Abstract
The present study reports the occurrence of parasitic copepods and isopods infecting marine teleost fishes from the Kerala coast, India. A total of 1795 fishes belonging to 38 species were collected from the fish landing centres across the Kerala Coast for 5 months. The isopod & copepod infection status of these fishes were assessed. The incidents of site-specific infection were documented for all parasites and a fecundity analysis was conducted in randomly selected species. The single or multiple parasitic crustacean infestation and host preference (single or multiple hosts) were also documented. A total of 32 species of copepods and 6 species of isopod were sourced out. However, the maximum prevalence was recorded for the family Lernanthropidae (71.43%) and the maximum intensity (14) was recorded for a Caligid copepod Euryphorus nordmannii. The predominantly targeted region of infection on the host fish appeared to be gill filament (52.15%). The fecundity showed significant differences between the tested species. Fourteen species of fishes showed multiple parasitic crustacean infestation and eight species of parasites infected multiple hosts. The present study demonstrates that over 68% of marine fish species of Kerala coast were under heavy infection either by isopods or copepods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crustaceans are one of the major Arthropod groups preferring aquatic life with diverse levels of adaptation. A significant proportion of crustaceans exhibit parasitism infecting both cultured and captive fishes (Athanassopoulou et al. 2001; Barber and Poulin 2002; Rijin et al. 2017; Başusta et al. 2017) and thus affect the behaviour, growth, health of the host fish (El-Rashidy and Boxshall 2010; Misganaw and Getu 2016). Mostly the infestation gets fatal employing the infectious injuries made by the parasites during its feeding of the host’s mucus, blood, muscles and other vital tissues (Margolis and Kabata 1988; Oğuz and Oktener 2007). Most known parasitic crustaceans comprise copepods, isopods, brachyuran, tantulocarids, and rhizocephalic cirripedes (Thamban et al. 2015). They exhibit close interrelatedness in the sustainability of their own with the host organism (Margolis and Kabata 1988; Raibaut and Trilles 1993; Raibaut et al. 1998; Williams and Boyko 2012). Parasitic crustacean diseases in fishes seriously affect aquaculture production and its economic viability (Aneesh et al. 2013; Rania and Rehab 2015).
Many ecologists now recognize that crustacean parasitism is one of the most important factors affecting the viability of captured and cultured fish populations and communities of different aquatic habitat. In the marine environment, it has been demonstrated that individual fish may suffer from parasitic crustaceans (Rohde 1994; Jithin et al. 2016). The possible effects of parasitic crustaceans on their fish hosts are difficult to assess or quantify, in natural conditions and it remains to be unexplained why some fish species have a higher parasite species richness than others, and how parasite communities build upon these hosts. Because of the inefficient study about the effects of macroparasites like crustaceans on the teleost host, the alteration in the fitness has rarely been quantified, and their ecological and evolutionary meanings are unknown (Trilles et al. 2011; Rania and Rehab 2015).
Like coastal countries in India, both marine fisheries and aquaculture have witnessed phenomenal growth during the last five decades both in terms of quantitative and qualitative levels (Flaherty et al. 2009). However, during the last decade’s aquaculture has been facing the problems posed by pathogens and parasites and their implications for fishery leading to constraints in aquaculture productivity (Sanil et al. 2009; Rijin et al. 2019). In this context, as a prelude, the present survey report on the parasitic copepods and isopods infecting marine fishes along the Kerala Coast is relevant.
Materials and methods
Ethics statement
This study doesn’t require ethical approval from an animal ethics committee since the fish were collected from food fish supply and doesn’t involve any actions against animal welfare.
Parasite collection
The fresh non-live fish- samples were collected from the fisherman of major fish landing centres including Vizhinjam harbour–Trivandrum (528 m Lat.8°22′37″ N Long.76°59′17″ E), Neendakara harbour–Kollam (743 m Lat.8°56′18″ N Long.76°32′20″ E), Thottappally harbour–Alappuzha (1025 m Lat.9°19′11″ N Long.76°22′43″ E), Beypore harbour–Kozhikode (3968 m Lat.11°10′18″ N Long.75°48′29″ E), Ayikkara harbour–Kannur (827 m Lat.11°51′22″N Long.75°22′32″ E) and Madakkara harbour–Kasaragod (730 m Lat.12°12′39″ N Long.75°07′43″ E) of Kerala Coast. The fish samples were collected from food fish suppliers, hence permission from harbour authorities for sample collection was not required.
The present study was conducted during the period from December 2017 to April 2018. The fresh or live fish for the present study were collected directly from the local fishermen twice a week throughout the study period. Soon after the collection, the fish were subjected to morphometric analysis such as total length, standard length and fork length of the host and the images of the fish were also captured. Then each collected fish was closely observed for the isopod and copepod infection. For the purpose, different parts of the body like fins, gill rack, gill cavity, gill filament, gill arch, opercular region, head, the surface of the body, etc. were observed using a hand lens. The recovered parasites and the host fishes were immediately brought to the laboratory. The parasites were subjected to detailed microscopic examination using a stereo-microscope (Leica-S6D), stereo zoom microscope (Radical RSM-8), compound microscope (Magnus MLX-DX) and photographed. The onsite photographs of macroscopic parasites and fishes were taken using Nikon Coolpix B700.
Species identification of parasites and host fishes
The primary identification of the parasites was carried out using standard taxonomic keys (Kirtisinghe 1964; Pillai 1985; Yamaguti 1985) and WoRMS (2020). Identification to the species level was aided by the available descriptions (Bassett-Smith 1898; Pillai 1964; Cressey and Cressey 1980; Kumar 1990). The identification of the fishes was based on the SeaLifeBase and Fish Base databases (Froese and Pauly 2018).
Preservation of parasite samples
The specimens were preserved in 70% ethanol and kept in the crustacean biology lab for future reference based on the species name and the number of parasites recovered from the respective host. The data were entered into Microsoft Excel spreadsheet for subsequent calculation.
Fecundity analysis
The fecundity of selected representatives of isopod Norileca indica and copepods Euryphorus nordmannii, Cybicola armatus, Penniculus fistula fistula, Brachiella otolithi of different families are calculated and compared. The total number of eggs was counted per selected individuals of the above-mentioned species, its average number of eggs were calculated and compared each other.
Data analysis
The data on the parasitic crustaceans and their respective host fish were subjected to statistical analysis. The fecundity of four copepods and one isopod species was analysed by a two-tailed student’s unpaired t test using GraphPad Prism version 5.01 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. The prevalence and intensity were calculated using the following equations:
where NFI is the number of fishes infested, NFO is the number of fish observed and TNPR is the total number of parasites recovered (Margolis et al. 1982; Bush et al. 1997).
Results and discussion
The occurrence of parasitic crustaceans
A total of 1795 fish were collected from the different collection sites Fig. 1, including 38 species, 35 genera, 22 families and 7 orders. Among them, 274 fish under 25 species, 25 genera, 12 families and 6 orders were found to be infected with 32 species of copepods and 6 species of isopods (Figs. 2, 3, 4, 5, 6, 7, 8). The details of fish hosts and corresponding parasites along with their family references are provided in Table 1—Supplementary file. The fish families of order Perciformes such as Scombridae, Carangidae, Sciaenidae and Sillaginidae were found to be more prone to infection with 26 copepods and 4 isopod species supporting the previously published reports (Yuniar et al. 2007; Rameshkumar and Ravichandran 2014; Helna et al. 2018). Among the isopods recovered, Norileca indica was recovered with the high prevalence from four host fishes Rastrelliger kanagurta (30.53%), Thryssa malabarica (2.86%), Saurida tumbil (1.96%) and Sillago lutea (1.20%) of family Scombridae, Engraulidae, Synodontidae and Sillaginidae, respectively. The present study adds three new host fishes (Thryssa malabarica, Saurida tumbil and Sillago lutea) for the Isopod parasite Norileca indica from Ayikkara harbour (Kannur), Neendakara harbour (Kollam) and Thottapally harbour (Alappuzha) as it was previously reported only from host fish Rastrelliger kanagurta of Malabar Coast (Ghosh et al. 2016; Panakkool-Thamban et al. 2016). Agarna malayi, another Isopod collected from the carangid fish Selar crumenophthalmus from Vizhinjam harbour (Trivandrum) with a prevalence of 1.69% also forms new host record for this parasitic isopod species as it was previously reported only from the clupeid host Tenualosa toli (Rijin et al. 2017) of Malabar Coast.
Family Caligidae a Synestius caliginus Steenstrup & Lütken, 1861; b Caligus robustus Bassett-Smith 1898; c Caligus kanagurta Pillai, 1961; d Caligus amblygenitalis Pillai, 1961; e Caligus coryphaenae Steenstrup & Lütken, 1861; f Caligus phipsoni Bassett-Smith 1898; g Caligus bonito bonito Wilson C.B., 1905; h Caligus rotundigenitalis Yü, 1933; i Euryphorus nordmannii Milne Edwards, 1840; j Caligodes laciniatus Krøyer, 1863; k Abasia platyrostris Pillai, 1963; l Hermilius pyriventris Heller, 1865
Family Lernanthropidae a: Lernanthropus giganteus Krøyer, 1863; b Lernanthropus corniger Yamaguti, 1954; c Lernanthropus otolithi Pillai, 1963; d Lernanthropus latis Yamaguti, 1954; e Lernanthropus tylosuri Richiardi, 1880; f Lernanthropus sillaginis Pillai, 1963; g Mitrapus oblongus Pillai 1964; h Lernanthropus opisthopteri Pillai 1964
Family Pseudocycnidae – A: Pseudocycnus appendiculatus Heller, 1865; B: Cybicola armatus Bassett-Smith 1898
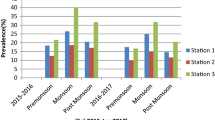
The occurrence of various Copepod families in the host fishes are shown in Fig. 9 and the foremost among them appears to be the family Pseudocycnidae. Among the parasitic copepods, Lernanthropus corniger infesting Megalaspis cordyla and Pseudocycnus appendiculatus infecting Thunnus tonggol exhibit the highest prevalence of about 71.43% and 66.67%, respectively. Meanwhile, Euryphorus nordmannii recovered from Coryphaena equiselis and Lernanthropus tylosuri recovered from Strongylura leiura shows highest parasitic intensity as 14 and 9.57 respectively. Although, it has been found out that, concerning other fish landing centres Thottappally fish landing centre (Alappuzha District) manifests comparatively less infestation of crustacean parasites. The widely prevalent fish, Sardinella longiceps observed from all regions coming under family Clupeidae showed very less infestation or even say uninfected as 154 fishes have been examined from various harbours of Kerala Coast. While considering the site of infestation of isopods, it is entirely different from that of copepods, where its prominent site of infection seems to be opercular wall, mouth, gill cavity etc. But in the case of copepods, the intensively affected site is the gill filament as represented in Fig. 10, which is corresponding to the previously published report (Panakkool-Thamban et al. 2016).
Differential fecundity in parasite species (egg sac observation and fecundity analysis)
The Fig. 11 shows the relative fecundity of selected representatives of isopods Norileca indica and copepods Euryphorus nordmannii, Cybicola armatus, Penniculus fistula fistula, Brachiella otolithi of different families are comparatively analysed. In the egg sac of Brachiella otolithi, eggs were in a multiseriate arrangement which is an exception from the remaining three copepods which shows uniseriate egg sacs. Uniseriate egg sac could accommodate a greater number of eggs, likely an adaptation for maximum progeny production.
Significant variations in fecundity were found between 7 parasite pairs out of 10 (for P < 0.05). The highest fecundity was noted in Cybicola armatus (466.0 ± 56.99) followed by Peniculus fistula fistula (256.2 ± 44.21) compared to other copepods. The average fecundity of Norileca indica was recorded as 262.0 ± 39.01. The significant values for various pairs are as follows. Cybicola armatus vs Euryphorus nordmannii (0.0024**), Cybicola armatus vs Brachiella otolithi (0.0001***), Cybicola armatus vs Penniculus fistula fistula (0.0196*), Cybicola armatus vs Norileca indica (0.0183*), Euryphorus nordmannii vs Brachiella otolithi (0.0110*), Brachiella otolithi vs Penniculus fistula fistula (0.0024**), and Brachiella otolithi vs Norileca indica (0.0010***). The fecundity has a relation with body size under the quality of water which has been reported earlier that the body size, as well as fecundity increases as the quality of water, improves (Tolba and Holdich 1981; Guha et al. 2013).
Multiple parasitism
The present study could also demonstrate various levels of parasitism seen on the host fish during the study period. Usually, to the best of knowledge, up to Quadruple Parasitism is being evident strongly. Surprisingly we got multiple infestations of five parasitic crustaceans seen in two hosts such as Sillago lutea of about 4 are copepods (Nothobomolochus lateolabracis, Lernanthropus sillaginis, Caligus phipsoni, Caligus bonito bonito) and the 1 left behind is an isopod (Norileca indica) also from the fish host Strongylura leiura, by three copepods(Caligodes laciniatus, Lernanthropus tylosuri & Bomolochus bellones) and two isopods (Mothocya renardi & Cymothoa frontalis) is also supporting the view of (Aneesh et al. 2014).
Along with this, quadruple parasitism was seen in one host, Otolithes cuvieri parasites observed comprises of four copepods (Brachiella otolithi, Lernanthropus otolithi, Lernanthropus latis, Bomolochus bellones). Likewise, triple parasitism was also observed in three hosts such as Parastromateus niger with three copepods (Synestius caliginus, Lernaeenicus stromatei, and Bomolochus megaceros), Otolithes ruber infected by three copepods (Catoessa boscii, Bomolochus bellones, Parabrachiella albida) and Anodontostoma chacunda is also infected by three copepods (Peniculus fistula fistula, Pseudorbitacolax varunae, Mitrapus oblongus). Along with this, double parasitism also exists in eight host fishes Saurida tumbil comprises an isopod (Norileca indica) and a copepod (Abasia platyrostris), Coryphaena equiselis infected by two copepods (Euryphorus nordmannii & Brachiella trichiuri), Thunnus tonggol through two copepods (Pseudocycnus appendiculatus and Caligus amblygenitalis), Katsuwonus pelamis infected by two copepods (Caligus coryphaenae and Pseudocycnus appendiculatus). Caranx sp. comprises two copepods (Lernanthropus giganteus and Bomolochus megaceros), Rastrelliger kanagurta with one isopod (Norileca indica) and one copepod (Caligus kanagurta), Mugil cephalus through two copepods (Caligus bonito bonito, Bomolochus nitidus) and in Euthynnus affinis with 2 copepods (Pseudocycnus appendiculatus, Caligus amblygenitalis). From a total of about 38 species of fish host species, 14 shows multiple parasitic crustacean infection and about 12 doesn’t show any infestation. Hence the 12 fish hosts left behind were under single parasitic crustacean infection.
Although the parasitic crustaceans are infecting the host in a wide variety of ways such as single, double, triple, quadruple, etc. irrespective of the infestation pathway, the parasitic crustacean harms the host due to its attachment and feeding habit, it is also responsible for any disease caused (Bharadhirajan et al. 2013; Heckmann 2015). One of the main facts about external body surface parasites, these are likely to be underestimated due to loss during capture and subsequent handling.
Conclusion
The present study could demonstrate that over 68% of marine fish species of Kerala Coast are under heavy infection by the parasitic crustaceans—isopods and copepods. Out of the 38 species of marine fish observed, 25 species were under the infestation of 32 species of copepods and 6 species of isopods. Among the fish species Scombridae, Sciaenidae, Carangidae, and Sillaginidae appeared to be more prone to parasitic crustacean infection. The Sillaginid fish, Sillago lutea and Strongylura leiura were found first-rate hosts for parasitic crustacean as we recovered 5 species of parasites each. The cymothoid Norileca indica shows multiple host infection and their hosts, Rastrelliger kanagurta, Thryssa malabarica, Saurida tumbil, and Sillago lutea, belonging to different families respectively Scombridae, Engraulidae, Synodontidae and Sillaginidae. The maximum prevalence was with R. kanagurta (30.53%). The present study adds three new host fishes (T. malabarica, S. tumbil, and S. lutea) for N. indica. The Carangid fish Selar crumenophthalmus also appears to be the new host for the A. malayi. Among 32 species of copepods, the Caligidae family forms the foremost comprising 12 species followed by the family Lernanthropidae with 8 species. Among the parasitic copepods Lernanthropus corniger infesting Megalaspis cordyla and Pseudocycnus appendiculatus infecting Thunnus tonggol exhibit highest prevalence of about 71.43% and 66.67% respectively. Simultaneously Euryphorus nordmannii recovered from Coryphaena equiselis and Lernanthropus tylosuri recovered from Strongylura leiura shows the highest parasitic intensity as 14 and 9.57 respectively. The study needs to be extended to the culture fishes as they are facing the threat of crustacean parasitism.
Availability of data and material
Not applicable.
References
Aneesh P-T, Sudha K, Arshad K, Anilkumar G, Trilles J-P (2013) Seasonal fluctuation of the prevalence of cymothoids representing the genus Nerocila (Crustacea, Isopoda), parasitizing commercially exploited marine fishes from the Malabar Coast, India. Acta Parasitologica. https://doi.org/10.2478/s11686-013-0112-3
Aneesh P-T, Kappalli S, Kottarathil Helna A, Anilkumar G, Trilles J-P (2014) Multiple parasitic crustacean infestation on belonid fish Strongylura strongylura. ZooKeys 457:339–353. https://doi.org/10.3897/zookeys.457.6817
Athanassopoulou F, Bouboulis D, Martinsen B (2001) In vitro treatments of deltamethrin against the isopod parasite Anilocra physodes, a pathogen of seabass Dicentrarchus labrax L. Bull Eur Ass Fish Pathol 21:4
Barber I, Poulin R (2002) Interactions Between Fish, Parasites and Disease. In: Hart PJB, Reynolds JD (eds) Handbook of Fish Biology and Fisheries, vol 1. Blackwell Publishing Ltd. Oxford, UK, pp 259–389
Bassett-Smith PW (1898) I.— Some new parasitic Copepods found on fish at Bombay. Ann Mag Natural History 1:1–17. https://doi.org/10.1080/00222939808677915
Başusta N, Mutlu E, Deval MC (2017) Parasitic isopods (Anilocra frontalis H. Milne Edwards, 1830 and Ceratothoa capri (Trilles, 1964)) from the Antalya Bay (Turkey) with new host records. Fırat Univ Turkish J Sci Technol 12:11–15
Bharadhirajan P, Gopalakrishnan A, Raja K, Murugan S, Vijayakumar R, Rahman MM (2013) Prevalence of copepod parasite (Lernaeenicus polynemi) infestation on Eleutheronema tetradactylum from Pazhayar coastal waters, southeast coast of India. JCLM 1:278–281. https://doi.org/10.12980/JCLM.1.20133D154
Bush AO, Lafferty KD, Lotz JM, Shostak AW et al (1997) Parasitology meets ecology on its own terms: margolis et al. Revisited. J Parasitol 83:575. https://doi.org/10.2307/3284227
Cressey RF, Cressey HB (1980) Parasitic copepods of mackerel- and tuna-like fishes (Scombridae) of the world. Smithsonian Contribut Zool. https://doi.org/10.5479/si.00810282.311.i
El-Rashidy H, Boxshall GA (2010) Parasitic copepods on immigrant and native clupeid fishes caught in Egyptian coastal waters off Alexandria. Syst Parasitol 76:19–38. https://doi.org/10.1007/s11230-010-9230-6
Flaherty Mark, Samal Kishor C, Pradhan Dolagobinda, Ray Subrata (2009) Coastal Aquaculture in India: Poverty. Concept Publishing Company, New Delhi, Environment and Rural Livelihood
Froese R, Pauly (2018). In: Fish base. World Wide Web electronic publication. http://www.Fishbase.org. Accessed 15 May 2018
Ghosh S, Rao MVH, Mahesh VU, Kumar MS, Rohit P (2016) Fishery, reproductive biology and stock status of the Indian mackerel Rastrelliger kanagurta (Cuvier, 1817), landed along the north-east coast of India. 63:10. https://doi.org/10.21077/ijf.2016.63.2.53399-05
Guha A, Aditya G, Saha SK (2013) Correlation between body size and fecundity in fish louse Argulus bengalensis Ramakrishna, 1951 (Crustacea: Branchiura). J Parasit Dis 37:118–124. https://doi.org/10.1007/s12639-012-0144-x
Heckmann R (2015) Other ectoparasites infesting fish; copepods, branchiurans, isopods, mites and bivalves. 8
Helna AK, Sudha K, Aneesh P-T (2018) Caligus cybii (Caligidae, Copepoda) Parasitising the Commercially Exploited Seer Fish, Scomberomorus commerson, from the Malabar Coast (India). Occurr Adaptat. https://doi.org/10.4194/1303-2712-v18_3_10
Jithin K, Swapna A, Kumar RR, Venu S, Helna AK, Sudha K (2016) Studies on Crustacean Parasites from Commercial Marine Fish Along the Andaman Coast in Comparison with Malabar Coast of Kerala of Indian EEZ. 8
Kirtisinghe P (1964) A Review of the parasitic copepods of fish recorded from Ceylon with description of additional forms. Bull Fish Res Station Ceylon 17:45–132
Kumar A (1990) Studies on Copepod Parasites on Elasmobranchs of Kerala Coast. 286
Margolis L, Kabata (eds) (1988) Guide to the parasites of fishes of Canada. 3: Acanthocephala. Cnidaria. Ottawa
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA (1982) The Use of Ecological Terms in Parasitology (Report of an Ad Hoc Committee of the American Society of Parasitologists). J Parasitol 68:131. https://doi.org/10.2307/3281335
Misganaw K, Getu A (2016) Review on Major Parasitic Crustacean in Fish. Fish Aquac J. https://doi.org/10.4172/2150-3508.1000175
Oğuz MC, Oktener A (2007) Four parasitic Crustacean species from marine fishes of Turkey. Turkiye Parazitolojii Dergisi 31:79–83
Panakkool-Thamban A, Ameri Kottarathil H, Kappalli S (2016) Branchial cymothoids infesting the marine food fishes of Malabar coast. J Parasit Dis 40:1270–1277. https://doi.org/10.1007/s12639-015-0666-0
Pillai NK (1964) Parasitic isopods of the family Cymothoidae from south Indian fishes. Parasitology 54:211–223. https://doi.org/10.1017/S003118200006786X
Pillai NK (1985) The fauna of India: copepod parasites of marine fishes. Zoological Survey of India, Calcutta
Raibaut A, Trilles JP (1993) The Sexuality of Parasitic Crustaceans. In: Advances in Parasitology. Elsevier, pp 367–444
Raibaut A, Combes C, Benoit F (1998) Analysis of the parasitic copepod species richness among Mediterranean fish. J Marine Syst 15(1–4):185–206
Rameshkumar G, Ravichandran S (2014) Problems caused by isopod parasites in commercial fishes. J Parasit Dis 38:138–141. https://doi.org/10.1007/s12639-012-0210-4
Rania AA, Rehab RA (2015) Some studies on parasitic isopods of some marine fishes. Egyptian J Chem Environ Health 1:400–420
Rijin K, Sudha K, Vineesh PJ, Anilkumar G (2017) Seasonal variation in the occurrence of parasitic isopods and copepods (Crustacea) Infecting the Clupeidaen Fishes of Malabar Coast, India. Turk J Fish Aquat Sci 19:241–249. https://doi.org/10.4194/1303-2712-v19_3_07
Rijin K, Mumthaz T, Sudha K, Gopinathan A (2019) Clupeid fish hosts a Peniculus sp. (Pennellidae, Siphonostomatoida, Copepoda)—First report on new host and season dependent prevalence. Acta Oceanol Sin 38:118–125. https://doi.org/10.1007/s13131-019-1517-0
Rohde K (1994) Niche restriction in parasites: proximate and ultimate causes. Parasitology 109:S69–S84. https://doi.org/10.1017/S0031182000085097
Sanil NK, Vikas PA, Ratheesh TB, George KC, Vijayan KK (2009) Mortalities caused by the crustacean isopod, Cirolana fluviatilis, in tropical, cage-cultured Asian seabass, Lates calcarifer: a case study from the southwest coast of India. Aquac Res 40:1626–1633. https://doi.org/10.1111/j.1365-2109.2009.02263.x
Thamban AP, Kappalli S, Kottarathil HA, Gopinathan A, Paul TJ (2015) Cymothoa frontalis, a cymothoid isopod parasitizing the belonid fish Strongylura strongylura from the Malabar Coast (Kerala, India): redescription, description, prevalence and life cycle. Zool Stud. https://doi.org/10.1186/s40555-015-0118-7
Tolba MR, Holdich DM (1981) The effect of water quality on the size and fecundity of Asellus aquaticus (crustacea:isopoda). 1:101–112
Trilles J-P, Ravichandran S, Rameshkumar G (2011) A checklist of the Cymothoidae (Crustacea, Isopoda) recorded from Indian fishes. Acta Parasitologica. https://doi.org/10.2478/s11686-011-0077-z
Williams JD, Boyko CB (2012) The global diversity of parasitic isopods associated with crustacean hosts (Isopoda: Bopyroidea and Cryptoniscoidea). PLoS ONE 7:e35350. https://doi.org/10.1371/journal.pone.0035350
WoRMS - World Register of Marine Species (2020). http://www.marinespecies.org/. Accessed 4 May 2020
Yamaguti S (1985) Parasitic copepod and branchiura of fishes. International books and periodicals supply service, New Delhi
Yuniar AT, Palm HW, Walter T (2007) Crustacean fish parasites from Segara Anakan Lagoon, Java, Indonesia. Parasitol Res 100:1193–1204. https://doi.org/10.1007/s00436-006-0391-9
Acknowledgement
Authors’ gratefully acknowledge Dr Santhosh B (Principal scientist, CMFRI Trivandrum) and Mrs Nikhila Reshmi (Research scholar, Dept. of Animal Science, Central University of Kerala) for the identification of copepod species. We thank Mr Arun Kumar K (Research Scholar, Dept. of Biochemistry and Molecular Biology) for his inputs in the statistical analysis of the data as well as Dr Priya Jose (Post-doctoral fellow, Dept. of Animal Science) for her valuable comments and corrections.
Funding
Department of Science and Technology of India (sanction order No. EMR/2016/001163), Kerala State Council for Science Technology and Environment (sanction order No. KSCSTE/5224/2017-SRSLS, (T) 093/SRS/2011/CSTE).
Author information
Authors and Affiliations
Contributions
SK initiated the study and supervised. TSN, MR and SCP collected and entered the data. SK & TSN analysed and interpreted the data and were involved in writing the manuscript. All authors read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or personal conflict that may have inappropriately influenced them in writing this article.
Ethics approval
No ethical approval required for the present study.
Research involving human participants and/or animals
This study does not include human participants and the parasitic crustaceans involved in this study doesn’t require any ethical approval.
Informed consent
Not applicable.
Consent to participate
All the co-authors have given their consent to participate in the study.
Consent for publication
All the co-authors have given their consent to publish the data of the study.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nizar, T.S., Raveendran, M., Chenkayi Parambil, S. et al. The occurrence of parasitic copepods and isopods infesting the marine teleost fishes of Kerala coast, India. J Parasit Dis 45, 78–88 (2021). https://doi.org/10.1007/s12639-020-01268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01268-8