Abstract
A total of 2100 sheep, slaughtered or spontaneously dead, from various areas of Kashmir valley were screened for the presence of hydatidosis. Out of 2100 cases, 85 were positive for hydatidosis. Histopathologicallly, the cyst wall consisted of the inner germinal, middle lamellated, and outer fibrous layer. Inflammatory reaction around the cyst was variable and was characterized by an inner zone of loosely arranged fibroblasts infiltrated with mononuclear cells and densely packed fibroblasts mixed with mononuclear cells; and an outer layer of concentrically packed fibrous connective tissue. Mast cell reaction was observed mainly in the bronchiolar, peribronchiolar, and inflammatory zones in the lungs; while in the liver, mast cells were noted in portal triads and bile ducts and were least evident in the vicinity of the cysts. Qualitative increase was observed for acid mucopolysaccharides in fibroblasts, inflammatory cells, and bronchial epithelium, especially hyperplastic epithelial cells. Masson’s trichrome revealed intense formation of collagen fibers in the pericystic connective tissue. The calcareous corpuscles of the protoscolices were distinct when stained with combined Alcian blue PAS and toluidine stains but were not visible when stained with H & E and Masson’s trichrome stains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis (CE) is a zoonotic infection which is caused by the larval forms (metacestodes) of a tapeworm Echinococcus, of which the adults are found in the small intestines of carnivorous animals (Endalew and Nurradis 2013; Ahmad et al. 2011). Humans are infected accidently and are not a part of the natural life cycle of parasite. The pathogenicity of CE highly depends upon the severity of the infection and the organ infected (Kebede et al. 2009; Ould et al. 2010). Echinococcosis has been declared as a neglected zoonosis subgroup in its recent strategic plans for the regulation of tropical diseases that are neglected by the WHO (Da-Silva 2010). Echinococcus granulosus is still an important and challenging medical problem and endemic in many countries (Young 2005).
The hydatid cyst has two layers which are parasite derived; an inner germinal layer which is nucleated and an outer acellular laminated layer which is then surrounded by a host-produced fibrous capsule. Nevertheless, cysts can cause an irreversible damage to the organs, and rupture or puncture of the cyst can infect several organs with their larvae and hence cause the anaphylactic reactions. The animals are sacrificed even before the cysts become mature enough to show various clinical signs, but when their entrails are given to the dogs, it continues the cycle. Animals having a long lifespan, such as horses, become ill.
Usually, CE may develop silently over years and even decades until it surfaces with clinical signs. Clinical signs are mainly related to the localization, size, and number of cysts (Torgerson 2003). Echinococcosis is diagnosed by different ways using X-ray, CT scan, and immunological and serological tests including modern diagnostic technique, i.e., polymerase chain reaction (PCR). Larval of forms of E. granulosus can usually be detected visually in organs. Special care has to be taken for specific diagnosis of E. granulosus in instances where Taenia hydatigena in sheep is also a problem. Microscopic examination of the tissue may confirm the diagnosis, i.e., differential diagnosis from other cysts and is helpful in highlighting the structural details of cysts and protoscolex. The presence of a periodic-acid-Schiff positive, acellular laminated layer with or without an internal cellular, nucleated germinal membrane can be regarded as a specific characteristic of metacestodes of Echinococcus. The present study was envisaged to study histopathological and histochemical changes associated with cystic echinococcosis in sheep.
Materials and methods
Study material
The present study was conducted from the year 2013–2016 on sheep, including both slaughtered and spontaneously dead, from local sheep farms, local abattoirs, and local butcher shops in different regions of Kashmir valley.
Histopathology
Representative tissue samples associated with hydatid cyst in different organs were collected and preserved in 10% formalin. These tissues were routinely processed through ascending grades of alcohol, cleared in benzene, and embedded in paraffin wax. The paraffin sections were cut at 4 to 5 μ thickness and stained by hematoxylin and eosin (H & E) method (Luna 1968).
Histochemistry
Parallel tissue sections, on the basis of histological examination, were selected and then stained for neutral or acid mucopolysaccharide by PAS technique using combined Alcian blue (Bancroft and Gamble 2002), connective tissue by Masson’s trichrome stain (Luna 1968), and mast cells by toluidine blue stain (Humason 1979).
Results
Histopathology
Lung
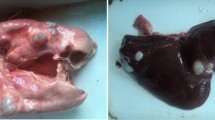
Out of total 2100 heads screened, only 85 animals showed one or more cysts in lungs and livers. The cysts were observed to have a thin inner germinal layer, a laminated layer eosinophilic in nature, and outer adventitial layer. Parasitic membranes (laminated membranes and germinal layers) were obvious in most examined sections, some were continuous and intact containing brood capsules with protoscolices (Fig. 1). Laminated membranes varied with respect to thickness and number of laminations. The inflammatory reaction, consisting of eosinophils, mononuclear cells immediate to the cyst layer and often extended into the surrounding alveoli and even to the adjacent terminal and small bronchioles. The laminated cyst wall was sometimes surrounded immediately by macrophage cell layer. The macrophages resembled the Langhan’s type giant cells. Alveolar emphysema was seen adjacent to atelectic alveoli in the vicinity of the cysts. Peribronchiolar lymphoid hyperplasia and bronchiolar epithelial hyperplasia were the additional findings.
Liver
The histological picture of the hydatid cyst in liver resembled to that in the lung. The hepatocytes revealed severe degenerative changes and pyknotic nuclei. Biliary hyperplasia and degenerative changes in biliary epithelium along with inflammatory cell infiltration was observed in the liver of some of the affected with hydatidosis.
Histochemistry
Lung
Combined PAS Alcian blue staining revealed that the laminated membranes, germinal layers, and brood capsules took very deep magenta color positive for neutral mucopolysaccharides; however, adventitia showed areas of positivity for acid mucopolysaccharides (Fig. 2). Protoscolices also showed positive specks of acid mucopolysaccharides corresponding to the areas of calcareous corpuscles. Intense acid mucopolysaccharide activity was noted sometimes in the inflammatory reactive area around the laminated membranes. Strong acid mucopolysaccharide activity was also evident in the macrophage layer immediate to laminated membrane.
Toluidine blue stained sections revealed presence of numerous mast cells in the fibro-cellular reaction adjacent to the cyst wall. The mast cells revealed metachromatically stained granules both within and outside the cells adjacent to these cells (Fig. 3). Strong mast cell response was also noted in the alveolar parenchyma of the hydatid cyst affected lungs. Lung sections stained with Masson’s trichrome method revealed intense formation of collagen fibers in the pericystic connective tissue reaction, while the laminated membrane appeared as acellular layers with slightly gray color, whereas germinal layers, brood capsules, and protoscolices were red in color (Fig. 4).
Section of the sheep lung affected with hydatidosis revealing marked formation of collagen fibers in the pericystic connective tissue reaction while the laminated membrane appeared acellular layers with gray color, whereas germinal layers, brood capsules, and protoscolices stained red in color. Masson’s trichrome—Original magnification x 100X
Liver
Combined Alcian blue PAS staining revealed areas of acid mucopolysaccharide in the adventitial area and inflammatory areas around the cysts. Wherever the cyst membrane had collapsed and the inflammatory cells had been trapped in between the cyst wall, it showed positivity for acid mucopolysaccharides. The associated bile ductules in the vicinity of the cysts revealed strong positivity for acid mucopolysaccharides.
Toluidine blue staining revealed presence of mast cells in the inflammatory areas of the liver adjacent to the hydatid cysts. Mast cell reaction was also evident in the portal triads of such livers.
Masson’s trichrome staining revealed extensive fibrosis around cyst wall which had even encroached the surrounding parenchyma (Fig. 5). In some cases, the laying down of collagen around the periphery of cyst was not uniformly visible.
Discussion
Histopathology
In the present study, the cyst wall in lung presented the characteristic trilaminar structure with germinal membranes and brood capsules, and free scolex as reported earlier by (Mitrea 1998; Thompson and Lymbery 1988; Ibrahim and Gameel 2014). Solcan et al. (2010) observed that in case of lungs, hydatid cyst wall, from inside to outside was composed of endocyst (proligerous membrane), ectocyst (laminated membrane), and pericyst. Sakamoto and Gutierrez (2005) reported that many granulocytes, mainly derived from infiltrating eosinophils, were in the border zone between the laminated and adventitial layers and the adventitial layer surrounding the echinococcal cyst comprised an anuclear fibrous zone and a connective tissue zone and hemorrhagic foci and congestion was in the border zone between the adventitia and the lung tissue. Histopathological picture of the cysts in the liver was similar to that observed in the lungs (Anwar et al. 1999; Rashed et al. 2004; Verma and Swamy 2009; Barnes et al. 2011; Kul and Yildiz 2010; Ibrahim and Gameel 2014).
Histochemistry
The acellular laminar layer is a carbohydrate-protein complex with galactose, galactosamine, and glucosamine as the principal component of the polysaccharide portion (Kilejian and Schwabe 1971). This layer is not present in young cysts until, i.e., below 14–18 days old, it later appears as a thin, clear layer on its outer margin (Taherkhani 2001). The germinal layer consists of distal cytoplasmic syncytium and a perinuclear layer containing tegument, glycogen, undifferentiated cells (Lascano et al. 1975; Thompson and Lymbery 1988; Gupta et al. 2014). In the present study, generally, laminated membranes, germinal layers, brood capsules, and major part of protoscolices in lung tissue took very deep magenta color, positive for neutral mucopolysaccharide. The actively proliferating cells like fibroblast cells, inflammatory cells, and bronchial epithelium, especially hyperplastic cells showed qualitative increased in acid mucopolysaccharide. These findings were in consonance with that of Lupu et al. (1959) who reported significant increase of acid mucopolysaccharides in alveolar macrophages in response to fibrogenic substances. Kilejian et al. (1962) reported that the laminated layer of the hydatid cyst stains strongly by Schiff’s reagent (PAS) and that is an excellent diagnostic marker in various histological studies. Rashed et al. (2004) revealed that the cystic wall consisted of three layers which were germinal, laminated, and fibrous layer, respectively. The glycogen and mucopolysaccharide content increases in these layers in infected sheep. Morseth (1967) observed that the components of the brood capsule are positive for PAS following their extraction using amylase. Qualitative increase in both acid and neutral mucopolysaccharides in and around the lesions may be attributed to inflammatory process (Ibrahim and Gameel 2014).The pericytic area showed a linear area of acid mucopolysaccharides which is in agreement to Solcan et al. (2010) who reported that between the pericyst and the ectocyst, there was a space through which tissue fluid and nutrient medium flows. The space between the pericyst and the ectocyst was the place where precipitation of neutral and acid polysaccharide was observed, especially in sheep.
Masson’s trichrome staining demonstrated collagen fibers in cyst wall in both lung and liver sections. Rashed et al. (2004) and Ibrahim and Gameel (2014) also demonstrated the fibrous tissue associated with hydatidosis in sheep. The occurrence of collagen reflects the inflammatory response to persistent irritation which may be ascribed to the consistent, slow exosmosis occurring in the hydatid cyst. This is essentially the basic protective response aimed at containment of the parasite which leads to formation of cyst wall. As already mentioned, the pronounced fibrosis and cirrhosis observed in certain cases may be due to the immunological reactions of the host tissue (Anwar 1997).
Occurrence of mast cell reaction predominantly in the vicinity of the developing cysts, peribronchial and peribronchiolar regions of lungs, was an indication of local type1 hypersensitivity and has also been frequently observed in other parasitic infections of lung (Darzi et al. 2003). Mast cells have a significant role in a myriad of inflammatory diseases and are also involved in remodeling of the tissues. Mast cell activation is triggered by tissue hypoxia which in turn releases various proteolytic enzymes, angiogenic factors, and growth factors which in turn mediate the tissue destruction and its remodeling in a variety of physiological as well as pathological conditions (Maxova et al. 2012). However, in case of hydatidosis, whether this reaction occurs primarily against migrating oncosphere or as a result of exudation of hydatid fluid from enlarging cyst remains to be determined. The hydatid fluid has been found to be highly antigenic leading to acute hypersensitivity reaction (Sanjay et al. 2003). Anaphylaxis following rupture of cyst has been frequently observed (Jakubowski and Barnard 1971). Mast cells secrete a huge amount of mediators having proteolytic, growth and proangiogenic effects and hence, they prove to be very important cell type involved in the pathogenesis of many diseases, from the bone marrow-derived cells (Pejler et al. 2010). Tucker et al. (1977) determined that mast cells are significantly altered, i.e., their lung density affected in pigs, rats, and sheep under chronically hypoxic conditions. In our study, metachromatic areas and mast cells accumulation was demonstrated in and around fibroplastic areas around the cyst. Migally et al. (1983) observed significant proliferation of perivascular mast cells and their enhanced secretory activity under chronic cases. Mediators that are released from the mast cells regulate the tissue remodeling thereby may also contribute to pulmonary fibroplasia. Wygrecka et al. (2013) investigated the role of interaction between mast cells and fibroblasts during the progression of lung fibrosis and observed an increased load of active mast cells near the fibroblast foci and alveolar type II cells. Veerappan et al. (2013) identified two mast cell mediators as fibrogenic: histamine and renin, via angiotensin (ANG II). Both human as well as rat lung fibroblasts express histamine H1 and renin receptor subtypes in activated condition; they promote transforming growth factor β 1 (TGF-β1) secretion, proliferation, and the collagen synthesis.
References
Ahmad ME, Rahim MA, Fatima MA (2011) Hydatid disease, a morbid drop needs awareness. Sudan Med J 47:1
Anwar Z (1997) The effect of echinococcosis on rabbit and sheep along with its control by indigenous plants of Pakistan. Ph.D. thesis. Department of Zoology, university of Punjab, Lahore
Anwar Z, Tanveer A, Bashir S (1999) Echinococcus granulosus: histopathology of naturally infected sheep liver. Punjab Univ. J Zool 14:105–111
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th edn. Harcout Publishers Limited, London, pp 181–182
Barnes TS, Hinds LA, Jenkins DJ, Bielefeldt-Ohmann H, Lightowlers MW, Coleman GT (2011) Comparative pathology of pulmonary hydatid cysts in macropods and sheep. J Comp Pathol 144(2-3):113–122. https://doi.org/10.1016/j.jcpa.2010.07.003
Darzi MM, Mir MS, Khan M (2003) Concurrent anthracosis and parasitic pneumonia in a sheep. SKUAST-J Res 5:213–216
Da-Silva A (2010) Human echinococcosis: a neglected disease. Gastroenterol Res Pract 2010:1–9. https://doi.org/10.1155/2010/583297
Endalew D, Nurradis I (2013) Prevalence and economic importance of echinococcosis in cattle slaughtered at North Gonder Elfora Abattoir. Eur. J Appl Sci 5:29–35
Gupta V, Kaira V, Sharma J, Sen R, Sangwaiya A (2014) Primary hydatid cyst of spleen: a rare entity. J Trop Dis 2:2
Humason GL (1979) Staining cellular elements. In animal tissue techniques, fourth Edn. San Francisco, W.H. Freeman pp. 319-320. Indian J Anim Res 37(1): 57–58
Ibrahim SEA, Gameel AA (2014) Pathological, histochemical and immunohistochemical studies of lungs and livers of cattle and sheep infected with hydatid disease. The 5th annual conference-agricultural and veterinary research - February 2014, Khartoum, Sudan, Conference Proceedings 2: 1–17
Jakubowski MS, Barnard DE (1971) Anaphylactic shock during operation for hydatid disease. Anesthesiol 34(2):197–199. https://doi.org/10.1097/00000542-197102000-00029
Kebede N, Mitiku A, Tilahun G (2009) Echinococcosis of slaughtered animals in Bahir Dar Abattoir, Northwestern Ethiopia. Trop Anim Health Prod 41(1):43–50. https://doi.org/10.1007/s11250-008-9152-3
Kilejian A, Kenneth S, Schwabe CW (1962) Host-parasite relationships in echinococcosis, VIII. Infrared spectra and chemical composition of the hydatid cyst. Exp Parasitol 12: 377–392
Kilejian A, Schwabe CW (1971) Host-parasite relationships in echinococcosis, VIII. Infrared spectra and chemical composition of the hydatid cyst. Exp Parasitol 12:377–392
Kul O, Yildiz K (2010) Multivesicular cysts in cattle: characterisation of unusual hydatid cyst morphology caused by Echinococcus granulosus. Vet Parasitol 170(1-2):162–166. https://doi.org/10.1016/j.vetpar.2010.01.045
Lascano EF, Coltorti EA, Varela-Diaz VM (1975) Fine structure of the germinal membrane of Echinococcus granulosus cysts. The. J Parasitol 61(5):853–860. https://doi.org/10.2307/3279219
Luna LG (1968) Manual of histologic staining methods of the Armed Forces, Institute of Pathology, 3rd edn. Mc Graw Hill Book Company, New York
Lupu NG, Velican D, Velican C, Olinescu V (1959) The action exerted by pneumoconiotic factors upon the acid mucopolysaccharide contents of pulmonary macrophages. Brit J Indutr Med 16:244
Maxova H, Herget J, Vízek M (2012) Lung mast cells and hypoxic pulmonary hypertension. Physiol Res 61(1):1–11
Migally NB, Tucker A, Greenlees K, Wright M, Zambernard J (1983) Density and ultrastructure of mast cells in lung vessels of aging rats exposed to and recovering from chronic hypoxia. Cell Tissue Res 232(3):601–608. https://doi.org/10.1007/BF00216432
Mitrea IL (1998) Research regarding immunodiagnostics, immune response and immune prophylaxis in hydatidosis in ruminants [In Romanian]. PhD thesis, USAMV Bucharest, Romania
Morseth DJ (1967) Fine structure of the hydatid cyst and protoscolex of Echinococcus granulosus. J Parasitol 53(2):312–325. https://doi.org/10.2307/3276582
Ould CB, Schneegans F, Chollet JY, Jemli MH (2010) Prevalence and aspects of lesions of echinococcosis in camel in Northern Mauritania. Rev Elev Méd Vét Pays Trop 63:23–28
Pejler G, Ronnberg E, Waern I, Wernersson S (2010) Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115(24):4981–4990. https://doi.org/10.1182/blood-2010-01-257287
Rashed AA, Omer HM, Fouad MAH, Al-Shareef AMF (2004) The effect of severe cystic hydatidosis on the liver of a Najdi sheep with special reference to the cyst histology and histochemistry. J Egypt Soc Parasitol 34(1):297–304
Sakamoto T, Gutierrez C (2005) Pulmonary complications of cystic echinococcosis in children in Uruguay. Pathol Int 55(8):497–503. https://doi.org/10.1111/j.1440-1827.2005.01859.x
Sanjay OP, Tauro DI, Kilpadi K (2003) Intra-operative anaphylaxis caused by a pulmonary hydatid cyst. J Thor Cardiov Surg 19(2):128–129. https://doi.org/10.1007/s12055-003-0029-9
Solcan C, Solcan G, Ioniţă M, Hristescu DV, Mitrea IL (2010) Histological aspects of cystic echinococcosis in goats. Sci Parasitol 11(4):191–198
Taherkhani H (2001) Analysis of the Echinococcus granulosus laminated layer carbohydrates by Lectin blotting. Iran Biomed J 5(1):47–51
Thompson RCA, Lymbery AJ (1988) The nature, extent and significance of variation within the genus Echinococcus. Adv Parasitol 27:209–258. https://doi.org/10.1016/S0065-308X(08)60356-5
Torgerson PR (2003) The economic effects of echinococcosis. Acta Trop 85(2):113–118. https://doi.org/10.1016/S0001-706X(02)00228-0
Tucker A, Mcmurtry I, Alexander AF, Reeves JT, Grover RF (1977) Lung mast cell density and distribution in chronically hypoxic animals. J Appl Physiol 42(2):174–178
Veerappan A, O’Connor NJ, Brazin J, Reid AC, Jung A, McGee D, Summers B, Branch-Elliman D, Stiles B, Worgall S, Kaner RJ, Silver RB (2013) Mast cells: a pivotal role in pulmonary fibrosis. DNA Cell Bio 32(4):206–218. https://doi.org/10.1089/dna.2013.2005
Verma Y, Swamy M (2009) Prevalence and pathology of hydatidosis in buffalo liver. Buffalo Bull 28(4):207–211
Wygrecka M, Dahal BK, Kosanovic D, Petersen F, Taborski B, Gerlach S, Didiasova M, Zakrzewicz D, Preissner KT, Schermuly RT, Philipp M (2013) Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis role of transmembrane SCF and the PAR-2/PKC-a/Raf-1/p44/42 signaling pathway. Am J Pathol 182(6):2094–2108. https://doi.org/10.1016/j.ajpath.2013.02.013
Young ED (2005) Brucella species. In: Mandell, Douglos, Bennett, editors. Principles and practice of infectious disease, 6th edn. Churchill Livingstone, Pensylvania, pp 3290–3292
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest with themselves or with any other organization.
Ethical approval
During all stages of our research, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. In addition, this article does not contain any studies with human participants performed by any of the authors. There has been no significant financial support for this research work.
Rights and permissions
About this article
Cite this article
Beigh, A.B., Darzi, M.M., Bashir, S. et al. Pathological and histochemical studies of the effects of cystic echinococcosis in sheep. Comp Clin Pathol 27, 407–412 (2018). https://doi.org/10.1007/s00580-017-2606-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2606-0