Abstract
The enteric nervous system is responsible for controlling the gastrointestinal tract (GIT) functions. Enteric neuropathies are highly correlated to the development of several intestinal disturbances. Fluoride (F) is extensively applied for dental health improvement and its ingestion can promote systemic toxicity with mild to severe GIT symptomatology and neurotoxicity. Although F harmful effects have been published, there is no information regarding noxiousness of a high acute F exposure (25 mg F/kg) on enteric neurons and levels of expression of intestinal proteins in the duodenum. Quantitative proteomics of the duodenum wall associated to morphometric and quantitative analysis of enteric neurons displayed F effects of a high acute exposure. F-induced myenteric neuroplasticity was characterized by a decrease in the density of nitrergic neurons and morphometric alterations in the general populations of neurons, nitrergic neurons, and substance P varicosities. Proteomics demonstrated F-induced alterations in levels of expression of 356 proteins correlated to striated muscle cell differentiation; generation of precursor metabolites and energy; NADH and glutathione metabolic process and purine ribonucleoside triphosphate biosynthesis. The neurochemical role of several intestinal proteins was discussed specially related to the modulation of enteric neuroplasticity. The results provide a new perspective on cell signaling pathways of gastrointestinal symptomatology promoted by acute F toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considered the most important compound for oral health improvement worldwide, fluoride (F) is largely ingested in a daily basis, especially through water and food consumption. Initially, F benefits strongly supported its addition to water supplies and dental products, but important symptomatology in the gastrointestinal tract (GIT) and nervous system has been insistently described for decades associated to its general toxic effects. Acute F exposure is mainly observed through accidental ingestion of a high F dose culminating in mild to relevant gastrointestinal symptoms, which can ultimately promote human and animal death (Whitford 1990). Doses of 0.1 to 0.8 mg F/kg from water supplies or accidental ingestion of F products can promote different levels of toxicity (Akiniwa 1997). Initially, the estimated lethal acute F dose for humans was described in values ranging from 2 to 5 mg F/kg or to even higher values of 8 mg F/kg (Whitford 1987). Finally, 5 mg F/kg was defined as the “probable toxic dose”, which represents the dose able to cause toxic effects varying from the first GIT symptoms to the death, not excluding the possibility of toxic effects of lower doses (Whitford 1990).
Due to the animal high metabolism, rats present F blood serum concentrations 10 times lower for the same F concentration ingested by humans, which means that the ingestion of a concentration of 100 ppm F results in serum concentrations for rats around to the same serum levels of humans consuming 5–10 ppm F (Smith et al. 1993). Therefore, a dose of 25 mg F/kg applied to an animal corresponds to the 2.5 mg F/kg administered to a human being. For that reason, in the present study, it was administered a dose of 25 mg F/kg, which is the equivalent of 2.5 mg F/kg, half of the “probable toxic dose” for humans (Whitford 1990). This allowed us to simulate the ingestion of a high F dose, which could promote symptomatology described in several publications after the ingestion of an acute F dose (Augenstein et al. 1991; Lidbeck et al. 1943).
F toxicity in the GIT leads to a broad range of symptoms from nausea, vomiting, diarrhea, abdominal pain, paresthesia, flatulence, constipation; to gastritis, ulcers, hemorrhage, severe epigastric pain, abdominal distension, appetite loss, and anorexia (Akiniwa 1997; Augenstein et al. 1991; Gessner et al. 1994; Shashi 2002; Vogt et al. 1982; Waldbott 1977; Waldbott and Lee 1978). This important gastrointestinal symptomatology is highly prevalent in fluorosis endemic areas (Susheela et al. 1993), especially exacerbated when associated to poor nutritional and dietary habits (Sharma et al. 2009) confirming F toxicity in high concentrations and doses on the GIT.
The Enteric Nervous System (ENS) is devoted to the control of the GIT function coordinating secretion, absorption, motility, blood flow, and the intestinal immune barrier through a large collection of nerves distributed in two major interconnected plexi in the walls of gastrointestinal organs (Bertrand 2003; Furness and Costa 1987; Gershon 1981; Gershon et al. 1994). As an organized neuronal network presenting several neurotransmitters, the ENS works independently to the CNS (Furness 2006; Gershon 1998) and presents the ability to adapt to local changes for the maintenance of its homeostasis from the mucous to the smooth muscle layers microenvironment (Schäfer et al. 2009). This capacity named neuroplasticity is a powerful tool of the ENS for preservation of the GIT function evoked in physiological or pathological states (Vasina et al. 2006). It is well defined that neuroplasticity involves broad changes affecting gene expression, cellular function, circuit formation, neuronal morphology, and behavior (Maze et al. 2013). Since the ENS is exposed to a variety of chemical and mechanical insults, as well as to foreign organisms, the question of plasticity and regeneration of enteric nerves is highly relevant (Hanani et al. 2003).
Several pathologies affecting enteric neurons are characterized by damage or loss of enteric neurons, contribute to the pathogenesis of important gastrointestinal abnormalities such as achalasia, idiopathic gastroparesis, congenital hypertrophic pyloric stenosis, chronic intestinal pseudoobstruction (CIPO), irritable bowel syndrome (IBS), Hirshsprung’s and Chagas’ disease (De Giorgio et al. 2004). In addition, gastrointestinal dysfunction associated with diabetes (Hermes-Uliana et al. 2014), or ageing process (Camilleri et al. 2008) are also connected to enteric neuronal impairment. The fact that F effects in the GIT present similar symptomatology to these significant pathologies is an important indicative of F-induced enteric neurotoxicity.
F toxicity in the Central and Peripheral Nervous System associated to the ingestion of high doses has been extensively described (Bhatnagar et al. 2006; Blaylock 2004; Mullenix et al. 1995; Shivarajashankara et al. 2002; Tsunoda et al. 2005; Vani and Reddy 2000; Varner et al. 1998). Neurotoxicity can be reported as the first sign of F harmful effects, even in the case of skeletal fluorosis development, compromising the nervous system previously to evident bone abnormalities of excessive ingestion (Mullenix et al. 1995). Other relevant effects, including manifestations of decreased intelligence quotient, cognition, memory, and learning ability (Madhusudhan et al. 2009), are described as fluoride-induced neurotoxic mechanisms in the CNS detected in specific brain areas (Niu et al. 2018), mainly guided by alteration in the expression of neurodevelopmental (Zhu et al. 2017) or synapse-associated proteins (Ge et al. 2018). All these evidences substantiate F toxicity to neuronal structures and interference in neuronal function.
As an active enzymatic inhibitor that can effectively compromise protein function (Everett 2011) and due to the fact that F affects levels of proteins in many different tissues (Araujo et al. 2019; Carvalho et al. 2013; Khan et al. 2016; Khan et al. 2019; Lobo et al. 2015; Melo et al. 2017; Pereira et al. 2013), F could also affect protein levels in the GIT that can be associated to enteric neurons and promote the significant gastrointestinal symptomatology observed in cases of an acute exposure to high doses of F. Since the ENS function is coordinated by different neurotransmitters, the evaluation of morphological and quantitative alterations in the total population and other subpopulations of enteric neurons to an acute exposure can contribute to finally illustrate the mechanistic action of F toxicity on the GIT. To uncover the outcome of an acute F exposure in proteins of the proximal small intestine, the first site of F absorption and systemic distribution after its ingestion, we undertook a large-scale quantitative proteome profiling strategy to investigate alterations in protein levels of expression of the duodenum and associated the proteomic result to findings of immunofluorescence techniques for morphometric and quantitative analysis of different enteric neuronal subpopulations. The results demonstrated F-induced myenteric neuroplasticity characterized by a decrease in the density of nitrergic neurons and morphometric alterations in the general populations of neurons, nitrergic neurons, and substance P varicosities. Proteomics demonstrated that F-induced alterations in levels of expression of 356 proteins correlated to striated muscle cell differentiation; generation of precursor metabolites and energy; NADH and glutathione metabolic process; and purine ribonucleoside triphosphate biosynthesis. Revealing F effects in the proximal segment of the small intestine can contribute to unveil the initial mechanistic action of F potentially involved in its toxicity on the GIT.
Methods
Animals, Experimental Procedures, and Analysis of Plasma F Concentrations
Ethical permission for the study was granted by the Research Ethics Committee of Bauru Dental School of the University of São Paulo (protocol 014/2011) according to the guidelines of the Brazilian National Research Council. Twelve male rats (Rattus norvegicus, Wistar type) with 60 days of age were kept housed individually in metabolic cages with water and food ad libitum at 12 h/12 h (light/dark cycle) and room temperature was controlled (around 22 °C). The animals received deionized water for 29 days; this period is used for acclimation of the animals to the metabolic cages (in which they are placed individually instead of in groups in the same cage) before starting the protocol of F exposure, promoting a pacific transition to the new individualized cage. At the 30th day, they were randomly divided into two groups (n = 6 per group): control and acute dose receiving 0 or 25 mg F/kg orally by gavage, respectively. After 2 h of the gavage procedure (experimental period of exposure), the animals had their blood harvested for confirmation of F exposure in plasma samples and the duodenum harvested for histological, immunofluorescence, and proteomic analysis.
Plasma F concentrations of the animals were analyzed to confirm the exposure of the animal to F doses. This is a standard analysis for F studies (Dionizio et al. 2018; Khan et al. 2016; Khan et al. 2019; Leite et al. 2014; Lobo et al. 2015; Melo et al. 2017; Pereira et al. 2013).
Plasm F concentrations were accessed through hexamethyldisiloxane (HMDS)-facilitated diffusion (Taves 1968). ANOVA and Tukey’s test (p<0.05%) were applied for statistical analysis using GraphPad InStat software, version 3.0 for Windows (GraphPad Software Inc., La Jolla, CA, USA).
Nano-LC Electrospray Ionization MS
A label-free proteomics strategy based on LC-MS was applied for global identification of proteins in duodenum samples with previously detailed methods described (Melo et al. 2017). Briefly, duodenum was collected, washed, frozen in liquid nitrogen, and posteriorly lyophilized in a cryogenic mill. Global proteins were extracted by sonication with lysis buffer followed by tryptic digestion. Separation of the peptides was carried out on a nanoAcquity UPLC-Xevo QTof MS system (Waters Corporation, Manchester, UK) loading a sample volume of 200 µL. Peptides in a solution of 0.1% formic acid and 3% acetonitrile were injected in the column with an 0.1% formic acid (mobile phase A) and 93% acetonitrile, 0.1% formic acid, and 100% acetonitrile (mobile phase B). A linear gradient of 7–85% of mobile phase was gradually applied for 70 min at a flow rate of 0.35 µL/min. Quantitative comparison of the proteome from different samples was achieved using bioinformatics analysis through peptide identification of raw files obtained from LC/MS based in a fasta file from Rattus norvegicus proteome provided by UniProtKB (https://www.uniprot.org/). Expression profiles among groups were obtained using the ProteinLynx Global Server (PLGS) software provided by Waters Corporation through calculation of peptide ratios and generation of a non-redundant list of proteins identified that had their ratio normalized and expressed as p < 0.05 for protein downregulation and 1 − p > 0.95 for upregulation expression levels. Gene ontology was determined with CYTOSCAPE plugin CLUEGO (Bindea et al. 2013; Bindea et al. 2009).
Enteric Neurons Immunofluorescence
Samples of the duodenum were prepared for immunofluorescence techniques as described previously (Dionizio et al. 2018; Melo et al. 2017). Briefly, after the duodenum was harvested, samples were fixed, washed in ethanol, dehydrated in ethanol, cleared with xylene, hydrated with ethanol, and stored in PBS. After microdissection of the intestinal walls, the tunica muscularis was obtained, which contains the myenteric plexus, responsible for controlling intestinal motility through neurons distributed between the longitudinal and circular smooth muscle layers. For the identification of the general population of neurons and nitrergic neurons, antibodies for the human neuronal protein (HuC/D) and the neuronal nitric oxide synthase (nNOS) were, respectively, used. For the myenteric varicosities immunoreactive (IR) to other important neurotransmitters investigated (vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), and substance P), specific antibodies anti-VIP, anti-CGRP, and anti-SP were applied. In the immunofluorescence protocol, samples were washed in PBS with Triton X-100, blocked for 1 h (PBS with 0.5% Triton X-100, 2% BSA, and 10% donkey serum). The double-labeling technique anti-HuC/D (mouse, 1:500) and anti-nNOS (rabbit, 1:500) were applied for 48 h and washed 3 times in PBS. Incubation in secondary antibodies [Alexa Fluor 488 (1:250), Alexa Fluor 568(1:500)] was conducted for 2 h and samples were washed and mounted with Prolong Gold antifade. Morphometric and quantitative analyses were performed as described in other studies from our group (Alves et al. 2010; Ferreira et al. 2013; Lopes et al. 2012; Pereira et al. 2011). Briefly, 40 images were captured for each animal and 100 neurons or 400 varicosities from the images were analyzed using the tools of Image Pro Plus 4 software (Media Cybernetics, Silver Spring, EUA). For the quantitative analysis, the density of the general population of neurons (HuC/D-IR) and nitrergic neurons (nNOS-IR) was calculated in neurons/cm2. For the morphometric analysis, measurement was performed of the average value of the areas (μm2) of the cell bodies of the general population of neurons and of the nitrergic neurons and areas of the varicosities VIP-IR, CGRP-IR, SP-IR. Antibodies used in the immunofluorescence techniques: nNOS (H-299, sc-8309 Santa Cruz, Dallas, TX, USA), Mouse anti-HuC/D (A-21271, Invitrogen, Waltham, MA, USA), Rabbit anti-CGRP (AB15360, Millipore, St Charles, MO, USA), Rabbit anti-VIP (V0390, Sigma-Aldrich, St. Louis, MO, USA), Goat anti-substance P (sc9758, Santa Cruz, Dallas, TX, USA), Anti-rabbit 546 (A10040, Invitrogen, Waltham, MA, USA), Anti-mouse 488 (A21202, Invitrogen, Waltham, MA, USA), Anti-rabbit 546 (A10040, Invitrogen, Waltham, MA, USA), and Anti-goat 568 (A11057, Invitrogen, Waltham, MAs, USA).
Histological Analysis
Duodenum samples were prepared for analysis of the thickness of the total wall and of the tunica muscularis. This is considered a relevant analysis since it can suggest the development of important processes, such as neoplastic, inflammatory, infectious, or ischemic conditions when associated to imaging techniques (Fernandes et al. 2014) being applied for diagnose (Serafini et al. 2017) allowing clinicians to identify several intestinal pathologies, such as inflammatory bowel disease, intestinal infection, ischemic colitis, and fibrosing colonopathy (Haber et al. 1997). The intention of using this analysis was seeking evidences of morphological changes caused by an acute F exposure, not directly indicating any specific pathology. Duodenum samples were washed with PBS, fixed in 10% formaldehyde, embedded in paraffin for sectioning at 5 μm of thickness. Hematoxylin/eosin (HE) staining was applied. Images were captured (10× objective) using a high-resolution camera (Moticam 2500, Motic China Group Co, Shanghai, China) coupled to a microscope (Olympus BX40, Olympus Co., Japan). The measurements of the thickness of the tunica muscularis and the total wall of the duodenum were obtained using Image-Pro Plus software (10 sections/animal distributed in 5 different regions of the duodenum (images not provided).
Results
Plasma F Concentrations Analysis
F exposure was confirmed through analysis of F concentrations in blood plasma harvested from the animals. Significant difference was found in the acute F exposure group in relation to the control group and presented in the Table 1.
Morphometric Analysis of the Duodenum Wall Thickness
After the morphometric analysis of the duodenum wall stained with HE, the total thickness presented and increase in the group exposed to F compared with the control, while in the mean (± SD) thickness of the tunica muscularis had a significant decrease in the exposed group in relation to the control group. This result shows that the acute F exposure affected the morphology of the intestinal wall, which may contribute to GIT symptoms observed due to F toxicity extensively mentioned in the literature.
Proteomics Analysis
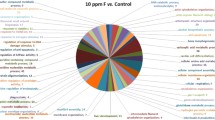
Merging the peptide identifications obtained after database searching resulted in a core set of 520 and 508 proteins in the control group and in the group exposed to an acute dose of 25 mg F/kg, respectively. In the quantitative analysis, the comparison between control vs. 25 mg F/kg, 356 proteins were considered statistically reliable hits with altered expression; most of the representative spectrum showed protein downregulation in the 25-mg F/kg group (Supplementary Table 3. Additionally, several proteins were identified exclusively in each group, 164 in the control and 152 in the 25 mg F/kg (Supplementary Table 1 and Supplementary Table 2, respectively). The distribution and interaction of several proteins identified initially are presented as a colored subnetwork obtained with CYTOSCAPE analysis (Fig. 1). From the total of identified proteins, only the proteins presenting interaction, according to CYTOSCAPE analysis, were represented in the subnetwork. Proteins with altered expression levels were distributed according to terms based on their gene ontology presenting the following categories of biological processes most affected by the acute F exposure: striated muscle cell differentiation (31%), generation of precursor metabolites and energy (27%), NADH metabolic process (21%), muscle structure development (18%), muscle system process (17%), purine ribonucleoside triphosphate biosynthetic process (15%), and glutathione metabolic process (15%) (Fig. 2). Proteins with relevant behavior for gastrointestinal activity or neuronal role had their function investigated and described in the discussion to suggest possible correlations with the findings of the quantitative and morphometric analysis of enteric neurons here performed. Relevant characteristics of proteins specially correlated to apoptosis, enzymatic activity, and energy metabolism were mentioned as well as their function in cascades and pathways in different tissues, GIT, and neurons.
Subnetworks generated by VizMapper based on the comparison of the acute F exposure group (25 mg F/kg) vs. control group. Color of node indicates the differential expression. Green, red, and gray nodes indicate upregulation, downregulation, and interaction proteins, respectively. The access number in the green node corresponds to T-complex protein 1 subunit alpha (P28480). The access numbers in gray nodes correspond to Coronin-1A (Q91ZN1), mitogen-activated protein kinase 3-MAPK3 (P21708), Synaptojanin-1 (Q62910), cystic fibrosis transmembrane conductance regulator (P13569), 14-3-3 protein zeta/delta (P63102), peptidylprolyl cis/trans isomerase (B0BNL2), Plastin-3 (Q63598), and serine/threonine-protein phosphatase 4 catalytic subunit (Q5BJ92). The access numbers in red nodes correspond to Ccdc94 protein (A2VD11), glyceraldehyde-3-phosphate dehydrogenase (B1WBQ8), 60S acidic ribosomal protein P2 (P02401), malate dehydrogenase, mitochondrial (P04636), L-lactate dehydrogenase A chain (P04642), glyceraldehyde-3-phosphate dehydrogenase (P04797), sodium/potassium-transporting ATPase subunit alpha-3 (P06687), carbamoyl-phosphate synthase [ammonia], mitochondrial (P07756), histone H2A.Z (P0C0S7), histone H2A type 1-C (P0C169), Histone H2A type 1-E (P0C170), ATP synthase subunit beta, mitochondrial (P10719), aspartate aminotransferase, cytoplasmic (P13221), phosphoglycerate kinase 1 (P16617), creatine kinase U-type, mitochondrial (P25809), cytochrome b-c1 complex subunit 2, mitochondrial (P32551), ATP synthase subunit delta, mitochondrial (P35434), adenine phosphoribosyltransferase (P36972), heat shock protein beta-1 (P42930), trifunctional enzyme subunit beta, mitochondrial (Q60587), major vault protein (Q62667), keratin, type I cytoskeletal 19 (Q63279), polyubiquitin-C (Q63429), peroxiredoxin-1 (Q63716), elongation factor 1-gamma (Q68FR6), histone H3.1 (Q6LED0), citrate synthase, mitochondrial (Q8VHF5), Na(+)/H(+) exchange regulatory cofactor NHE-RF1 (Q9JJ19)
Functional distribution of proteins identified with differential expression in the duodenum of rats exposed to an acute F dose of 25 mg F/kg vs. control group (0 mg F/kg). Categories were described based on GO Biological Process. Terms significant (kappa = 0.04) and distribution according to percentage of number of genes association. ClueGo® and Cytoscape® were used for gene ontology evaluation
Quantitative and Morphometric Assessment of Myenteric Neurons of the Duodenum
The acute F exposure did not cause alteration in the density of the general population of neurons (HuC/D-IR), but promoted a significant decrease in the density of nitrergic neurons (nNOS-IR) (Fig. 3). In the myenteric plexus of the ENS, NO promotes the relaxation of the smooth muscle during intestinal activity (Rivera et al. 2011). Damage to nitrergic neurotransmission in the GIT is correlated to several pathologies, such as diabetes (Pereira et al. 2016), which contribute to gastrointestinal motility disturbances. Additionally, a decrease in the nitrergic neuronal subpopulation suggests a significant neurochemical alteration (Bódi et al. 2019). Regarding the morphometric analysis, the acute F exposure caused an increase in the area of the neuronal cell bodies of the general population of neurons and a decrease in the area of the nitrergic neurons in the group of the acute exposure in relation to the control (Table 3). About the analysis of the varicosities immunoreactive to CGRP, VIP, and SP, morphometric changes only in the SP-IR varicosities as a significant increase in the average value of the area of the varicosities were observed (Fig. 4). In the myenteric plexus of the ENS, VIP is an inhibitory neurotransmitter of the smooth muscle contraction, which means that it promotes relaxation of gastrointestinal smooth muscle (Belai et al. 1997; Dick et al. 2000; Furness et al. 1992; Furness and Costa 1979; Murthy et al. 1996) and present acomplementary effect to NO, which is also inhibitory (Chino et al. 2002). SP is an excitatory neurotransmitter of the smooth muscle, inducing directly and indirectly the contraction of the intestinal muscle layers through the stimulation of cholinergic neurons (Holzer and Lembeck 1980). Additionally, SP is also an important vasodilator affecting the blood flow of the intestinal wall (Llewellyn-Smith et al. 1984). CGRP also presents and excitatory effect on the smooth muscle of the GIT (Ohtani; Kaneko; Kline et al. 1989) presenting neuronal projections distributed in the muscle layers (Belai and Burnstock 1987; Ekblad et al. 1987) contributing to the regulation of the intestinal motility (Holzer et al. 1989). Therefore, in our study, it was possible to evaluate F effects on two subpopulations of inhibitory and two subpopulations of excitatory enteric neurons, to better understand how F can affect the intestinal motility. These morphological alterations observed suggest that F induced enteric neuroplastic changes, which can be associated to disturbance in the GIT function. Trying to connect these results with the proteomics analysis, in the discussion section, we describe the role of many proteins that present functional correlation to neuronal and GIT physiology, such as creatine kinase U-type mitochondrial (P25809, CKMT1B), 60S acidic ribosomal protein P2 (P02401), Ccdc94 protein (A2VD11), peroxiredoxin-1 (Q63716; Prdx1), creatine kinase U-type mitochondrial (P25809, CKMT1B), glyceraldehyde-3-phosphate dehydrogenase (B1WBQ8, GAPDH, P04797), phosphoglycerate kinase 1 (P16617), carbamoyl-phosphate synthase mitochondrial (P07756, CPS1), phosphoglycerate kinase 1 (P16617; PGK1), L-lactate dehydrogenase A chain (LDHA, P04642), adenine phosphoribosyltransferase (P36972; APRT), Na+/K+ ATPase subunit alpha-3 (P06687, NKAα3, ATP1α3), citrate synthase mitochondrial (Q8VHF5), and aspartate aminotransferase cytoplasmic (P13221; AspAT). Aspartate aminotransferase cytoplasmic, for example, suggests a correlation of F effects on SP-IR varicosities represented in our findings; GAPDH is involved in development of pathologies affecting neuronal function and citrate synthase mitochondrial enhanced NO signaling and could affect nitrergic neurons. All these roles are explained in detail in the discussion.
Discussion
In this study, we applied proteomics to comprehensively screen differentially expressed proteins associated to morphological changes in enteric neurons of the duodenum after acute F exposure. The duodenal wall is the first site of intestinal metabolism presenting a significant absorptive capacity, and the acute F dose administered during the experiments is considered an elevated exposure, able to promote high systemic toxicity with similar effects of F poisoning caused by accidental ingestion of high F doses. Since a prevalent symptomatology of the GIT is observed due to F exposure in different levels (Akiniwa 1997; Augenstein et al. 1991; Gessner et al. 1994; Shashi 2002; Susheela et al. 1993; Vogt et al. 1982), we assumed that the dose applied in this study could affect intestinal homeostasis in protein levels disturbing the physiology of the GIT including the function of the ENS. Description of protein behavior in the CNS is also provided here, since high mechanistic similarity between the CNS and ENS is observed and some principles established for the CNS can be applied in the enteric context (Gershon and Ratcliffe 2004). Furthermore, ENS and CNS normally communicate bidirectionally (Rao and Gershon 2018), and some roles in the central environment could help to explain the F-induced toxicity on enteric neurons.
The proteomic profile provided associative networks displaying interactions of distinct peptides with corresponding gene ontology strongly correlated to processes such as striated muscle cell differentiation (31%), NADH metabolic process (21%), muscle structure development (18%), muscle system process (17%), purine ribonucleoside triphosphate biosynthetic process (15%), glutathione metabolic process (15%), and purine ribonucleoside triphosphate biosynthetic process (15%). Cellular alterations associated with skeletal muscle differentiation share a high degree of similarity with phenotypic changes correlated to apoptosis (Fernando et al. 2002); since skeletal muscle differentiation was the most affected biological process observed with the proteomics, we may suggest that the acute F dose could promote apoptosis of intestinal cells, but specific experiments to prove this hypothesis were not tested in the present study to confirm this argument.
Regarding to the specific proteins with altered expression observed in the proteomic approach, some presented involvement in the apoptotic process in a diversity of tissues, and adequate citations of their roles are described below. Creatine kinase U-type mitochondrial (P25809, CKMT1B), one of the transcribed copies of the creatine mitochondrial kinase 1 (CKMT1), which catalyzes the high energy phosphate transfer from mitochondrial to cytosolic creatine, a mechanism present in tissue with large oscillating energy demand (Zhang et al. 2007) enzymes of mitochondrial membrane pore (Beutner et al. 1996), it disturbs the process of pore opening affecting apoptosis induction through cytochrome c release (Vyssokikh and Brdiczka 2003). On this way, the decrease in CKMT1B expression due to the ingestion of the acute F dose could facilitate apoptosis by a disturbance in cell structure. 60S acidic ribosomal protein P2 (P02401), other protein identified among our findings, corresponds to one of the three ubiquitous P ribosomal acidic phosphoproteins (P0, P1, P2) composing the 60S ribosomal subunit and contributing to protein synthesis (Briani et al. 2009), whose downregulation can compromise total cell protein synthesis, disturbing many cellular functions (Zandi et al. 2009) and causing cell death (Remacha et al. 1995). The high specificity of P protein antigen is observed in neurons of different rat brain areas and associated to apoptosis induction (Matus; Burgos et al. 2007). About the role of this protein in the intestinal environment, P2 down-regulation has been described as a subject to be investigated in the carcinogenesis of colorectal as well as other cancers (Cao et al. 1997). Other cancer- and apoptosis-related protein here identified, Ccdc94 protein (A2VD11), has been described as a radioprotective gene, whose inactivation makes cells more sensitive to radiation-induced cell death, contributing to the Prp19 complex action, known for regulating gene expression and repair of damaged DNA (Sorrells et al. 2012). Upon the Prp19 role, this complex represses radiation-induced cell death by inhibition of the p53 gene, a critical mediator of DNA damage-induced cell death. It is possible that the downregulation of this protein in the group that received the acute F dose could contribute to the mechanisms that induce to cell death in the intestine. Peroxiredoxin-1 (Q63716; Prdx1), other protein involved with apoptosis, was observed downregulated in our findings. Belonging to a family of antioxidant enzymes working in a defense mechanism against oxidative stress in the brain, peroxiredoxin inhibition leads to free peroxide accumulation and oxidation of key proteins that induce activation of the apoptosis signaling (Jin et al. 2005). In this cascade, Prdx1 functions as a peroxide receptor in response to extracellular H2O2 inhibiting apoptosis by transducing the peroxide signal (Jarvis et al. 2012), confirming Prdx1 protective role by controlling oxidative stress (Abbas et al. 2008). About its expression in the GIT, on the same way, Prxd1 has an significant function in gastric mucosal protection against oxidative injury induced by H. pylori infection (Sato; Yanaka et al. 2008). Therefore, down regulation of Prdx1 due to the acute F dose could impact the defense mechanism against oxidative stress contributing to processes such as lipid peroxidation and apoptosis in the duodenum. Altogether, these proteins that regulate apoptosis could have some involvement with the decrease in the nitrergic neuronal density observed in our study promoting important symptomatology, since nitrergic neurons inhibit the activity of the intestinal smooth muscle layers. To confirm these effects, further experiments would be necessary.
Conjointly with basic mechanistic cellular processes, other alterations due to acute F exposure were observed in molecules that dictate energy metabolism justifying NADH metabolic process description in the biological processes mostly associated with the proteomic analysis in the present study. Mitochondrial malate dehydrogenase (P04636) is a polypeptide that contributes to the tricarboxylic acid (TCA) cycle and associated to the cytosolic malate dehydrogenase transfers NADH into the mitochondria to access the electron transport chain for ATP production. In the small intestine of rats, the steady state of mitochondrial malate dehydrogenase mRNA levels is high due to a large energy requirement (Kelly 1989). Other enzyme involved in the TCA cycle, citrate synthase mitochondrial (Q8VHF5), also had reduced expression in our study. This catalytic enzyme of the first cycle reaction participates in the respiratory metabolism linked to ATP output and indicates unaffected mitochondria (Stetler et al. 2012), classified as a marker of increase in mitochondrial content and increased availability of an energy substrate (Hughes et al. 2014). In neurons, enhanced NO signaling increased citrate synthase activity and was correlated with enhanced reserve respiratory capacity and survival in neurons (Cerqueira 2012). The fact that the nitrergic neurons morphologically evaluated in the present study presented a decrease in the average value of the neuronal area and a decrease in their density in the duodenum could be correlated to a decrease in the concentration of NO signalized by citrate synthase decreased expression and activity. Additionally, pathophysiology of GI abnormalities has been associated with mitochondrial (Rose et al. 2017) and enteric neuronal function (Lomax 2006) confirming the possibility of association between citrate synthase reduction and impairment in enteric neuronal activity through an alteration in mitochondrial activity. Citrate synthase can also be inhibited by nucleotides and polycarboxylate compounds that are products of the cellular metabolic pathway, in which citrate synthase is responsible for the first reaction (Srere 1974). Therefore, its downregulation could be also correlated with changes in the concentration of ATP, NADH, and especially acyl CoA and oxaloacetate affecting energy metabolism described as highly affected in our findings.
Energy production in the duodenum can also be affected by modified expression of glyceraldehyde-3-phosphate dehydrogenase (B1WBQ8, GAPDH, P04797), described as an abundant cell protein with a central role in energy production, GAPDH functions as a glycolysis catalytic enzyme and frequently is used as a reliable house-keeping gene, characterized as an invariant control in studies of gene expression analysis (Sirover 1999). Besides the glycolytic function, GAPDH is involved in membrane fusion, microtubule bundling, nuclear RNA transport, regulation of Ca2+ homeostasis, transcription, apoptosis (Nakajima et al. 2007), translational control of gene expression, DNA replication, and DNA repair (Sirover, 1999). Especially its role in apoptosis has been evaluated in age-related human neuronal disorders as Alzheimer’s (Schulze et al. 1993) and huntington’s diseases (Burke et al. 1996), in which GAPDH interacts with other proteins directly related to the development of these pathologies affecting neuronal function. The oxidative stress-induced neuronal cell death is also another mechanism mediated by GAPDH, which is correlated to neurodegenerative and neuropsychiatric disorders (Itakura et al. 2015). In glioma cells, suppression of GAPDH mRNA induces apoptosis (Appelskog et al. 2004), indicating the neurotoxic potential of alteration in GAPDH expression. Its downregulation could reflect a mechanism involving enteric neurons and the induced neuronal death detected as well as an energy synthesis disturbance produced by F toxicity.
Other peptide involved in the glycolytic pathway, phosphoglycerate kinase 1 (P16617; PGK1), catalyzes 1,3-diphosphoglycerate to 3-phosphoglycerate conversion to the first molecule of ATP (Boyd; Tu; Shorrock; Groen et al. 2017). PGK has increased expression in different malignant tumors including colon cancer (Ahmad et al. 2013) and gastric cancer (Zieker et al. 2008; Zieker et al. 2010), in which it is associated with poor prognostic (Ding et al. 2014). Its mutations promote CNS defects in humans (Flanagan 2006) and PGK1 deficiency is correlated with the development of early-onset parkinsonism (Sakaue et al. 2017). High PGK1 expression is found in the cytoplasm of ‘disease-resistant’ motor neurons and axon terminals, suggesting that PGK1 could present a significant role in neuronal energy pathways (Boyd et al. 2017). Likewise, enhanced expression of PGK1 has been associated with neuroprotective effect through energy metabolism and glycolysis pathway activation (Han 2010). Reduction in ATP intracellular levels causes neuronal cell death, culminating with the development of significant neurodegenerative pathologies as Alzheimer’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease. On the same way, the decreased PGK1 expression observed due to the ingestion of the acute F dose could compromise the ENS in a similar way to the CNS, disturbing enteric neuronal function or even promoting neuronal cell death by decrease in ATP generation, an event that could explain the decrease in the nitrergic neuronal density here mentioned.
Downregulation of other catalytic enzymes was observed, such as L-lactate dehydrogenase A chain (LDHA, P04642), a catalytic cytosolic enzyme that contributes to anaerobic and aerobic glycolysis (the Warburg effect). NAD+ is normally produced through oxidative phosphorylation, but when the oxygen is limited, NAD+ is regenerated from NADH in a reaction catalyzed by LDHA with the objective of maintaining glycolysis process. This process is classified as anaerobic glycolysis, and although it is not efficient as the aerobic pathway, it can provide energy in a faster way in conditions of low levels or oxygen, but with a high demand of glucose (Valvona; Fillmore; Nunn; Pilkington 2016). As the acute F dose caused a decrease in the expression of LDHA, it is possible that in the duodenum cells, it could have predominantly to use the oxidative phosphorylation to produce ATP during the exposure, a process that increases the generation of mitochondrial ROS, conducting to increase in the oxidative stress, mechanism described for other authors as a consequence F accumulation in many tissues (Chouhan and Flora 2008; Ranjan et al. 2009). Other possible effects of LDHA downregulation in the intestine caused by an increase in the oxidative phosphorylation activity can be a reduction of ATP production rate, especially in hypoxia condition (Miao et al. 2013) and an increase in the cell dependency on oxygen for the glycolysis process (Valvona 2016).
Still discussing the group of proteins related to energy metabolism here identified, other downregulated can be mentioned, such as ATP synthase subunit beta mitochondrial (P10719), ATP synthase subunit delta mitochondrial (P35434), phosphoglycerate kinase 1 (P16617; PGK1), creatine kinase U-type mitochondrial (P25809; CKMT1B), cytochrome b-c1 complex subunit 2 mitochondrial (P32551; UQCRC2), aspartate aminotransferase cytoplasmic (P13221; AspAT), cytochrome b-c1 complex subunit 2 mitochondrial (P32551; UQCRC2), and Na+/K+ ATPase subunit alpha-3 (P06687, NKAα3, ATP1α3). The last one, for example, has its expression distributed among several neuronal populations exclusively in neurons, especially in neuronal projections mostly correlated to movement control (Bøttger et al. 2011). Its differential expression affects the pattern of neuronal excitability (Edwards et al. 2013) with mutations in motor neurons implicated in movement disorders such as Rapid-Onset Dystonia-Parkinsonism (Edwards et al. 2013).
Carbamoyl-phosphate synthase mitochondrial (P07756, CPS1), recognized as an important intestinal protein, had also decreased expression. Identified in intestinal enterocytes (Ryall 1986), it has a role in the conversion of ammonia into citrulline for urea synthesis and the local production of arginine and consequently formation of NO (Van Beers et al. 1998). Produced especially in the proximal part of the small intestine, citrulline is correlated with the integrity of the intestinal epithelium specifically related to enterocyte function (Crenn; Messing; Cynober 2008) and the gravidity of intestinal pathologies (Moinard and Cynober 2007). Therefore, the decrease in the CPS1 expression observed after the acute F exposure can compromise epithelium mechanisms in the duodenum, especially involving enterocytes, disturbing the function in the proximal segment of the small intestine (Chouhan and Flora 2008; 2010; Das et al. 1994; Rocha et al. 2013; Rocha et al. 2012). Another mechanism that can be affected by the downregulation of CPS1 is NO production, which could reflect the alterations observed in nitrergic neuron morphology and density that could reflect in the intestinal motility and contribute to important symptoms as diarrhea, frequently described due to F toxicity.
According to previous results of our group (Melo et al. 2017), F exposure can affect other mechanisms that contribute to protein synthesis, such as histones expression. In the CNS, the existence of different variants indicates the relevance of these proteins and also suggests a cell type-specific distribution (Maze et al. 2014). In the present study, three variants presented downregulation: histone H2A.Z (P0C0S7), histone H2A type 1-C (P0C169), histone H2A type 1-E (P0C170), and histone H3.1 (Q6LED0). Alterations in histone expression can reflect changes in epigenetic mechanisms that can be correlated with neuronal disorders (Kazakevych et al. 2017) and could affect enteric neuronal function as well.
The impact of F absorption in the duodenum can be further extended to other sites through the impairment of global proteins such as adenine phosphoribosyltransferase (P36972; APRT). Distributing in all mice tissues, APRT catalyzes the formation of 5′-adenosine monophosphate and inorganic pyrophosphate from adenine and 5-phosphoribosyl-1-pyrophosphate (Bollée et al. 2012). When this process is compromised by the lack of APRT, adenine is oxidized by xanthine dehydrogenase, and then converted by 8-hydroxyadenine, which is metabolized to DHA (Engle et al. 1996). In the lack of functional APRT, DHA high urinary levels are observed, and due to its insolubility in urine, it is converted in crystals that can form stones leading to the crystalline nephropathy (Bollée et al. 2012) and renal failure (Engle et al. 1996). Lower expression of APRT increases the formation of DHA and can disturb kidney function. In mice, this protein is ubiquitously expressed, but in humans, it is found in the liver and intestinal mucosa (Engle et al. 1996). Therefore, in humans, F effects of an ingested acute dose could increase the production of DHA from the intestine and correlate kidney failure with F intestinal toxicity, as cited in other studies of our group (Carvalho et al. 2013; Kobayashi et al. 2009).
In retrospect of our findings, acute F effects on the duodenum encompass the possibility of the impairment of distinct functions in the cellular machinery of different tissues, affecting from basic enzymatic activity to energy metabolism. Regarding to the ENS, although marked differences distinguish ENS from CNS, analogy between both neuronal sets can be adopted due to their similarities, allowing the findings of the CNS mechanisms to be adjusted to the ENS environment (Gershon and Ratcliffe 2004), confirming the vulnerability of enteric neurons to the effects of an acute F exposure in a protein expression level.
Considering that the expression of several proteins involved in the energy production, defense response, oxidative stress, and metabolic processes were altered, it is clear that high acute F dose can breakdown intestinal homeostasis compromising also several other cell types present in the intestinal wall and promoting a general effect affecting its thickness as mentioned in the results (Table 2). The combination of these intricate cellular mechanisms affected by the differential peptide expression can impair enteric neurons behavior and explain the symptoms described after acute F exposure such as spastic bowels, severe epigastric pain, abdominal pain, diarrhea, and abdominal distension (Akiniwa 1997; Shashi 2002; Waldbott and Lee 1978; Whitford and Pashley 1984). Neuroplastic alterations are indicated not only by the morphological changes but also by the fact that precursors and compounds of important neurotransmitters and neuronal molecules presented altered expression after the ingestion of the acute F dose. For example, the aspartate aminotransferase, the enzyme responsible for glutamate production that was downregulated in the group that received the acute F dose, indicates potential effects of F on enteric glutamatergic neurons. All glutamatergic neurons co-express substance P (Liu et al. 1997) and the fact that the SP-IR varicosities were morphologically altered by the acute F dose can suggest a correlation with F effects on glutamatergic neurons that consequently reflected on SP-IR varicosities represented in our findings. SP is characterized as an excitatory neurotransmitter to intestinal motility, indicating that the acute F exposure could produce an increase in intestinal motility. It is also suggested that glutamatergic neurons are probably sensory neurons transferring sensory information from the mucosa to the enteric plexuses (Kirchgessner et al. 1992). Therefore, since morphological alterations can reflect a compromised function probably the enteric sensory neurotransmission through the submucous and myenteric plexi can be also disturbed.
Given that the ingestion of an acute high F dose can affect the physiology of the duodenum especially compromising through myenteric neurons and global protein levels, a parsimonious mechanistic explanation for the GIT symptomatology described for decades in consequence of F toxicity. We cannot rule out that other biological processes cannot be affected by F. However, we can assert the correlation of this acute F exposure to changes in specific intestinal structures and molecules compelling evidence for the local and systemic toxic effects of the ingestion of an acute F dose, mainly distributed from the proximal small intestine.
References
Abbas K, Breton J, Drapier JC (2008) The interplay between nitric oxide and peroxiredoxins. Immunobiology 213(9–10):815–822
Ahmad SS, Glatzle J, Bajaeifer K, Bühler S et al (2013) Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer. Int J Oncol 43(2):586–590
Akiniwa K (1997) Re-examination of acute toxicity of fluoride. Fluoride 30(2):89–104
Alves EP, Alves AM, Pereira RV (Feb ) De Miranda Neto MH et al Immunohistochemical study of vasoactive intestinal peptide (VIP) enteric neurons in diabetic rats supplemented with L-glutamine. Nutr Neurosci 13(1):43–51
Appelskog IB, Ammerpohl O, Svechnikova IG, Lui WO et al (2004) Histone deacetylase inhibitor 4-phenylbutyrate suppresses GAPDH mRNA expression in glioma cells. Int J Oncol 24(6):1419–1425
Araujo TT (2019) Barbosa Silva Pereira HA, Dionizio A, Sanchez CDC et al Changes in energy metabolism induced by fluoride: Insights from inside the mitochondria. Chemosphere 236:124357
Augenstein WL, Spoerke DG, Kulig KW, Hall AH et al (1991) Fluoride ingestion in children: A review of 87 cases. Pediatrics 88(5):907–912
Belai A, Boulos PB, Robson T, Burnstock G (1997) Neurochemical coding in the small intestine of patients with Crohn’s disease. Gut 40(6):767–774
Belai A, Burnstock G (1987) Selective damage of intrinsic calcitonin gene-related peptide-like immunoreactive enteric nerve fibers in streptozotocin-induced diabetic rats. Gastroenterology 92(3):730–734
Bertrand PP (2003) ATP and sensory transduction in the enteric nervous system. Neuroscientist 9(4):243–260
Beutner G, Ruck A, Riede B, Welte W et al (1996) Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett 396(2–3):189–195
Bhatnagar M, Rao P, Saxena A, Bhatnagar R et al (2006) Biochemical changes in brain and other tissues of young adult female mice from fluoride in their drinking water. Fluoride 39(4):280–284
Bindea G, Galon J, Mlecnik B (2013) CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 29(5):661–663
Bindea G, Mlecnik B, Hackl H, Charoentong P et al (2009) ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25(8):1091–1093
Blayblock RL (2004) Excitotoxicity: A possible central mechanism in fluoride neurotoxicity. Fluoride 37:301–314
Bollée G, Harambat J, Bensman A, Knebelmann B et al (2012) A denine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol 7(9):1521–1527
Boyd PJ, Tu WY, Shorrock HK, Groen EJN (2017) et al Bioenergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy. PLoS Genet 13(4):e1006744
Briani C, Lucchetta M, Ghirardello A, Toffanin E et al (2009) Neurolupus is associated with anti-ribosomal P protein antibodies: An inception cohort study. J Autoimmun 32(2):79–84
Burke JR, Enghild JJ, Martin ME, Jou YS et al (1996) Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nat Med 2(3):347–350
Bódi N, Szalai Z, Bagyánszki M (2019) Nitrergic enteric neurons in health and disease-focus on animal models. Int J Mol Sci 20(8)
Bøttger P, Tracz Z, Heuck A, Nissen P et al (2011) Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol 519(2):376–404
Camilleri M, Cowen T, Koch TR (2008) Enteric neurodegeneration in ageing. Neurogastroenterol Motil 20(4):418–429
Cao J, Cai X, Zheng L, Geng L et al (1997) Characterization of colorectal-cancer-related cDNA clones obtained by subtractive hybridization screening. J Cancer Res Clin Oncol 123(8):447–451
Carvalho JG, Leite AEL, Peres-Buzalaf C, Salvato F et al (2013) Renal proteome in mice with different susceptibilities to fluorosis. PLoS One 8(1):e53261
Cerqueira FM, Cunha FM, Laurindo FR, Kowaltowski AJ (2012) Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO•-mediated mechanism: Impact on neuronal survival. Free Radic Biol Med 52(7):1236–1241
Chino Y, Fujimura M, Kitahama K, Fujimiya M (2002) Colocalization of NO and VIP in neurons of the submucous plexus in the rat intestine. Peptides 23(12):2245–2250
Chouhan S, Flora SJ (2008) Effects of fluoride on the tissue oxidative stress and apoptosis in rats: Biochemical assays supported by IR spectroscopy data. Toxicology 254(1–2):61–67
Chouhan S, Flora SJ (2010) Arsenic and fluoride: Two major ground water pollutants. Indian J Exp Biol 48(7):666–678
Crenn P, Messing B, Cynober L (2008) Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 27(3):328–339
Das TK, Susheela AK, Gupta IP, Dasarathy S et al (1994) Toxic effects of chronic fluoride ingestion on the upper gastrointestinal tract. J Clin Gastroenterol 18(3):194–199
de Giorgio R, Guerrini S, Barbara G, Cremon C et al (2004) New insights into human enteric neuropathies. Neurogastroenterol Motil 16(Suppl 1):143–147
Dick JM, van Geldre LA, Timmermans JP, Lefebvre RA (2000) Investigation of the interaction between nitric oxide and vasoactive intestinal polypeptide in the guinea-pig gastric fundus. Br J Pharmacol 129(4):751–763
Ding H, Cheng YJ, Yan H, Zhang R et al (2014) Phosphoglycerate kinase 1 promotes radioresistance in U251 human glioma cells. Oncol Rep 31(2):894–900
Dionizio AS, Melo CGS, Sabino-Arias IT, Ventura TMS et al (2018) Chronic treatment with fluoride affects the jejunum: Insights from proteomics and enteric innervation analysis. Sci Rep 8(1):3180
Edwards IJ, Bruce G, Lawrenson C, Howe L et al (2013) Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons. J Neurosci 33(24):9913–9919
Ekblad E, Winther C, Ekman R, Håkanson R et al (1987) Projections of peptide-containing neurons in rat small intestine. Neuroscience 20(1):169–188
Engle SJ, Stockelman MG, Chen J, Boivin G et al (1996) Adenine phosphoribosyltransferase-deficient mice develop 2,8-dihydroxyadenine nephrolithiasis. Proc Natl Acad Sci U S A 93(11):5307–5312
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90(5):552–560
Fernandes T, Oliveira MI, Castro R, Araújo B et al (2014) Bowel wall thickening at CT: Simplifying the diagnosis. Insights Imaging 5(2):195–208
Fernando P, Kelly JF, Balazsi K, Slack RS et al (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A 99(17):11025–11030
Ferreira PE, Lopes CR, Alves AM, Alves É et al (2013) Diabetic neuropathy: An evaluation of the use of quercetin in the cecum of rats. World J Gastroenterol 19(38):6416–6426
Flanagan JM, Rhodes M, Wilson M, Beutler E (2006) The identification of a recurrent phosphoglycerate kinase mutation associated with chronic haemolytic anaemia and neurological dysfunction in a family from USA. Br J Haematol 134(2):233–237
Furness JB (2006) The Enteric Nervous System. Oxford: Blackwell Publishing.
Furness JB, Bornstein JC, Murphy R, Pompolo S (1992) Roles of peptides in transmission in the enteric nervous system. Trends Neurosci 15(2):66–71
Furness JB, Costa M (1979) Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett 15(2–3):199–204
Furness JB, Costa M (1987) The enteric nervous system. Churchill Livingstone, Edinburgh
Ge Y, Chen L, Yin Z, Song X et al (2018) Fluoride-induced alterations of synapse-related proteins in the cerebral cortex of ICR offspring mouse brain. Chemosphere 201:874–883
Gershon MD (1981) The enteric nervous system. Annu Rev Neurosci 4:227–272
Gershon MD (1998) The Second Brain. Harper Collins, New York
Gershon MD, Kirchgessner AL (1994) Wade PR Functional anatomy of the enteric nervous system. In: Johnson LR (ed) Physiology of the Gastrointestinal Tract, 3rd edn. Raven Press, New York, NY, pp 381–422
Gershon MD, Ratcliffe EM (2004) Developmental biology of the enteric nervous system: Pathogenesis of Hirschsprung’s disease and other congenital dysmotilities. Semin Pediatr Surg 13(4):224–235
Gessner BD, Beller M, Middaugh JP, Whitford GM (1994) Acute fluoride poisoning from a public water system. N Engl J Med 330(2):95–99
Haber HP, Benda N, Fitzke G, Lang A et al (1997) Colonic wall thickness measured by ultrasound: Striking differences in patients with cystic fibrosis versus healthy controls. Gut 40(3):406–411
Han J, Miyamae Y, Shigemori H, Isoda H (2010) Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 169(3):1039–1045
Hanani M, Ledder O, Yutkin V, Abu-Dalu R et al (2003) Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol 462(3):315–327
Hermes-Uliana C, Panizzon CP, Trevizan AR, Sehaber CC et al (2014) Is L-glutathione more effective than L-glutamine in preventing enteric diabetic neuropathy? Dig Dis Sci 59(5):937–948
Holzer P, Barthó L, Matusák O, Bauer V (1989) Calcitonin gene-related peptide action on intestinal circular muscle. Am J Physiol 256(3 Pt 1):G546-552
Holzer P, Lembeck F (1980) Neurally mediated contraction of ileal longitudinal muscle by substance P. Neurosci Lett 17(1–2):101–105
Hughes SD, Kanabus M, Anderson G, Hargreaves IP et al (2014) The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem 129(3):426–433
Itakura M, Nakajima H, Semi Y, Higashida S et al (2015) Glyceraldehyde-3-phosphate dehydrogenase aggregation inhibitor peptide: A potential therapeutic strategy against oxidative stress-induced cell death. Biochem Biophys Res Commun 467(2):373–376
Jarvis RM, Hughes SM, Ledgerwood EC (2012) Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic Biol Med 53(7):1522–1530
Jin MH, Lee YH, Kim JM, Sun HN et al (2005) Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett 381(3):252–257
Kazakevych J, Sayols S, Messner B, Krienke C et al (2017) Dynamic changes in chromatin states during specification and differentiation of adult intestinal stem cells. Nucleic Acids Res 45(10):5770–5784
Kelly DP, Gordon JI, Alpers R, Strauss AW (1989) The tissue-specific expression and developmental regulation of two nuclear genes encoding rat mitochondrial proteins. Medium chain acyl-CoA dehydrogenase and mitochondrial malate dehydrogenase. J Biol Chem 264(32):18921–18925
Khan ZN, Leite AL Charone S, Sabino IT et al (2016) Liver proteome of mice with different genetic susceptibilities to the effects of fluoride. J Appl Oral Sci 24(3):250–257
Khan ZN, Sabino IT, De Souza Melo CG, Martini T et al (2019) Liver Proteome of Mice with Distinct Genetic Susceptibilities to Fluorosis Treated with Different Concentrations of F in the Drinking Water. Biol Trace Elem Res 187(1):107–119
Kirchgessner AL, Tamir H, Gershon MD (1992) Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: Activity-induced expression of Fos immunoreactivity. J Neurosci 12(1):235–248
Kobayashi CA, Leite AL, Silva TL, Santos LD et al (2009) Proteomic analysis of kidney in rats chronically exposed to fluoride. Chem Biol Interact 180(2):305–311
Leite AL, Lobo Gualiume Vaz Madureira, J, Barbosa Da Silva Pereira HA, Silva Fernandes M, et al (2014) Proteomic analysis of gastrocnemius muscle in rats with streptozotocin-induced diabetes and chronically exposed to fluoride. PLoS One 9(9):e106646
Lidbeck WL, Hill IB, Beeman JA (1943) Acute sodium fluoride poisoning. JAMA 121(11):826–827
Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL (1997) Glutamatergic enteric neurons. J Neurosci 17(12):4764–4784
Llewellyn-Smith IJ, Furness JB, Murphy R, O'Brien PE et al (1984) Substance P-containing nerves in the human small intestine. Distribution, ultrastructure, and characterization of the immunoreactive peptide. Gastroenterology 86(3):421–435
Lobo JG, Leite AL, Pereira HA, Fernandes MS et al (2015) Low-level fluoride exposure increases insulin sensitivity in experimental diabetes. J Dent Res 94(7):990–997
Lomax AE, Linden DR, Mawe GM, Sharkey KA (2006) Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127:250–257
Lopes CR, Ferreira PE, Zanoni JN, Alves AM et al (2012) Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Dig Dis Sci 57(12):3106–3115
Madhusudhan N, Basha PM, Begum S, Ahmed F (2009) Fluoride induced neuronal oxidative stress and its amelioration by antioxidants in developing rats. Fluoride 42(3):179–187
Matus S, Burgos PV, Bravo-Zehnder M, Kraft R et al (2007) Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med 204(13):3221–3234
Maze I, Noh KM, Allis CD (2013) Histone regulation in the CNS: Basic principles of epigenetic plasticity. Neuropsychopharmacology 38(1):3–22
Maze I, Noh KM, Soshnev AA, Allis CD (2014) Every amino acid matters: Essential contributions of histone variants to mammalian development and disease. Nat Rev Genet 15(4):259–271
Melo CGS, Perles JVCM, Zanoni JN, Souza SRG et al (2017) Enteric innervation combined with proteomics for the evaluation of the effects of chronic fluoride exposure on the duodenum of rats. Sci Rep 7(1):1070
Miao P, Sheng S, Sun X, Liu J et al (2013) Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy. IUBMB Life 65(11):904–910
Moinard C, Cynober L (2007) Citrulline: A new player in the control of nitrogen homeostasis. J Nutr 137(6 Suppl 2):1621S-1625S
Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ (1995) Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol 17(2):169–177
Murthy KS, Grider JR, Jin JG, Makhlouf GM (1996) Interplay of VIP and nitric oxide in the regulation of neuromuscular function in the gut. Ann N Y Acad Sci 805:355–362; discussion 362–353
Nakajima H, Amano W, Fujita A, Fukuhara A et al (2007) The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem 282(36):26562–26574
Niu Q, Chen J, Xia T, Li P et al (2018) Excessive ER stress and the resulting autophagic flux dysfunction contribute to fluoride-induced neurotoxicity. Environ Pollut 233:889–899
Ohtani R, Kaneko T, Kline LW, Labedz T et al (1989) Localization of calcitonin gene-related peptide in the small intestine of various vertebrate species. Cell Tissue Res 258(1):35–42
Pereira HA, Leite AEL, Charone S, Lobo JG et al (2013) Proteomic analysis of liver in rats chronically exposed to fluoride. PLoS One 8(9):e75343
Pereira RV, Linden DR, Miranda-Neto MH, Zanoni JN (2016) Differential effects in CGRPergic, nitrergic, and VIPergic myenteric innervation in diabetic rats supplemented with 2% L-glutamine. An Acad Bras Cienc 88(Suppl 1):609–622
Pereira RV, Tronchini EA, Tashima CM, Alves EP et al (2011) L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci 56(12):3507–3516
Ranjan R, Swarup D, Patra R (2009) Oxidative stress indices in erythrocytes, liver, and kidneys of fluoride-exposed rabbits. Fluoride 42(2):88–93
Rao M, Gershon MD (2018) Enteric nervous system development: What could possibly go wrong? Nat Rev Neurosci 19(9):552–565
Remacha M, Jimenez-Diaz A, Santos C, Briones E et al (1995) Proteins P1, P2, and P0, components of the eukaryotic ribosome stalk. New structural and functional aspects. Biochem Cell Biol 73(11–12):959–968
Rivera LR, Poole DP, Thacker M, Furness JB (2011) The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 23(11):980–988
Rocha RA, Devesa V, Vélez D (2013) In vitro study of intestinal transport of fluoride using the Caco-2 cell line. Food Chem Toxicol 55:156–163
Rocha RA, Vélez D, Devesa V (2012) In vitro evaluation of intestinal fluoride absorption using different cell models. Toxicol Lett 210(3):311–317
Rose S, Bennuri SC, Murray KF, Buie T et al (2017) Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: A blinded case-control study. PLoS One 12(10):e0186377
Ryall JC, Quantz MA, Shore GC (1986) Rat liver and intestinal mucosa differ in the developmental pattern and hormonal regulation of carbamoyl-phosphate synthetase I and ornithine carbamoyl transferase gene expression. Eur J Biochem 156(3):453–458
Sakaue S, Kasai T, Mizuta I, Suematsu M et al (2017) Early-onset parkinsonism in a pedigree with phosphoglycerate kinase deficiency and a heterozygous carrier: Do PGK-1 mutations contribute to vulnerability to parkinsonism? NPJ Parkinsons Dis 3:13
Sato D, Yanaka A, Shibahara T, Matsui H et al (2008) Peroxiredoxin I protects gastric mucosa from oxidative injury induced by H. pylori infection. J Gastroenterol Hepatol 23(4):652–659
Schulze H, Schuler A, Stüber D, Döbeli H et al (1993) Rat brain glyceraldehyde-3-phosphate dehydrogenase interacts with the recombinant cytoplasmic domain of Alzheimer’s beta-amyloid precursor protein. J Neurochem 60(5):1915–1922
Schäfer KH, van Ginneken C, Copray S (2009) Plasticity and neural stem cells in the enteric nervous system. Anat Rec (Hoboken) 292(12):1940–1952
Serafini S, Santos MM, Aoun Tannuri AC, Zerbini MCN et al (2017) Is hematoxylin-eosin staining in rectal mucosal and submucosal biopsies still useful for the diagnosis of Hirschsprung disease? Diagn Pathol 12(1):84
Sharma JD, Jain P & Sohu D (2009) Gastric discomforts from fluoride in drinking water in Sanganer Tehsil, Rajasthan, India. Fluoride 42(4):286
Shashi A (2002) Histopathological effects of sodium fluoride on the duodenum of rabbits. Fluoride 35(1):28–37
Shivarajashankara YM, Shivashankara AR, Bhat GP, Rao SM et al (2002) Histological changes in rat brain of young fluoride intoxicated rats. Fluoride 35:12–21
Sirover MA (1999) New insights into an old protein: The functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta 1432(2):159–184
Smith CE, Nanci A, Denbesten PK (1993) Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec 237:243–258
Sorrells S, Carbonneau S, Harrington E, Chen AT et al (2012) Ccdc94 protects cells from ionizing radiation by inhibiting the expression of p53. PLoS Genet 8(8):e1002922
Srere PA (1974) Controls of citrate synthase activity. Life Sci 15(10):1695–1710
Stetler RA, Leak RK, Yin W, Zhang L et al (2012) Mitochondrial biogenesis contributes to ischemic neuroprotection afforded by LPS pre-conditioning. J Neurochem 123(Suppl 2):125–137
Susheela AK, Kumar A, Bhatnagar M, Bahadur R (1993) Prevalence of endemic Fluorosis with gastrointestinal manifestations in People living in some North-Indian villages. Fluoride 26(2):97–104
Taves DR (1968) Separation of fluoride by rapid diffusion using hexamethyldisiloxane. Talanta 15(9):969–974
Tsunoda M, Aizawa Y, Nakana K, Liu Y et al (2005) Changes in fluoride levels in the liver, kidney and brain and in neurotransmitters of mice after subacute administration of fluoride. Fluoride 38:284–292
Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ (2016) The regulation and function of lactate dehydrogenase A: Therapeutic potential in brain tumor. Brain Pathol 26(1):3–17
Van Beers EH, Rings EH, Posthuma G, Dingemanse MA et al (1998) Intestinal carbamoyl phosphate synthase I in human and rat. Expression during development shows species differences and mosaic expression in duodenum of both species. J Histochem Cytochem 46(2):231–240
Vani LM, Reddy PK (2000) Effect of fluoride accumulation on some enzymes of brain and gastrocnemius muscles of mice. Fluoride 33:17–26
Varner JA, Jensen KF, Horvath W, Isaacson RL (1998) Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: Alterations in neuronal and cerebrovascular integrity. Brain Res 784(1–2):284–298
Vasina V, Barbara G, Talamonti L, Stanghellini V et al (2006) Enteric neuroplasticity evoked by inflammation. Auton Neurosci 126–127:264–272
Vogt RL, Witherell L, Larue D, Klaucke DN (1982) Acute fluoride poisoning associated with an on-site fluoridator in a Vermont elementary school. Am J Public Health 72(10):1168–1169
Vyssokikh MY, Brdiczka D (2003) The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol 50(2):389–404
Waldbott GL (1977) Gastric ulcer and fluoride. Fluoride 10:149–151
Waldbott GL, Lee JR (1978) Toxicity from repeated low-grade exposure to hydrogen fluoride–case report. Clin Toxicol 13(3):391–402
Whitford GM (1987) Fluoride in dental products: Safety considerations. J Dent Res 66(5):1056–1060
Whitford GM (1990) The physiological and toxicological characteristics of fluoride. J Dent Res 69:539–549; discussion 556–537
Whitford GM, Pashley DH (1984) Fluoride absorption: The influence of gastric acidity. Calcif Tissue Int 36(3):302–307
Zandi F, Eslami N, Soheili M, Fayaz A et al (2009) Proteomics analysis of BHK-21 cells infected with a fixed strain of rabies virus. Proteomics 9(9):2399–2407
Zhang Y, Malekpour M, Al-Madani N, Kahrizi K et al (2007) Sensorineural deafness and male infertility: A contiguous gene deletion syndrome. J Med Genet 44(4):233–240
Zhu YP, Xi SH, Li MY, Ding TT et al (2017) Fluoride and arsenic exposure affects spatial memory and activates the ERK/CREB signaling pathway in offspring rats. Neurotoxicology 59:56–64
Zieker D, Königsrainer I, Traub F, Nieselt K et al (2008) PGK1 a potential marker for peritoneal dissemination in gastric cancer. Cell Physiol Biochem 21(5–6):429–436
Zieker D, Königsrainer I, Tritschler I, Löffler M et al (2010) Phosphoglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer. Int J Cancer 126(6):1513–1520
Acknowledgments
The authors thank Dr. Antonio de Castro Rodrigues and Dr. Jesus Carlos Andreo (Human Anatomy, Department of Biological Sciences, FOB/USP/Bauru, Brazil) for offering laboratory support. Laboratory Specialists: Márcia S. Z. Graeff and Rafaela A. da Silva (Integrated Research Center-CIP, FOB/USP/Bauru, Brazil) for the technical support during the image capture.
Funding
This work was supported by the Sao Paulo Research Foundation/FAPESP, Sao Paulo, Brazil (Processes No 2011/10233-7, 2012/16840-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guimaraes de Souza Melo, C., Nelisis Zanoni, J., Raquel Garcia de Souza, S. et al. Global Proteomic Profile Integrated to Quantitative and Morphometric Assessment of Enteric Neurons: Investigation of the Mechanisms Involved in the Toxicity Induced by Acute Fluoride Exposure in the Duodenum. Neurotox Res 39, 800–814 (2021). https://doi.org/10.1007/s12640-020-00296-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00296-9