vs ISS + SOE, green symbol vs ISS + P. Values are expressed as the mean ± SEM
Figure 6 depicts the effects of probiotics on several motor parameters in an LPS-induced parkinsonian model. Pre-treatment with probiotic significantly improved the stand phase at 13 days post LPS injection (LPS + SOE vs LPS + P, F12,3.410 = 3.41, p < 0.01) but did not affect the swing phase or step cycle.
Effects of probiotics on step cycle in an LPS-induced parkinsonian model. Probiotic treatment modified the support phase 13 days after injection (a) but did not modify the swing phase (b) or the step cycle (c). The vertical dotted line (a, b) indicates the day of stereotaxic surgery. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). Statistical analysis was performed using a mixed effects model for repeated measures and a post-Tukey test. *p < 0.05. Red symbol vs ISS + SOE, green symbol vs ISS + P. Values are expressed as the mean ± SEM
It is important to highlight that evaluation of the motor behavior can be affected by various factors related to the physical characteristics of the subject (e.g., age, weight, height, leg length). Since normalization of all these factors is untenable, the statistical analysis in the present study only accounted for parameters related to speed (Baker 2013; Batka et al. 2014).
Figure 7 depicts the effects of probiotics on the number of tyrosine hydroxylase (TH+) neurons in SNpc. Pre-treatment with probiotic did not affect this parameter suggesting that the probiotic did not prevent LPS-induced dopaminergic degeneration in the SNpc (F4,25 = 12.06, p > 0.05).
Effects of probiotics on dopaminergic toxicity in an LPS-induced parkinsonian model. Probiotic treatment did not prevent dopaminergic degeneration in the SNpc. (a) Schematic representation of the evaluation site. (b–f) Photomicrographs of nigral sections stained for TH. (g) The number of nigral TH+ neurons in the ipsilateral hemisphere relative to the contralateral. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). The bar in the lower magnification photograph (5 ×) indicates 500 microns. The bar in the higher magnification (100x) indicates 50 microns. Statistical analysis was performed by a one-way ANOVA and a post-Tukey. **p < 0.01, ***p < 0.001 vs LPS, red symbol vs ISS + SOE, green symbol vs ISS + P. Values are expressed as the mean ± SEM
Figure 8 depicts the effects of probiotics on striatal microgliosis in an LPS-induced parkinsonian model. As seen, LPS-induced microgliosis in the striatum (F4,33 = 20.56, p < 0.001). In contrast, pre-treatment with probiotics significantly reduced this effect (approx. 30%, p < 0.05). Interestingly, SOE also had a similar effect (p < 0.05).
Effects of probiotics on striatal microgliosis in an LPS-induced parkinsonian model. Both probiotic and SOE prevented striatal microgliosis. (a) Schematic representation of the injection site in the striatum and the evaluation site. (b–f) Photomicrographs of sections of the striatum stained with Iba-1.+ (g). The proportion of striatal Iba-1- + cells in the ipsilateral hemisphere relative to the contralateral. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). The bar in the lower magnification photograph (20 ×) indicates 100 microns. The line in higher magnification (40 ×) indicates 50 microns. Statistical analysis was performed by a one-way ANOVA and a post-Tukey. *p < 0.05, ***p < 0.001, red symbol vs ISS + SOE, green symbol vs ISS + P. Values are expressed as the mean ± standard error of the mean (SEM)
Figure 9 depicts the effects of probiotics on nigral microgliosis in an LPS-induced parkinsonian model. In contrast to the effect on the striatum, pre-treatment with probiotic did not prevent microgliosis in SNpc (F4,32 = 2.774, p > 0.05).
Effects of probiotics on nigral microgliosis in an LPS-induced parkinsonian model. Probiotic treatment did not prevent nigral microgliosis. (a) Schematic representation of the evaluation site in the SNpc. (b–f) Photomicrographs of ipsilateral SNpc sections stained with iba-1+. (g) The proportion of striatal Iba-1 + cells in the ipsilateral hemisphere relative to the contralateral. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). The bar in the lower magnification photograph (5 ×) indicates 500 microns. The bar in the higher magnification (40 ×) indicates 50 microns. Statistical analysis was performed by a one-way ANOVA and a post-Tukey, p < 0.05. Values are expressed as the mean ± SEM
Figure 10 depicts the effects of probiotic on microglial morphology in the striatum and SNpc in an LPS-induced parkinsonian model. Pre-treatment with probiotic prevented LPS-induced aberrant activation of microglia in the striatum (F4,165 = 21.9, activated: p < 0.001; amoeboid: p < 0.005), but not in SNpc (F3,128 = 11.58, p > 0.05).
Effects of probiotics on microglial morphology in the striatum and SNpc in an LPS-induced parkinsonian model. Probiotic treatment prevented an aberrant activation of microglia in the striatum but not in SNpc. (a–d) Representative photomicrographs of microglial morphology and their classification as branched, primed, activated, amoeboid, and macrophage-like in the striatum. (b) Total number of Iba-1+ cells in the contralateral and ipsilateral striatum and (e) SNpc. Both probiotic and sunflower oil + vitamin E prevented the aberrant growth of amoeboid cells. (c) Ipsilateral/contralateral ratio of the number of total Iba-1+ cells in the striatum and (f) SNpc. Probiotic treatment also prevented the aberrant increase of activated and amoeboid morphologies. ISS, isotonic saline solution; P, probiotic; SOE, sunflower oil + vitamin E. ISS + SOE (n = 6); ISS + P (n = 6); LPS only (n = 6); LPS + SOE (n = 9); LPS + P (n = 11). The bar in the micrograph is 10 μm. Statistical analysis was performed by a two-way ANOVA and a post-Tukey. *p < 0.05, **p < 0.01, ***p < 0.001 amoeboid and activated LPS vs LPS + P, and activated LPS vs LPS + SOE. Values are expressed as the mean ± SEM
Discussion
The results of this study suggest that treatment with probiotics could be of therapeutic value in PD. This is based on our findings that 2 weeks of pre-treatment with a combination of 2 probiotics (Microbiot®) countered several behavioral and toxicological markers of centrally administered LPS. These improvements were observed at different time-points after intrastriatal injection of LPS. For example, in the beam test, significant increases in avoidance of type 3 error were observed at 7 days after LPS injection, whereas improvements in stand phase were observed 13 days after LPS injection. Concomitantly, pre-treatment with probiotic significantly reduced LPS-induced microgliosis as well as LPS-induced aberrant activation of microglia in the striatum. It is of importance to note that the probiotic treatment was carried out solely prior to intrastriatal administration of LPS. Thus, potential beneficial effects of the continuation of such treatment on motor functions and amelioration of neuronal damage have to be further investigated. Moreover, the improvements we observed were confined to specific indices of motor function made possible by detailed (frame by frame) analysis of motor behavior. Similarly, intricate evaluation of the microglia’s function including glial morphology as applied here should be considered in follow-up studies.
The utility of probiotics in neuropsychiatric/neurodegenerative diseases such as depression, bipolar disorder, and particularly in PD, is a subject of intense research. Thus, a number of preclinical studies show that administration of probiotics can prevent dopaminergic degeneration by preserving TH+ cells of the SNpc and concomitantly reduce motor asymmetry, and improve walking, coordination, and even fine motor movements (Hsieh et al. 2020; Perez Visñuk et al. 2020; Alipour Nosrani et al. 2021; Dwyer et al. 2021; Nurrahma et al. 2021; Sun et al. 2021; Alfonsetti et al. 2022; Cuevas-Carbonell et al. 2022). Moreover, in addition to mood regulation and effectiveness as antidepressants (Getachew et al. 2018, 2019b; Getachew and Tizabi 2019; Tizabi et al. 2021; McGuinness et al. 2022; Mitrea et al. 2022; Zhang et al. 2022), probiotic benefits may extend to a variety of other non-motor functions such as cognitive behavior (Xie and Prasad 2020), appetite and temperature control (Gabanyi et al. 2022), and addiction to substances such as alcohol (Ezquer et al. 2022). Our observation of the animal’s behavior in narrow beam situated at 1 m above the ground suggests a potential antianxiety effect of the probiotic, although this has to be verified in specific models of anxiety. The utility of probiotics in such diverse conditions or diseases mentioned above is not surprising given the recently discovered critical role of microbiota in maintenance of homeostasis and regulation of the immune system. As mentioned earlier, a major culprit in practically all neuropsychiatric and/or neurodegenerative diseases is dysregulation of the immune system and induction of neuroinflammation, which are intimately tied to dysbiosis (Mou et al. 2022; Mitrea et al. 2022). Thus, the immune regulatory effects of probiotic in general, and their anti-inflammatory properties in particular, along with their ability to activate the signaling cascades related to cell survival and production of growth factors (Srivastav et al. 2019; Castelli et al. 2020; Wang et al. 2022), may be the underlying contributor to their therapeutic utility.
It is now well established that microglia are the central mediators of the immune response and that their overactivation can lead to neuroinflammation and eventual disruption of circuitries involved in cognitive function, mood regulation, and motor behavior (Doorn et al. 2012; Aono et al. 2017). Our finding of reduced LPS-induced microgliosis as well as reduction of LPS-induced aberrant microglia in the striatum by the probiotics is in line with this hypothesis. However, further investigation on utility of other probiotics or longer application of probiotics used in this study should be considered.
In summary, our results support usefulness of probiotics in neuroinflammatory-induced motor impairments. Nonetheless, further mechanistic evaluation of Microbiot® or other probiotics in neurodegenerative diseases in general, and PD in particular, is warranted.
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D (2021) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7(1):47. https://doi.org/10.1038/s41572-021-00280-3
Aceves M, Dietz VA, Dulin JN, Jeffery U, Jeffery ND (2020) An analysis of variability in "CatWalk" locomotor measurements to aid experimental design and interpretation. eNeuro 7(4). https://doi.org/10.1523/ENEURO.0092-20.2020
Alfonsetti M, Castelli V, d'Angelo M (2022) Are we what we eat? Impact of diet on the gut-brain axis in Parkinson's disease. Nutrients 14(2). https://doi.org/10.3390/nu14020380
AlipourNosrani E, Tamtaji OR, Alibolandi Z, Sarkar P, Ghazanfari M, AzamiTameh A, NaderiTaheri M (2021) Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine: a behavioral, biochemical, and histological study. J Immunoassay Immunochem 42(2):106–120. https://doi.org/10.1080/15321819.2020.1833917
Aono H, Choudhury ME, Higaki H, Miyanishi K, Kigami Y, Fujita K, Tanaka J (2017) Microglia may compensate for dopaminergic neuron loss in experimental Parkinsonism through selective elimination of glutamatergic synapses from the subthalamic nucleus. Glia 65(11):1833–1847. https://doi.org/10.1002/glia.23199
Avila-Luna A, Gálvez-Rosas A, Alfaro-Rodríguez A, Reyes-Legorreta C, Garza-Montaño P, González-Piña R, Bueno-Nava A (2018) Dopamine D. Behav Brain Res 336:145–150. https://doi.org/10.1016/j.bbr.2017.08.026
Baker R (2013) Measuring walking : a handbook of clinical gait analysis. Mac Keith Press
Batka RJ, Brown TJ, Mcmillan KP, Meadows RM, Jones KJ, Haulcomb MM (2014) The need for speed in rodent locomotion analyses. Anat Rec (hoboken) 297(10):1839–1864. https://doi.org/10.1002/ar.22955
Boix J, von Hieber D, Connor B (2018) Gait analysis for early detection of motor symptoms in the 6-OHDA rat model of Parkinson’s disease. Front Behav Neurosci 12:39. https://doi.org/10.3389/fnbeh.2018.00039
Brailowsky S, Knight RT, Blood K, Scabini D (1986) Gamma-Aminobutyric acid-induced potentiation of cortical hemiplegia. Brain Res 362(2):322–330. https://doi.org/10.1016/0006-8993(86)90457-9
Bueno-Nava A, Gonzalez-Pina R, Alfaro-Rodriguez A, Nekrassov-Protasova V, Durand-Rivera A, Montes S, Ayala-Guerrero F (2010) Recovery of motor deficit, cerebellar serotonin and lipid peroxidation levels in the cortex of injured rats. Neurochem Res 35(10):1538–1545. https://doi.org/10.1007/s11064-010-0213-4
Castelli V, d'Angelo M, Lombardi F, Alfonsetti M, Antonosante A, Catanesi M, Cimini A (2020) Effects of the probiotic formulation SLAB51 in. Aging (Albany NY) 12(5), 4641–4659. https://doi.org/10.18632/aging.102927
Chang JW, Wachtel SR, Young D, Kang UJ (1999) Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88(2):617–628
Clarke KA, Parker AJ (1986) A quantitative study of normal locomotion in the rat. Physiol Behav 38(3):345–351. https://doi.org/10.1016/0031-9384(86)90105-8
Cuevas-Carbonell SG, Vásquez-Celaya L, García-López D, Granados-Patrón D, García-Miss MDR, Álvarez-Cervera FJ, Góngora-Alfaro JL (2022) Chronic treatment with the probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis BB12 attenuates motor impairment, striatal microglial activation, and dopaminergic loss in rats with 6-hydroxydopamine-induced hemiparkinsonism. Neuroscience. https://doi.org/10.1016/j.neuroscience.2022.11.004
Doorn KJ, Lucassen PJ, Boddeke HW, Prins M, Berendse HW, Drukarch B, van Dam AM (2012) Emerging roles of microglial activation and non-motor symptoms in Parkinson’s disease. Prog Neurobiol 98(2):222–238. https://doi.org/10.1016/j.pneurobio.2012.06.005
Dorszewska J, Kowalska M, Prendecki M, Piekut T, Kozłowska J, Kozubski W (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391. https://doi.org/10.4103/1673-5374.300980
Dwyer Z, Chaiquin M, Landrigan J, Ayoub K, Shail P, Rocha J, Hayley S (2021) The impact of dextran sodium sulphate and probiotic pre-treatment in a murine model of Parkinson’s disease. J Neuroinflammation 18(1):20. https://doi.org/10.1186/s12974-020-02062-2
Ezquer F, Quintanilla ME, Morales P, Santapau D, Munita JM, Moya-Flores F, Israel Y (2022) A dual treatment blocks alcohol binge-drinking relapse: microbiota as a new player. Drug Alcohol Depend 236:109466. https://doi.org/10.1016/j.drugalcdep.2022.109466
Gabanyi I, Lepousez G, Wheeler R, Vieites-Prado A, Nissant A, Wagner S, Lledo PM (2022) Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science, 376(6590), eabj3986. https://doi.org/10.1126/science.abj3986
Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabiy Y (2018) Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol 18(1):222. https://doi.org/10.1186/s12866-018-1373-7
Getachew B, Csoka AB, Bhatti A, Copeland RL, Tizabi Y (2020) Butyrate protects against salsolinol-induced toxicity in SHSY5Y Cells: Implication for Parkinson’s Disease. Neurotox Res 38(3):596–602. https://doi.org/10.1007/s12640-020-00238-5
Getachew B, Mendieta L, Csoka AB, Aguilera J, Tizabi Y (2019a) Antidepressant effects of C-Terminal domain of the heavy chain of tetanus toxin in a rat model of depression. Behav Brain Res 370:111968. https://doi.org/10.1016/j.bbr.2019.111968
Getachew B, Reyes RE, Davies DL, Tizabi Y (2019b) Moxidectin effects on gut microbiota of Wistar-Kyoto Rats: relevance to depressive-like behavior. Clin Pharmacol Transl Med 3(1):134–142
Getachew B, Tizabi Y (2019) Effects of C-terminal domain of the heavy chain of tetanus toxin on gut microbiota in a rat model of depression. Clin Pharmacol Transl Med 3(2):152–159
Gonzalez-Latapi P, Marotta N, Mencacci NE (2021) Emerging and converging molecular mechanisms in dystonia. J Neural Transm (vienna) 128(4):483–498. https://doi.org/10.1007/s00702-020-02290-z
Hamers FP, Koopmans GC, Joosten EA (2006) CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma 23(3–4):537–548. https://doi.org/10.1089/neu.2006.23.537
Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141(4):527–545. https://doi.org/10.1007/s00401-021-02268-5
Hsieh TH, Chen JJ, Chen LH, Chiang PT, Lee HY (2011) Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav Brain Res 222(1):1–9. https://doi.org/10.1016/j.bbr.2011.03.031
Hsieh TH, Kuo CW, Hsieh KH, Shieh MJ, Peng CW, Chen YC, Chen HY (2020) Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson's disease. Brain Sci 10(4). https://doi.org/10.3390/brainsci10040206
Klann EM, Dissanayake U, Gurrala A, Farrer M, Shukla AW, Ramirez-Zamora A, Vedam-Mai V (2021) The gut-brain axis and its relation to Parkinson’s disease: a review. Front Aging Neurosci 13:782082. https://doi.org/10.3389/fnagi.2021.782082
Koopmans GC, Deumens R, Brook G, Gerver J, Honig WM, Hamers FP, Joosten EA (2007) Strain and locomotor speed affect over-ground locomotion in intact rats. Physiol Behav 92(5):993–1001. https://doi.org/10.1016/j.physbeh.2007.07.018
Lee SW, Gajavelli S, Spurlock MS, Andreoni C, de RiveroVaccari JP, Bullock MR, Dietrich WD (2018) Microglial inflammasome activation in penetrating ballistic-like brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2017.5530
McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O’Hely M, Jacka FN (2022) A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01456-3
Mendieta L, Granado N, Aguilera J, Tizabi Y, Moratalla R (2016) Fragment C domain of tetanus toxin mitigates methamphetamine neurotoxicity and its motor consequences in mice. Int J Neuropsychopharmacol 19(8). https://doi.org/10.1093/ijnp/pyw021
Mitrea L, Nemeş SA, Szabo K, Teleky BE, Vodnar DC (2022) Guts imbalance imbalances the brain: a review of gut microbiota association with neurological and psychiatric disorders. Front Med (lausanne) 9:813204. https://doi.org/10.3389/fmed.2022.813204
Miyanishi K, Choudhury ME, Watanabe M, Kubo M, Nomoto M, Yano H, Tanaka J (2019) Behavioral tests predicting striatal dopamine level in a rat hemi-Parkinson’s disease model. Neurochem Int 122:38–46. https://doi.org/10.1016/j.neuint.2018.11.005
Mou Y, Du Y, Zhou L, Yue J, Hu X, Liu Y, Dong B (2022) Gut microbiota interact with the brain through systemic chronic inflammation: implications on neuroinflammation, neurodegeneration, and aging. Front Immunol 13:796288. https://doi.org/10.3389/fimmu.2022.796288
NOM-062-ZOO-1999 (1999) Norma oficial mexicana para el manejo de animales en el laboratorio. In
Nurrahma BA, Tsao SP, Wu CH, Yeh TH, Hsieh PS, Panunggal B, Huang HY (2021) Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front Aging Neurosci 13:668775. https://doi.org/10.3389/fnagi.2021.668775
Nuzum ND, Loughman A, Szymlek-Gay EA, Teo WP, Hendy AM, Macpherson H (2022) To the gut microbiome and beyond: the brain-first or body-first hypothesis in Parkinson’s disease. Front Microbiol 13:791213. https://doi.org/10.3389/fmicb.2022.791213
Parra I, Martínez I, Ramírez-García G, Tizabi Y, Mendieta L (2020) Differential effects of LPS and 6-OHDA on microglia’s morphology in rats: implications for inflammatory model of Parkinson’s disease. Neurotox Res 37(1):1–11. https://doi.org/10.1007/s12640-019-00104-z
Parra-Paz VG, Calderón-Sauri A, Granados-Patrón D, Cuevas-Carbonell SG, García-López D, Dawn-Ojeda A, Góngora-Alfaro JL (2021) Chronic feeding with 3% dried raw blueberries (V. corymbosum) reduces apomorphine-induced rotations and striatal dopaminergic loss in hemiparkinsonian rats. Food Res Int 140:110066. https://doi.org/10.1016/j.foodres.2020.110066
Paxinos G, Watson C (2007) The rat brain. Press, San Diego, California, U. S. A, In stereotaxic coordinates
Perez Visñuk D, Savoy de Giori G, LeBlanc JG, de Moreno de LeBlanc A (2020) Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition 79–80:110995. https://doi.org/10.1016/j.nut.2020.110995
Rani L, Mondal AC (2021) Unravelling the role of gut microbiota in Parkinson’s disease progression: pathogenic and therapeutic implications. Neurosci Res 168:100–112. https://doi.org/10.1016/j.neures.2021.01.001
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39(5):777–787. https://doi.org/10.1016/s0028-3908(00)00005-8
Schallert T, Tillerson JL (2000) Intervention strategies for degeneration of dopamine neurons in parkinsonism. In: EDF, Da RL, SPR (eds) Central Nervous System Diseases. Contemporary Neuroscience. Humana Press, Totowa, pp 131–151. https://doi.org/10.1007/978-1-59259-691-1_8
Shannon KM (2022) Gut-derived sterile inflammation and Parkinson’s disease. Front Neurol 13:831090. https://doi.org/10.3389/fneur.2022.831090
Smith L, Schapira AHV (2022) Variants and Parkinson disease: mechanisms and treatments. Cells 11(8). https://doi.org/10.3390/cells11081261
Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H, Choi DY (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem 69:73–86. https://doi.org/10.1016/j.jnutbio.2019.03.021
Sun J, Li H, Jin Y, Yu J, Mao S, Su KP, Liu J (2021) Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun 91:703–715. https://doi.org/10.1016/j.bbi.2020.10.014
Tan AH, Hor JW, Chong CW, Lim SY (2021) Probiotics for Parkinson’s disease: current evidence and future directions. JGH Open : an Open Access Journal of Gastroenterology and Hepatology 5(4):414–419. https://doi.org/10.1002/jgh3.12450
Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T (2001) Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 21(12):4427–4435
Tizabi Y, Getachew B, Aschner M (2021) Novel pharmacotherapies in Parkinson’s disease. Neurotox Res 39(4):1381–1390. https://doi.org/10.1007/s12640-021-00375-5
Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Mechawar N (2014) Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation 11:12. https://doi.org/10.1186/1742-2094-11-12
Wang L, Zhao Z, Zhao L, Zhao Y, Yang G, Wang C, Li S (2022) DP189 reduces α-SYN aggravation in MPTP-induced Parkinson’s disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder. J Agric Food Chem 70(4):1163–1173. https://doi.org/10.1021/acs.jafc.1c07711
Xie C, Prasad AA (2020) Probiotics treatment improves hippocampal dependent cognition in a rodent model of Parkinson's disease. Microorganisms 8(11). https://doi.org/10.3390/microorganisms8111661
Zhang P, Kong L, Huang H, Pan Y, Zhang D, Jiang J, Hu S (2022) Gut microbiota - a potential contributor in the pathogenesis of bipolar disorder. Front Neurosci 16:830748. https://doi.org/10.3389/fnins.2022.830748
Acknowledgements
The authors thankfully acknowledge IBRO’s support.
Funding
This work was supported by IBRO Early Career Awards 2020 grant, and it was partially supported by CONACYT-Mexico grant No. 256878 to JLGA, and VIEP-BUAP (2021–2022). I. Parra was supported by a scholarship from CONACYT-Mexico (1024175). L. Vásquez-Celaya was supported with a postdoctoral fellowship from PRODEP-SEP (Official document 511–6/2020–2912, ref. CCPIyV/043/20).
Author information
Authors and Affiliations
Contributions
LM, IMG, and JLGA designed the study. IP and LVC performed the experiments. IP, IMG, LVC, YT, and LM contributed to data analysis and the writing of the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
All animal procedures were carried out in accordance with the Mexican Council for Care and Use of Laboratory Animals and the Norma Oficial Mexicana NOM-062-ZOO-1999.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parra, I., Martínez, I., Vásquez-Celaya, L. et al. Neuroprotective and Immunomodulatory Effects of Probiotics in a Rat Model of Parkinson’s Disease. Neurotox Res 41, 187–200 (2023). https://doi.org/10.1007/s12640-022-00627-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00627-y