Abstract
Hydatid cysts formed by the metacestodes of Echinococcus granulosus. Cattle suffering from hydatid cyst shows fluid-filled structures, especially in liver. These parasite-induced cysts localized by forming fibrous capsules in the liver. Fibrogenesis is the host immune response in the liver against these parasites. Hepatic stellate cells (HSCs) are localized perisinusoidal space also known as vitamin A-storing cells, characterize the important fibrogenic cell type. In this study, livers from 15 animals with hydatid cyst and 8 healthy animals were used. Hematoxylin and Eosin, masson trichrome staining were performed on the prepared liver sections. Microscopically, cysts were bordered eosinophilic necrotic debris blended with degenerate neutrophils, macrophages, eosinophils, lymphocytes, plasma cells and multinucleated giant cells, which extend into the adjacent fibrous connective tissue. In Masson trichrome staining, the fibrous connective tissue was observed surrounding of hydatid cyst. Glial fibrillary acidic protein (GFAP), collagen I, GFAP/collagen I, positive cells were investigated using either indirect single- or double-labeling immunohistochemical staining. The results indicated that anti-GFAP-positive staining was seen in areas including fibrous tissue just under the foreign body giant cells surrounding the cyst wall. In double immunohistochemical staining, it was observed that HSCs labeled with anti-GFAP antibody in the fibrous connective tissue also labeled anti-collagen I antibody. This study shows that HSCs may responsible for synthesis the collagen I in the development of parasitic fibrosis in cystic echinococcosis in the liver of cattle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis is a disease of some animal species including cattle that affects humans caused by larval stage of Echinococcus granulosus, which called as hydatid cyst (Yildiz and Tunçer 2005). The main macroscopic findings are generated the hydatid cysts. They are responsible for the disease symptoms. The hydatid cysts are slow growing fluid-filled structures. They contain protoscoleces and are frequently placed in the liver or lungs. Hydatid cysts develop slowly in the tissues that can disturb the function of the tissues (Eckert et al. 2001; Uzal et al. 2015).
Fibrosis; is a pathological finding manifested by abnormal and ongoing wound healing process defined in many important parasitic diseases. Hydatid cysts formed by the metacestodes of E. granulosus, also Schistosoma mansoni and Schistosoma japonicum eggs are localized by forming fibrous capsules in the liver. The host immune response in the liver is fibrogenesis against these parasites (Ross et al. 2002). The fibrosis formed in the liver is a barrier with a peri-parasitic extracellular matrix and tries to prevent further growth of the parasite. This situation also negatively affects drug and drug interactions (Ross et al. 2002; Vuitton 2003). Liver fibrosis is characterized by imbalance between the extracellular matrix components and the degradation of the matrix metalloproteases (Friedman 2003). This is; resulting from the complex interaction between hepatocytes, Kupffer cells and hepatic stellate cells (HSCs). HSCs; the mesenchymal cells, formerly called the Ito cell, are located in the perisinusoidal space and store vitamins A (Friedman 2003). As a result of stimulation, HSCs are transformed from vitamin A storage cells into myofibroblast phenotypic cells (Friedman 2008) and they represent the key fibrogenic cell type (Mederacke et al. 2013).
In the healthy liver, extracellular matrix components (EMCs) produced at physiological limits by inactive HSCs. They undergo transformation into proliferation after the liver damage. Also, pass into fibrogenic and myofibroblast forms that generate enormous EMCs (Iredale 2003).
In the chronic liver diseases, inflammatory processes end in disproportion between EMCs’ production and degradation, favoring their stabilization via enzymatic bonding (Ricard-Blum et al. 1996). In murine alveolar echinococcosis, collagen type I joins the late phase of infection, but pro-collagen type III joins in the initial phase of infection. However, in the experimental study of Schistosoma mansoni in mice, type I collagen prevails in the initial phase of infection (Olds et al. 1985). In Schistosoma japonicum infection, Bartley et al. (2006) showed a contributive function for activated HSCs in the dynamics of egg-induced fibrosis.
To our knowledge, the role of HSC’s collagen production and HSCs localization in cattle liver infected with hydatid cyst has not been investigated before. In this study, HSCs were investigated whether a possible role in producing collagen in the development of fibrous tissue in cattle infected with cystic echinococcosis by immunohistochemistry.
Materials and methods
Sample collecting and tissue processing
The liver samples were obtained from the slaughterhouse in Kirikkale province. In the slaughterhouse visits made at regular intervals, samples were taken from healthy liver and the infected liver which were detected with hydatid cyst macroscopically after the slaughter. A total of 23 cattle liver samples were used, 15 were with hydatid cyst and 8 were healthy liver. Samples were fixed for 48–72 h in the 4% buffered Paraformaldehyde solution. After the fixation, tissues were routinely processed to paraffin sections. 4–5 µm thickness sections taken from each liver with a microtome and stored for histopathological and single and double immunohistochemical staining.
Macroscopy and histopathology
Macroscopic photos were taken from the liver samples. Hematoxylin and eosin (H&E), Masson Trichrome staining were performed on the prepared sections. Digital microphotographs were captured with a DP25 camera attached Olympus BX51 microscope (Japan).
Antibodies
Details of antibodies used in this study were given in Table 1.
GFAP and collagen I immunohistochemistry
All immunohistochemical tests were performed using a streptavidin–biotin kit (Thermo Fisher Scientific). Diaminobenzidine (DAB) chromogen was used for labeling, and Mayer’s hematoxylin was used for background staining. Normal mouse serum was used in negative control staining procedure. After deparaffinization step, endogenous peroxidase blocking process was done with 3% hydrogen peroxide in methyl alcohol for 9–10 min. Antigen retrieval was done using Tris-buffered saline (TBS, pH 6.0) in a pressure cooker for 30 min. Then, the protein blocking step was carried out for 8 min. After then, the slides were treated with primary antibody (GFAP and collagen I) for 1 h, after, 30 min secondary antibody step and 30 min streptavidin step was done. Slides were washed lightly twice with PBS for 5 min in every step. Sections were treated in a controlled under microscope with DAB for 10 min. Then slides were evaluated under light microscope after counterstained with Mayer’s hematoxylin.
GFAP/collagen I double immunohistochemistry
Co-localization of antibodies in this study (GFAP/collagen I) was showed with Double-labeling immunohistochemistry technique. Horseradish peroxidase (HRP) kit (Dako, Glostrup, Denmark) and an alkaline phosphatase kit (Lab Vision, Fremont, USA) were used. Antigen retrieval was done (same with Single-labeling immunohistochemistry) for 30 min and protein blocking step was carried out for 8 min. After then, the slides were treated with anti- GFAP antibody for overnight. Next day, the biotinylated polyvalent secondary antibody was added for 15 min, then streptavidin conjugated with HRP for 10 min. After a period of time, sections were treated in a controlled under a microscope with DAB for 15 min and were washed out with distilled water. The next step of co-localization was done for collagen I labeling. For this labeling, the washed out slides were incubated with collagen I antibody for 1 h and then were incubated with a biotinylated polyvalent anti-rabbit secondary antibody for 10 min followed by incubation with an alkaline phosphatase-conjugated anti-streptavidin antibody (Lab Vision, Fremont, USA) for 10 min. Then, the sections were treated with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT; ThermoScientific) for 30 min for color development. Brown and blue-purple colors were indicated the positive labelings. Counterstaining was not used at this stage.
Results
Macroscopy and histopathology
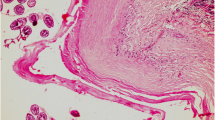
Macroscopically, liver containing hydatid cysts which are irregular oval to round, fluid-filled, gray-white colored areas were seen. In the sections of these regions, 4–7 cm diameter (Fig. 1A), the typical hydatid cysts with thick bands of fibrous connective tissue were determined.
A Macroscopic view of hydatid cysts in fixed liver section. B Microscopic view of hydatid cyst. Cyst wall (arrow) surrounded by multinucleated foreign body giant cells (arrowhead), the cyst is immediately surrounded by inflammation cells (a) and dense collagen (b). Hepatocytes have vacuolar degeneration and pyknosis (c). There is fibrosis, few inflammatory cells and hyperplastic bile ducts (d). Hydatid cyst space (asterisk). Hematoxylin- Eosin staining. Magnification: ×100. C Microscopic view of hyperplastic bile ducts (arrows). Hematoxylin- Eosin staining. Magnification: ×100. D Microscopic view of hydatid cyst. Cyst wall (arrow), hydatid cyst space (asterisk) and dense collagen (b), hyperplastic bile ducts (d). Masson Trichrome staining. Magnification: ×100
Hematoxylin and eosin staining; cysts were bordered eosinophilic necrotic debris blended with degenerate neutrophils, macrophages, eosinophils, lymphocytes, plasma cells and multinucleated giant cells, which extend into the adjacent fibrous connective tissue (Fig. 1B, C). It was noted that some cysts degenerated in the liver and the cyst structures were dispersed and caseous or calcified. The presence of degenerative and necrotic areas was found just outside the granulomatous tissue. In these areas, the liver parenchyma; in some hepatocytes, pycnotic findings were found with vacuolar degeneration. Periportal region is thickened with cellular growth. The biliary ductal reaction was seen (Fig. 1B, C). Definition of morphological diagnosis in all sampled animals was hydatid cyst, with multifocal fibrosis, necrotizing hepatitis and biliary hyperplasia, etiology consistent with E. granulosus.
In Masson trichrome staining, the fibrous connective tissue was observed surrounding of hydatid cyst. However, the hydatid cyst wall was also stained with the same color as the fibrous connective tissue (Fig. 1D)
Single- and double-labeling immunohistochemistry
Anti-GFAP immunopositivity was not detected in liver samples taken from healthy cattle. Livers taken from cattle with hydatid cysts; anti-GFAP-positive staining was seen in areas including fibrous tissue just under the foreign body giant cells surrounding the cyst wall (Fig. 2A, B).
A and B Anti-GFAP immunolabeling. Anti-GFAP immunopositive cells (brown) (arrows). Foreign body giant cells (arrow head). Hydatid cyst lumen (asteriks). Counter staining: hematoxylin. Magnification: ×200. C Anti-collagen I immunolabeling. Anti-collagen I immunopositive area (brown) (arrows). Hydatid cyst lumen (asteriks). Counter staining: hematoxylin. Magnification: ×200. D Anti-collagen I immunolabeling. Anti-collagen I immunopositive area (brown) (arrows) in dense collagen, periportal area. Counter staining: hematoxylin. Magnification: ×200
Anti-collagen I immunopositivity is not found in healthy animals; anti-collagen I immunopositivity was clearly observed in the liver of animals infected with hydatid cyst which are stained as collagen with H&E staining and Masson Trichrome staining. In addition, this positivity was also observed around the thickened periportal regions (Fig. 2C, D).
In double immunohistochemical staining, it was observed that HSCs labeled with anti-GFAP antibody in the fibrous connective tissue also labeled anti-collagen I (Fig. 3A–D).
A–D Anti- GFAP and anti- collagen I double immunolabeling. Anti-GFAP immunopositive cells (brown) express anti-collagen I (blue) in surrounded cyst and periportal area. Hydatid cyst lumen (asteriks). No counter staining. Magnification: ×100. E Anti-GFAP immunolabeling in healthy animal, no positive immunolabeling except for a few cells. Counter staining: hematoxylin. Magnification: ×100. F Anti- collagen I immunolabeling, no positive immunolabeling. Counter staining: hematoxylin. Magnification: ×100
Discussion
To our knowledge, the main character of granulomatous inflammation, which is dominant in the lesions formed by the E. granulosus in the cattle liver, was shown in this study for the first time that connective tissue elements were produced by HSCs placed perisinusoidal space in the liver.
HSCs are expressed by vimentin, desmin and alpha-sma in terms of morphological characteristics and mesenchymal cells. In addition, GFAP is expressed with nestin, neurotropin receptor and synaptophysin (Moreira 2007). It was demonstrated that GFAP, desmin and vimentin were positive for HSCs in cattle (Carollo et al. 2012). In this study, we used the GFAP antibody which was used before in our laboratory for another study to demonstrate the HSCs (Atmaca et al. 2013).
HSCs play a role in the production of collagen in the regenerative activities against liver damage (Friedman 2008). This clearly shows that it plays an important role in the development of fibrosis and the further stage of cirrhosis (Rastogi et al. 2012). Alveolar granulomatous inflammation and fibrosis in alveolar echinococcosis caused by E. multilocularis are important in the host against the growth of parasitic cysts (Guerret et al. 1998). However, HSCs play an important role in the synthesis of extracellular matrix components in the case of parasitic fibrosis and cirrhosis in bovine liver infected with Fasciola hepatica and Dicrocoelium dendriticum (Kukolj et al. 2009). Fascioloides magna infected livers in deers were reported that parasitic granulomatous lesions were surrounded by collagen I positive areas (Marinković et al. 2013). Similarly, granulomatous lesions in liver infected with Mesocestoides vogae in mice, were also surrounded by collagen I positive areas and these areas increased as the infection progressed (Hrčkova et al. 2010).
Cystic echinococcosis, a zoonotic disease that leads to remove of the liver parenchyma in the peri-parasitic area. Also, it leads fibrosis in portal spaces as a characteristic finding of the disease. Fibrosis allows the host to block the growth of the parasite. However, it also disrupts the liver tissue and may cause bile ducts and vessels to contract. Moreover, this condition has been shown to cause secondary biliary cirrhosis (Vuitton et al. 1986; Ricard-Blum et al. 1996).
Past experimental models of alveolar echinococcosis, have suggested that fibrosis progresses with an early deposition of type III collagen pro-peptide and type III collagen at the periphery of the granulomas, and subsequent remodeling of fibrosis with bundles of type I collagen in the periparasitic central area (Guerret et al. 1998). This study, on the other hand, has determined collagen type I expression in the liver that is infected with echinococcosis, especially around the parasitic cysts. Furthermore, in collagen positive fibrous area, GFAP positive HSCs were observed.
In the present study, it was clear that collagen I positive areas were present in the fibrous tissue around the hydatid cysts. Therefore, HSCs were determined by GFAP immunohistochemistry for the determination of the cells responsible for the produce of the collagen. HSCs were clearly observed in these collagen positive areas. Activated HSCs via Kupffer cells, triggered the conversion to myofibroblasts and eventually followed in collagen synthesis resulting fibrogenic immune response on the host is observed (De Minicis et al. 2007; Pradere et al. 2013). Myofibroblasts that derived from HSCs have been detected in echinococcosis in the liver in humans (Vuitton et al. 1986). These cells also have been demonstrated in experimental alveolar echinococcosis model in mice (Guerret et al. 1998).
It has been evidenced that there is a complex interaction between various cell types including hepatocytes, Kupffer cells, epithelial cells, SECs, platelets, neutrophils and HSCs in the liver. A subset of mediators with different effects on HSCs will be secreted by each of these cell types (Moreira 2007). Whether HSCs release mediators to decrease or increase or suppress the collagen production is still a matter of interest.
Further studies to be undertaken to better understand liver diseases of different etiologies and stages through immunohistochemical markers and molecular studies of HSCs are required to approve these initial observations. Future studies in this direction will also be helpful in elucidating liver injury and repair mechanisms as well as improving therapeutic interventions in liver diseases associated with cystic echinococcosis.
References
Atmaca H, Gazyagcı A, Canpolat S, Kul O (2013) Hepatic stellate cells increase in toxoplasma gondii infection in mice. Parasit Vectors 6:135. https://doi.org/10.1186/1756-3305-6-135
Bartley PB, Ramm GA, Jones MK et al (2006) A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol 36:993–1001. https://doi.org/10.1016/J.IJPARA.2006.04.015
Carollo V, Di Giancamillo A, Vitari F et al (2012) Immunohistochemical aspects of Ito and Kupffer cells in the liver of domesticated and wild ruminants. Open J Vet Med 02:129–136. https://doi.org/10.4236/ojvm.2012.23022
De Minicis S, Seki E, Uchinami H et al (2007) Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132:1937–1946. https://doi.org/10.1053/j.gastro.2007.02.033
Eckert J, International Office of Epizootics., World Health Organization (2001) WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris
Friedman SL (2003) Liver fibrosis—from bench to bedside. J Hepatol 38(Suppl 1):S38–S53
Friedman SL (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669. https://doi.org/10.1053/j.gastro.2008.03.003
Guerret S, Vuitton DA, Liance M et al (1998) Echinococcus multilocularis: relationship between susceptibility/resistance and liver fibrogenesis in experimental mice. Parasitol Res 84:657–667
Hrčkova G, Velebný S, Solár P (2010) Dynamics of hepatic stellate cells, collagen types I and III synthesis and gene expression of selected cytokines during hepatic fibrogenesis following Mesocestoides vogae (Cestoda) infection in mice. Int J Parasitol 40:163–174. https://doi.org/10.1016/j.ijpara.2009.06.008
Iredale JP (2003) Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ 327:143–147. https://doi.org/10.1136/bmj.327.7407.143
Kukolj V, Aleksić-Kovačević S, Jovanovic M, Knežević M (2009) Distribution of stellate cells in cattle liver with parasitic fibrosis and cirrhosis. J Comp Pathol 141:295. https://doi.org/10.1016/j.jcpa.2009.08.070
Marinković D, Kukolj V, Aleksić-Kovačević S et al (2013) The role of hepatic myofibroblasts in liver cirrhosis in fallow deer (Dama dama) naturally infected with giant liver fluke (Fascioloides magna). BMC Vet Res 9:45. https://doi.org/10.1186/1746-6148-9-45
Mederacke I, Hsu CC, Troeger JS et al (2013) Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 1:1. https://doi.org/10.1038/ncomms3823
Moreira RK (2007) Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med 131:1728–1734. https://doi.org/10.1043/1543-2165(2007)131%5b1728:HSCALF%5d2.0.CO;2
Olds GR, Griffin A, Kresina TF (1985) Dynamics of collagen accumulation and polymorphism in murine Schistosoma japonicum. Gastroenterology 89:617–624. https://doi.org/10.1016/0016-5085(85)90459-7
Pradere JP, Kluwe J, De Minicis S et al (2013) Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. https://doi.org/10.1002/hep.26429
Rastogi A, Bihari C, Maiwall R et al (2012) Hepatic stellate cells are involved in the pathogenesis of acute-on-chronic liver failure (ACLF). Virchows Arch 461:393–398. https://doi.org/10.1007/s00428-012-1291-2
Ricard-Blum S, Bresson-Hadni S, Guerret S et al (1996) Mechanism of collagen network stabilization in human irreversible granulomatous liver fibrosis. Gastroenterology 111:172–182
Ross AGP, Bartley PB, Sleigh AC et al (2002) Schistosomiasis. N Engl J Med 346:1212–1220. https://doi.org/10.1056/NEJMra012396
Uzal FA, Plattner BL, Hostetter JM (2015) Alimentary system. In: Maxie MG (ed) Jubb, Kennedy & Palmer's pathology of domestic animals, 6th edn. Saunders Ltd., Edingurgh, pp 1.e2–257.e2
Vuitton DA (2003) The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop 85:119–132
Vuitton DA, Guerret-Stocker S, Carbillet JP et al (1986) Collagen immunotyping of the hepatic fibrosis in human alveolar echinococcosis. Z Parasitenkd 72:97–104
Yildiz K, Tunçer C (2005) Prevalence of hydatid cysts in cattle in the province of Kirikkale. Turk Parazitol Derg 29:247–250
Author information
Authors and Affiliations
Contributions
HTA, study conception, analysis of results and manuscript writing. ANG, OST and TS, sample preparation, macroscopic and microscopic analysis, and manuscript writing. All authors have participated actively in the study and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atmaca, H.T., Gazyagci, A.N., Terzi, O.S. et al. Role of stellate cells in hepatic echinococcosis in cattle. J Parasit Dis 43, 576–582 (2019). https://doi.org/10.1007/s12639-019-01129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-019-01129-z