Abstract
This survey designed to assess the in vitro and in vivo activity of α-pinene, a monoterpene commonly originated in essential oils on Toxoplasma gondii. The in vitro effect of various concentration of α-pinene against tachyzoites of T. gondii Rh strain was assessed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The activity of α-pinene on the stimulation of apoptosis in tachyzoites of T. gondii was also examined using the caspase 3 colorimetric activity assay. In vivo assay, mice were orally received α-pinene at 2 and 4 mg/kg/day for 14 days, then, pre-treated mice were daily tested and the rate of death was recorded. α-pinene meaningfully declined (p < 0.001) the tachyzoites viability with the IC50 value of 23.3 µg/mL. α-pinene induced the apoptosis through increasing the caspase-3 activity by 35.6%. Oral treatment with α-pinene significantly (p < 0.01) improved the survival rate infected mice with by 9th day. α-pinene + atovauone (50 mg/kg) significantly (p < 0.01) improved the survival rate infected mice up to 11 days compared with the control groups. α-pinene especially in combined atovaquone at 50 mg/kg for 2 weeks meaningfully (p < 0.05) declined oxidative stress. We found the promising in vitro anti-Toxoplasma effects of α-pinene on T. gondii RH strain. In addition, we found that α-pinene therapy particularly along with the reference drug declined the mortality rate of infected mice. Although, we just confirmed the stimulation of apoptosis and anti-inflammatory effects as the main anti-Toxoplasma mechanisms of α-pinene; however, more surveys concerning the accurate mechanisms, toxicity, and efficacy on other T. gondii strains are required to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is a forced protozoan parasite which multiplies in all nucleated cells of vertebrate hosts and its main host is the cat (Innes 2010). Routinely, humans being infected by eating raw or cooked infected meat (especially beef and pork), by eating oocysts in water and food contaminated with cat feces, and probably transmitted through the placenta during pregnancy (Hill et al. 2005). Toxoplasmosis occurs in a variety of forms, from an asymptomatic self-limiting infection to a fatal disease in patients with congenital infections and patients with special conditions (Saadatnia and Golkar 2012). In healthy people with normal immune systems, infection with this protozoan usually has no clinical symptoms and in some cases may be dangerous due to the parasite tending to the host's eyes and brain and the formation of cysts in these organs (Weiss and Dubey 2009); but in people with defective immune systems, and the same pregnant women, the parasite causes severe complications (Wang et al. 2017). In immunocompromised individuals, chronic infection with T. gondii can reactivate and cause encephalitis, chorioretinitis, or death (Wang et al. 2017; Fallahi et al. 2017; Hanifehpour et al. 2019). The same congenital toxoplasmosis that results from the parasite passing through the placenta during primary maternal infection can cause miscarriage, fetal death in the womb, or severe congenital complications, e.g. hydrocephalus and chorioretinitis (Goldstein et al. 2008; Kheirandish et al. 2019).
One of the main problems of toxoplasmosis is its therapeutic limitation (Dunay et al. 2018). The drug of choice for toxoplasmosis is a synergistic mixture of pyrimethamine and sulfonamide, which in addition to having side effects, do not have the ability to kill parasites in the cyst and eradicate the infection (Dunay et al. 2018). This treatment regimen is usually associated with many complications, e.g. blood poisoning, hypersensitivity, intolerance, bone marrow suppression and teratogenic effects in the first trimester of pregnancy (Smith et al. 2021).
One of the research priorities of toxoplasmosis is to obtain an anti-Toxoplasma drugs with the desired effect and with the least side effects (Arab-Mazar et al. 2017; Cheraghipour et al. 2021). Medicinal plant products are one of these options that have been mentioned in traditional medicine about the antiparasitic effects of some of these plants. The use of natural compounds to treat diseases has a long history (Al-Snafi, 2016). Today, although most drugs are of chemical origin, it is estimated that about one third of all medicinal products are of plant origin or have been deformed after extraction from the plant (Bauri et al. 2015). Alpha-pinene (α-pinene, C10H16), an organic compound of the terpenoid hydrocarbon, is broadly found in essential oils a large number of medicinal herbs (Allenspach and Steuer 2021). In modern medicine, α-pinene have been displayed various therapeutic and pharmacological properties, e.g. anti-inflammatory, gastroprotective, neuroprotective, anticancer, anticoagulant, antinociceptive, antioxidant, and antimicrobial effect (Allenspach and Steuer 2021; Koziol et al. 2014; Santos et al. 2011). By anti-parasitic effects, studies have displayed the favorable effects of α-pinene against various parasite species such as Leishmania spp, and Plasmodium spp, (Allenspach and Steuer 2021). Based on the potent pharmacological properties of α –pinene, we intended to the in vitro and in vivo effects of α-pinene against acute T. gondii infection.
Materials and methods
Drugs and reagents
Atovaquone (≥ 98% purity), α-pinene (≥ 98% purity), and MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) powder was procured from Sigma Chemical Co. (St. Louis, MO, USA).
Parasite and cells
T. gondii RH tachyzoites were kindly provided from Department of Medical Parasitology, Khorramabad, Iran. Tachyzoites collected from BALB/c mice peritoneum and adjusted into 10,000 tachyzoites/mL for tests (Cheraghipour et al. 2022; Saadatmand et al. 2021). The Vero cells were procured from the Pasteur Institute of Iran cell flask and cultured in RPMI1640 fluid culture medium containing 10% of inactivated bovine fetal serum (Merck, Germany) and 1% of the antibiotics of penicillin and streptomycin. The cells were incubated in sterile flasks at 37 °C in 5% CO2 and 95% humidity (Kareshk et al. 2015).
In vitro cell viability assay
To do this, 0.2 mL of tachyzoites (106 parasites per each mL) were incubated with various concentrations of α-pinene (12.5–100 µg/mL) in 96-well plates for 24 and 48 min at 37 °C. After incubation time, 50 µl of MTT suspension (5 mg/ml) was added and incubated again at 37 °C for 4 h. After 4 h incubation, by adding the isopropanol (200 μL) formazan crystals were eluted. As the last step of experiment, the solution absorbance was assessed at 570 nm by ELISA reader. Tachyzoites treated with the tween-80 aqueous solution were considered as the negative control. The 50% inhibitory concentrations (IC50) was also measured. To increase the validity of the tests, all tests were accomplished in triplicate (Asgari et al. 2013).
Activity of α-pinene on the stimulation of apoptosis
The activity of α-pinene on the stimulation of apoptosis in tachyzoites was examined using the kits of Caspase 3 colorimetric activity test (Sigma-Aldrich, Germany) based on the dye obtained from the activity of caspase-3 enzyme. Initially, T. gondii RH tachyzoites were treated with α-pinene at 1/3 IC50, 1/2 IC50, and IC50. After centrifuging, and lysing the pellet cells, they were then centrifuged at 16,000 rpm for 15 min. Next, 5 μL of superior was mixed by 10 μL of caspase 3 substrate and 85 μL buffer solution and the combination was incubated for two hours min at 37 °C. As the final step, the absorbance of mixture was measured by the ELISA reader at 410 nm (Ezzatkhah et al. 2021).
In vivo effects on acute toxoplasmosis in mice
Animals
Sixty male BALB/c mice (40 to 60 days’ age) and weight of 20–25 g purchased from Animal Breeding Center of Pasteur Institute, Iran and were selected for in vivo evaluation on acute toxoplasmosis in mice. Storage conditions were the same for all mice and were considered as 12 h of light / dark and an ambient temperature of 21 ± 2 °C in special cages and in a bed of straw. Diet and water were also freely available. During the study, the maintenance and testing of animals and the destruction of animals were in accordance with standard methods of work and principles of ethics with animals.
Ethics
The procedure was permitted by Lorestan University of Medical Sciences Ethical Committee, Khorramabad, Iran (IR.LUMS.1401.014).
Treatment of mice
After random division of mice into 5 groups (12 mice per each), mice were orally received 500 µL of tween-80 aqueous solution, atovaquone 100 mg/kg, α-pinene 2 and 4 mg/kg, as well as α-pinene (2 mg/kg) + atovaquone (50 mg/kg), one time per day for two weeks. It should be mentioned that the selection of these doses for in vitro and in vivo tests was based on the primary experiments as well as previous study (Felipe et al. 2019).
Induction of acute toxoplasmosis in mice
One day after 14 days’ treatment, all mice were intraperitoneally infected with 100 µL of T. gondii tachyzoites (10,000 tachyzoites/mL) (Kareshk et al. 2015).
Evaluation of the oxidative stress markers
To study the effects of α-pinene therapy on reducing the oxidative stress markers in infected mice, on the third day after toxoplasmosis induction, six mice from each group were euthanized using sodium pentobarbital. The serum level of malondialdehyde (MDA) as a main marker of lipid peroxidation (LPO) was measured according to the technique defined elsewhere (Hagege et al. 1990). In summary, mice sera were separately added to the Thiobarbituric acid (0.06%) and phosphoric acid (1%) and the suspension was incubated in for 45 min. In the next step, after adding the n-buthanol to the mixture, the serum level of MDA was read at 532 nm by ELISA reader.
Assessment of mortality rate
Treated mice were everyday checked and the frequency of death was studied for mice in tested groups (Mahmoudvand et al. 2020).
Parasitological examination
To do this, three days’ post-infection, after collecting the peritoneal fluids of mice in all groups, the number of the collected tachyzoites of each mice were recorded by a light microscopic (19).
Statistical analysis
Finally, the collected outcomes were analyzed using SPSS software version 22.0 and one-way analysis of variance (ANOVA). Tukey's test was applied to compare the means of the data. The difference in the level of probability was considered to be less than 0.05.
Results
In vitro effects on cell viability of tachyzoites
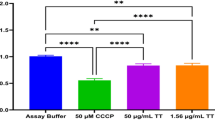
Figure 1 shows the results of in vitro cell viability assay used to evaluate the effect of various concentrations of α-pinene (12.5–100 µg/mL) on tachyzoites. The results of MTT assay revealed that α-pinene significantly reduced (p < 0.001) the tachyzoites viability as dose-dependent response, in comparision with the control group. The IC50 value for α-pinene and atovaquone was 23.3 and 17.6 µg/mL, respectively (Fig. 1).
Effects of α-pinene on the stimulation of apoptosis
The activity of α-pinene on apoptosis stimulation in tachyzoites was examined using the Caspase 3 colorimetric activity assay. Based on the obtained findings after treatment of T. gondii RH tachyzoites with α-pinene at 1/3 IC50, 1/2 IC50, and IC50, induced the apoptosis through increasing the caspase-3 activity by 16.3, 27.4, and 35.6%, respectively (Fig. 2).
In vivo effects on mortality rate of T. gondii mice infected
Treatment with α-pinene at 2, and 4 mg/kg considerably (p < 0.01) improved the survival rate infected mice with by the 8th, and 9th day p.i, respectively. In addition, pre-treatment of mice infected with atovaquone increased survival to 9 days. α-pinene at 4 mg/kg in combination with atovauone (50 mg/kg) meaningfully (p < 0.01) improved the survival rate infected mice up to 11 days compared with the control group and atovaquone at 100 mg/kg (Fig. 3).
Effect on the number of tachyzoites isolated from infected mice
The results showed that treatment with α-pinene at doses of 2 and 4 mg/kg, the mean number of peritoneal tachyzoites collected from infected mice was significantly (p < 0.001) decreased by 17.2 × 104 and 11.3 × 104, respectively. In addition, pre-treatment of infected mice with atovaquone at a dose of 100 mg/kg considerably (p < 0.001) declined the mean number of peritoneal tachyzoites collected from infected mice at 8.7 × 104. The results also showed that α-pinene (4 mg/kg) in combination with atovaquone (50 mg/kg) markedly (p < 0.001) declined the average number of tachyzoites in infected mice up to 8.7 × 104 compared with the control group and atovaquone (100 mg/kg) (Fig. 4).
Evaluation of the oxidative stress markers
Figure 5 indicates the effects of α-pinene therapy on the oxidative stress markers in infected mice. The results showed that in T. gondii infected mice the level of serum MDA was significantly increased (p < 0.001). On the other hand, treatment of infected mice with α-pinene especially in combined atovaquone at 50 mg/kg for 2 weeks meaningfully (p < 0.05) declined oxidative stress.
Discussion
Natural compounds are still considered an important resources for drug discovery (Bauri et al. 2015; Rasoulian et al. 2019). α-Pinene, as one of the famous secondary metabolite (monoterpenes) derived from plants is of high attention for medicinal, industrial, and commercial use (Silva et al. 2012). Today, studies have reported various pharmacological and therapeutic of α-pinene properties for treating diseases (Allenspach and Steuer 2021; Koziol et al. 2014; Santos et al. 2011). Today, the main treatment regimens for toxoplasmosis are frequently accompanying with various side effects such as blood poisoning, hypersensitivity, intolerance, bone marrow suppression and teratogenic effects in the first trimester of pregnancy (Cheraghipour et al. 2020; Keyhani et al. 2020; Smith et al. 2021). Therefore, finding an anti-Toxoplasma agent with the desired effect and with the least side effects especially from natural products seems necessary for researchers and clinicians (Cheraghipour et al. 2021). Based on the potent pharmacological properties of α –pinene, we intended to evaluate the effects of α-pinene against acute T. gondii infection.
By in vitro assay, our results showed that that α-pinene significantly reduced (p < 0.001) the viability of tachyzoites as dose-dependent response. The IC50 value for α-pinene and atovaquone was 23.3 and 17.6 µg/mL, respectively. Here, we studied the effect of α-pinene on the apoptosis stimulation in tachyzoites of T. gondii RH strain was examined using the caspase 3 colorimetric activity assay. Our findings exhibited that after treatment of T. gondii RH tachyzoites with α-pinene at 1/3 IC50, 1/2 IC50, and IC50, provoked the apoptosis through increasing the caspase-3 activity by 16.3, 27.4, and 35.6%, respectively. In line with our findings, Hou et al. (2019) and Matsuo et al. (2011) exhibited that α-pinene significantly elevated the level of caspase-3 in murine melanoma cell line and human ovary cell lines.
In view of the antimicrobial effects, investigations showed the relevant antibacterial effects of α-pinene against various pathogenic bacteria (e.g. Staphylococcus aureus, S. pyogenes, Streptococcus pneumonia, Klebsiella pneumonia, Haemophilus influenza) and fungal pathogenic strain (e.g. Candida spp., and Aspergillus spp.) with minimum inhibitory concentration (MIC) values 1.024 to 256 μg/mL (Nóbrega et al. 2021; Utegenova et al. 2018; Yang et al. 2015). da Franca Rodrigues et al. (2015) reported that α-pinene had potent anti-parasitic activity against promastigote, axenic amastigotes, and intracellular amastigote forms of Leishmania amazonensis, after 48 h incubation with IC50 values of 19.7, 16.1, and 15.6 μg/mL, respectively. In another study, Van Zyl et al. (2006) have revealed the considerable antimalarial effects of α-pinene against Chloroquine-resistant Plasmodium falciparum (FCR-3) with the IC50 value of 1.2 μM. Wang et al. (2019) have also reveal that after incubation of Bursaphelenchus xylophilus (pinewood nematode) with α-pinene (98% purity) at 4, 8, and 16 mL, the mortality rate of nematode was considerably increased by 51.1, 60.6, and 60.2%, respectively. Concerning the antimicrobial mechanisms of action of monoterpenes compounds, investigations reported that these compounds presented their antimicrobial efficacy through some cellular mechanisms, e.g. cell wall interruption, disruption of cellular oxygen consumption, induction of apoptosis, and deactivation of pathogenic virulence factors (Anand et al. 2019). Other study also showed that α-pinene displayed its antimicrobial mechanisms by structural changes in cell morphology, disruption of protein, DNA, and RNA synthesis, and the reactive oxygen species (ROS) creation (Li et al. 2014; Melkina et al. 2021). These reasons indicate the direct antiparasitic effects of α-pinene on the viability of tachyzoites of T. gondii RH strain by the mentioned mechanisms.
By in vivo assay, our findings revealed that oral administration of α-pinene at 2, and 4 mg/kg/day, especially along with atovaquone (50 mg/kg) significantly improved the survival rate infected mice; whereas, the mean number of peritoneal tachyzoites was significantly decreased. Previous studies showed that α-pinene had promising anti-inflammatory, antioxidant, neuroprotective through some mechanisms such as suppressing of inflammatory markers, reducing the inducible cyclooxygenase-2 and nitric oxide synthase, and inhibition of lipid peroxidation and oxidative stress, and inhibition of acetylcholinesterase (Karthikeyan et al. 2018; Kim et al. 2015; Miyazawa and Yamafuji, 2005). These reasons suggest that α-pinene, through its anti-inflammatory, antioxidant, and neuroprotective effects has been able to increase survival rate of infected mice.
It has been proven one of the frequent mechanisms of complicated in the pathogenesis of hepatic damage during acute toxoplasmosis is increasing the oxidative stress through the producing free radicals (Karaman et al. 2008). LPO is as an indicator of oxidative stress, which results in destruction of the cell membrane and subsequently discharge of indicator enzymes of hepatotoxicity (Tonin et al. 2014). Our results revealed that in T. gondii infected mice the level of serum MDA was considerably elevated (p < 0.001). On the other hand, treatment of infected mice with α-pinene especially in combined atovaquone at 50 mg/kg for 2 weeks meaningfully (p < 0.05) declined oxidative stress. Consequently, it may be claimed that α-pinene therapy by its anti-inflammatory activity protects the liver from injuries provoked with T. gondii. Considering the toxicity of α-pinene, in the study conducted by Felipe et al. (2019), the results showed that 50% lethal dose (LD50) for both monoterpenes of α-pinene and β-pinene was determined higher than 2000 mg/kg in tested mice, where oral administration of α-pinene and β-pinene at the doses of 100, 200 and 400 mg/kg had no toxicity in mice; indicating that α-pinene at the doses used in the present study had minimal toxicity in tested mice.
Conclusion
Here, we revealed the promising the in vitro anti-Toxoplasma activity of α-pinene on T. gondii tachyzoites. In addition, we found that treatment of T. gondii infected mice with α-pinene especially in combination with the reference drug markedly declined the mortality rate of infected mice. Although, we just confirmed the induction of apoptosis and anti-inflammatory activity as the main anti-Toxoplasma mechanisms of α-pinene; however, more surveys concerning the accurate mechanisms, toxicity, and efficacy on other T. gondii strains are necessary to confirm these findings.
References
Allenspach M, Steuer C (2021) α-Pinene: a never-ending story. Phytochemistry 1(190):112857
Al-Snafi AE (2016) Antiparasitic effects of medicinal plants (part 1): a review. IOSR J Pharm 6(10):51–66
Anand U, Jacobo-Herrera NJ, Altemimi AB, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimi-crobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9:258. https://doi.org/10.3390/metabo9110258
Arab-Mazar Z, Kheirandish F, Rajaeian S (2017) Anti-toxoplasmosis activity of herbal medicines: narrative review. Herb Med J pp 43–9
Asgari Q, Keshavarz H, Shojaee S, Motazedian MH, Mohebali M, Miri R, Mehrabani D, Rezaeian M (2013) In vitro and in vivo potential of RH strain of Toxoplasma gondii (Type I) in tissue cyst forming. Iran J Parasitol 8(3):367
Bauri RK, Tigga MN, Kullu SS (2015) A review on use of medicinal plants to control parasites. Indian J Nat Prod Resour 6(4):268–277
Cheraghipour K, Masoori L, Ezzatkhah F, Salimikia I, Amiri S, Makenali AS, Taherpour F, Mahmoudvand H (2020) Effect of chitosan on Toxoplasma gondii infection: a systematic review. Parasite Epidemiol Control 11:e00189
Cheraghipour K, Masoori L, Ezzatpour B, Roozbehani M, Sheikhian A, Malekara V, Niazi M, Mardanshah O, Moradpour K, Mahmoudvand H (2021) The experimental role of medicinal plants in treatment of toxoplasma gondii infection: a systematic review. Acta Parasitol 66(2):303–328
Cheraghipour K, Zivdari M, Beiranvand M, Shakib P, Kheirandish F, Pour MZ, Ghafarypour M, Marzban A, Alhameedawi AK (2022) Encapsulation of Nepeta cataria essential oils in a chitosan nanocomposite with lethality potential against Toxoplasma gondii. Emergent Mater 14:1–1
da Franca Rodrigues KA, Amorim LV, Dias CN, Moraes DF, Carneiro SM, de Amorim Carvalho FA (2015) Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol 160:32–40
Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG (2018) Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev 31(4):e00057-e117
Ezzatkhah F, Khalaf AK, Mahmoudvand H (2021) Copper nanoparticles: Biosynthesis, characterization, and protoscolicidal effects alone and combined with albendazole against hydatid cyst protoscoleces. Biomed Pharmacother 136:111257
Fallahi S, Rostami A, Birjandi M, Zebardast N, Kheirandish F, Spotin A (2017) Parkinson’s disease and Toxoplasma gondii infection: sero-molecular assess the possible link among patients. Acta Trop 173:97–101
Felipe CF, Albuquerque AM, de Pontes JL, de Melo JÍ, Rodrigues TC, de Sousa AM, Monteiro ÁB, Ribeiro AE, Lopes JP, de Menezes IR, de Almeida RN (2019) Comparative study of alpha-and beta-pinene effect on PTZ-induced convulsions in mice. Fundam Clin Pharmacol 33(2):181–190
Goldstein EJ, Montoya JG, Remington JS (2008) Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 47(4):554–566
Hagege D, Nouvelot A, Boucaud J et al (1990) Malondialdehyde titration with thiobarbiturate in plant extracts: avoidance of pigment interference. Phytochem Anal 1(2):86–89
Hanifehpour H, Shariat SK, Ghafari MS, Kheirandish F, Saber V, Fallahi S (2019) Serological and molecular diagnosis of Toxoplasma gondii infections in thalassemia patients. Iran J Parasitol 14(1):20
Hill DE, Chirukandoth S, Dubey JP (2005) Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev 6(1):41–61
Hou J, Zhang Y, Zhu Y, Zhou B, Ren C, Liang S, Guo Y (2019) α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med Sci Monitor 25:6631
Innes EA (2010) A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 57(1):1–7
Karaman U, Celik T, Kiran TR, Colak C, Daldal NU (2008) Malondialdehyde, glutathione, and nitric oxide levels in Toxoplasma gondii seropositive patients. Korean J Parasitol 46(4):293
Kareshk AT, Keyhani A, Mahmoudvand H, Oliaei RT, Asadi A, Andishmand M, Azzizian H, Babaei Z, Zia-Ali N (2015) Efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice model. Iran J Parasitol 10(4):625
Karthikeyan R, Kanimozhi G, Prasad NR, Agilan B, Ganesan M, Srithar G (2018) Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci 212:150–158
Keyhani A, Ziaali N, Shakibaie M, Kareshk AT, Shojaee S, Asadi-Shekaari M, Sepahvand M, Mahmoudvand H (2020) Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J Med Microbiol 69(1):104–110
Kheirandish F, Ezatpour B, Fallahi S, Tarahi MJ, Hosseini P, Rouzbahani AK, Tabaei SJ, Akbari S (2019) Toxoplasma serology status and risk of miscarriage, a case-control study among women with a history of spontaneous abortion. Int J Fertility Sterility 13(3):184
Kim DS, Lee HJ, Jeon YD, Han YH, Kee JY, Kim HJ, Shin HJ, Kang J, Lee BS, Kim SH, Kim SJ (2015) Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med 43(04):731–742
Koziol A, Stryjewska A, Librowski T, Salat K, Gawel M, Moniczewski A, Lochynski S (2014) An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem 14(14):1156–1168
Li L, Shi C, Yin Z, Jia R, Peng L, Kang S, Li Z (2014) Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz J Microbiol 45(4):1409–1413
Mahmoudvand H, Kareshk AT, Moradi MN, Fidalgo LM, Mirbadie SR, Niazi M, Khatami M (2020) Efficacy and safety of Zataria multiflora Boiss essential oil against acute toxoplasmosis in mice. Iran J Parasitol 15(1):22
Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JA, Massaoka MH, Travassos LR, Sartorelli P, Lago JH (2011) α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun 411(2):449–454
Melkina OE, Plyuta VA, Khmel IA, Zavilgelsky GB (2021) The mode of action of cyclic monoterpenes (−)-Limoneneand (+)-α-Pinene on bacterial cells. Biomolecules 11(6):806
Miyazawa M, Yamafuji C (2005) Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J Agric Food Chem 53(5):1765–1768
Nóbrega JR, Silva DD, Andrade Júnior FP, Sousa PM, Figueiredo PT, Cordeiro LV, Lima ED (2021) Antifungal action of α-pinene against Candida spp. isolated from patients with otomycosis and effects of its association with boric acid. Nat Prod Res 35(24):6190–6193
Rasoulian B, Hajializadeh Z, Esmaeili-Mahani S, Rashidipour M, Fatemi I, Kaeidi A (2019) Neuroprotective and antinociceptive effects of rosemary (Rosmarinus officinalis L.) extract in rats with painful diabetic neuropathy. J Physiol Sci 69(1):57–64
Saadatmand M, Al-Awsi GR, Alanazi AD, Sepahvand A, Shakibaie M, Shojaee S, Mohammadi R, Mahmoudvand H (2021) Green synthesis of zinc nanoparticles using Lavandula angustifolia Vera. Extract by microwave method and its prophylactic effects on Toxoplasma gondii infection. Saudi J Biol Sci 28(11):6454–6460
Saadatnia G, Golkar M (2012) A review on human toxoplasmosis. Scand J Infect Dis 44(11):805–814
Santos MR, Moreira FV, Fraga BP, Souza DP, Bonjardim LR, Quintans-Junior LJ (2011) Cardiovascular effects of monoterpenes: a review. Rev Bras 21(4):764–771
Silva AC, Lopes PM, Azevedo MM, Costa DC, Alviano CS, Alviano DS (2012) Biological activities of a-pinene and β-pinene enantiomers. Molecules 17(6):6305–6316
Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG (2021) Control of human toxoplasmosis. Int J Parasitol 51(2–3):95–121
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30(12–13):1217–1258
Tonin AA, Da Silva AS, Thomé GR, Sangoi MB, Oliveira LS, Flores MM, Schetinger MR, Fighera RA, Moresco RN, Camillo G, Vogel FS (2014) Influence of toxoplasmosis on acetylcholinesterase activity, nitric oxide levels and cellular lesion on the brain of mice. Pathol Res Practice 210(8):526–532
Utegenova GA, Pallister KB, Kushnarenko SV, Özek G, Özek T, Abidkulova KT, Kirpotina LN, Schepetkin IA, Quinn MT, Voyich JM (2018) Chemical composition and antibacterial activity of essential oils from Ferula L. species against methicillin-resistant Staphylococcus aureus. Molecules 23(7):1679
Van Zyl RL, Seatlholo ST, Van Vuuren SF, Viljoen AM (2006) The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res 18(sup1):129–133
Wang ZD, Liu HH, Ma ZX, Ma HY, Li ZY, Yang ZB, Zhu XQ, Xu B, Wei F, Liu Q (2017) Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol 8:389
Wang X, Yu Y, Ge J, Xie B, Zhu S, Cheng X (2019) Effects of α-pinene on the pinewood nematode (Bursaphelenchus xylophilus) and its symbiotic bacteria. PLoS ONE 14(8):e0221099
Weiss LM, Dubey JP (2009) Toxoplasmosis: a history of clinical observations. Int J Parasitol 39(8):895–901
Yang C, Hu DH, Feng Y (2015) Antibacterial activity and mode of action of the Artemisia capillaris essential oil and its constituents against respiratory tract infection-causing pathogens. Mol Med Rep 11(4):2852–2860
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kharazmkia, A., Al-Abodi, H.R., Yadegari, J.G. et al. Potential effects of alpha-pinene, a monoterpene commonly found in essential oils against Toxoplasma gondii infection; an in vitro and in vivo study. J Parasit Dis 46, 1055–1061 (2022). https://doi.org/10.1007/s12639-022-01514-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-022-01514-1