Abstract
Purpose
It has been suggested that a larger heparin dose during cardiopulmonary bypass (CPB) is associated with reduced perioperative coagulopathy and thromboembolic complications. We investigated the effect of different heparin doses during routine elective cardiac surgery. Our primary outcomes include blood loss and transfusion and secondary outcomes investigate the effects on coagulation biomarkers.
Methods
In this prospective pilot trial, we allocated 60 patients undergoing cardiac surgery on CPB in a single tertiary cardiac centre into three groups to receive an initial dose of 300, 400, or 500 units (U) per kilogram of intravenous heparin prior to the commencement of CPB. Blood was sampled after induction of anesthesia, at 30 and 60 min of CPB, and three minutes after heparin reversal with protamine. Samples were analyzed for fibrinopeptide A (FPA), fibrinopeptide B (FPB), D-dimer, and thrombin-antithrombin (TAT) complexes. Postoperative blood loss and transfusion was measured for the first 24-hr period after surgery.
Results
The total mean (95% CI) administered heparin dose in the 300 U·kg−1, 400 U·kg−1, and 500 U·kg−1 groups were 39,975 (36,528 to 43,421) U, 43,195 (36,940 to 49,449) U and 47,900 (44,807 to 50,992) U, respectively. There were no statistically significant differences in FPA, FPB or D-dimer levels at the measured time intervals. There was a trend towards lower TAT levels while on CPB with greater heparin dosing, which was statistically significant after the administration of protamine. The clinical significance appears to be negligible, as there is no difference in overall blood loss and amount of packed red blood cell transfusion or other blood product transfusion.
Conclusion
This pilot study indicates that, while larger heparin dosing for routine cardiac surgery results in subtle biochemical changes in coagulation, there is no demonstrable benefit in postoperative blood loss or transfusion requirements.
Résumé
Objectif
Il a été suggéré qu’une dose plus élevée d’héparine pendant la circulation extracorporelle (CEC) serait associée à une réduction de la coagulopathie périopératoire et des complications thromboemboliques. Nous avons étudié l’effet de différentes doses d’héparine au cours d’une chirurgie cardiaque non urgente de routine. Nos critères d’évaluation principaux comprenaient la perte de sang et la transfusion, et les critères d’évaluation secondaires exploraient les effets sur les biomarqueurs de la coagulation.
Méthode
Dans cette étude pilote prospective, nous avons réparti 60 patient·es bénéficiant d’une chirurgie cardiaque sous CEC dans un seul centre cardiaque tertiaire en trois groupes à recevoir une dose initiale de 300, 400 ou 500 unités (U) par kilogramme d’héparine intraveineuse avant le début de la CEC. Le sang a été prélevé après l’induction de l’anesthésie, à 30 et 60 minutes de CEC, et trois minutes après la neutralisation de l’héparine avec la protamine. Les échantillons ont été analysés pour les complexes fibrinopeptide A (FPA), fibrinopeptide B (FPB), D-dimère et thrombine-antithrombine (TAT). La perte de sang postopératoire et la transfusion ont été mesurées pendant la première période de 24 heures après la chirurgie.
Résultats
La dose moyenne totale (IC 95 %) d’héparine administrée dans les 300 U·kg−1, 400 U·kg−1, et 500 U·kg−1 était de 39 975 (36 528 à 43 421) U, 43 195 (36 940 à 49 449) U et 47 900 (44 807 à 50 992) U, respectivement. Il n’y avait aucune différence statistiquement significative dans les taux de FPA, FPB ou D-dimères aux intervalles de temps mesurés. Une tendance à des niveaux de TAT plus bas pendant la CEC a été observée avec une dose d’héparine plus élevée, ce qui était statistiquement significatif après l’administration de protamine. La signification clinique semble négligeable, car il n’y a pas de différence dans la perte de sang globale et la quantité de transfusion de concentrés globulaires ou d’autres produits sanguins.
Conclusion
Cette étude pilote indique que, bien qu’une dose plus importante d’héparine pour la chirurgie cardiaque de routine entraîne des changements biochimiques subtils dans la coagulation, il n’y a aucun avantage démontrable en matière de saignement postopératoire ou de besoins transfusionnels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bleeding after cardiac surgery is a serious complication associated with significant morbidity and mortality.1 Despite advances in modern circuit design, cardiopulmonary bypass (CPB)-associated coagulopathy is considered to be a significant contributor to perioperative blood loss after cardiac surgery.2,3,4 Reliable suppression of the coagulation system is mandatory in preparation for and throughout CPB. Thromboembolic events during CPB are often fatal or lead to significant morbidity. Unfractionated heparin is universally the most used anticoagulant during CPB, but, despite its widespread use, optimal dosing of heparin remains contentious.5 There is no clear consensus regarding the ideal dose of heparin or the target concentration for safe conduct of CPB. Although highly unspecific, the activated clotting time (ACT) is the most commonly used test to assess adequacy of anticoagulation for CPB.6 Global practice favours an initial fixed weight-based dose of 250 up to 500 units (U) per kilogram of unfractionated heparin, targeting an ACT of 450 to 550 sec.6 After repeat sampling, further adjustments in heparin doses are guided by the measured ACT.
Studies have suggested a paradoxical trend towards lower bleeding and rate of transfusion when larger heparin dosing strategies were used for CPB.7,8 A greater heparin dose is thought to potentially reduce activation of contact factor, consumption of downstream clotting factors (IX, X, XI, XII),9 thrombin activation, platelet aggregation, and fibrinogen depletion, thereby reducing coagulopathy after CPB by preserving the integrity of the coagulation system. During CPB, the ACT is typically measured every 20 to 30 min. Greater upfront dosing of heparin can yield more stable and consistent “safe range” ACTs, making fewer additional bolus doses on CPB necessary. This may avoid critical troughs in the levels of anticoagulation with low ACT readings in the interval between two measurements, during which there can be periods of potentially significant activation of the coagulation cascade before additional heparin can be administered. Early markers of coagulation activation, such as thrombin-antithrombin complexes, D-dimer, and fibrinopeptides can detect such subclinical activation.2
No data about the presence of early markers of coagulation at different doses of heparin exist. We conducted this pilot as a feasibility study for a potential large-scale randomized control trial investigating different heparin dosing regimens for safe conduct of CPB. We hypothesized that a larger upfront heparin dose will lead to lower postoperative bleeding and transfusion and will be more reliable at suppressing activation of the coagulation cascade.
Methods
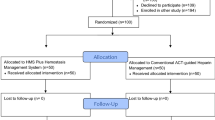
We performed a prospective, open-labelled, cohort pilot study involving patients undergoing uncomplicated open-heart surgery at the Royal Papworth Hospital, a specialized cardiothoracic centre in the UK. A data analysis and statistical plan was written and filed with the Royal Papworth Hospital Tissue Bank committee, which authorizes ethical approval for studies involving tissue and/or blood samples obtained during surgical procedures. Ethics approval was granted by the Cambridge Research Ethics Committee (18/EE/0269) and Royal Papworth Hospital Tissue Bank committee. All patients gave written informed consent prior to participating.
Study population
Between December 2019 and November 2020, 60 patients were included in this study. Exclusion criteria included age less than 18 yr, known or suspected blood dyscrasia, platelet dysfunction or coagulopathy, abnormal preoperative clotting screen, preoperative hemoglobin concentration less than 120 g·L−1, total body weight less than 45 kg or greater than 130 kg, end-stage renal failure (on dialysis), and previous thromboembolic events. Patient were also excluded if they were on unfractionated or low molecular weight heparin until surgery or did not cease P2Y12 antagonist, vitamin K antagonist, or direct oral anticoagulant treatment within seven, five, and three days respectively. Surgical exclusion factors encompassed emergency surgery, complex surgical requirements (anticipated CPB duration greater than two hours, redo sternotomy, aortic arch surgery, solid organ transplantation, pulmonary thromboendarterectomy), and therapeutic hypothermia less than 32 ºC.
Study intervention and coagulation management
Sixty patients were allocated to one of three different groups and were administered an intravenous bolus dose of either 300 U·kg−1, 400 U·kg−1, or 500 U·kg−1 of heparin (heparin sodium, Fannin, UK) according to the consultant anesthesiologist’s usual heparin dosing preference. Patients were excluded at this point of the study if they failed to achieve our institution’s bypass threshold ACT (Hemochron™ Signature Elite, Werfen, Barcelona, Spain) of greater than 400 sec and required additional boluses of heparin before CPB was initiated. All patients in the study achieved this target ACT and none were excluded. Antifibrinolytic treatment with tranexamic acid (2 g) was given prior to heparinization. An additional 5,000 U of heparin was added to the CPB pump prime. The CPB circuit for all included cases comprised nonheparin-bonded LivaNova tubing and an Inspire 8F oxygenator (LivaNova PLC, London, UK).
Additional doses of 5,000–10,000 U of heparin were given in collaboration between the anesthesiologist and the perfusionist if the ACT dropped below 450 sec during CPB. While the anesthesiologist was aware of the heparin dose, the surgeon and the intensive care unit were blinded. Conduct of anesthesia was at the discretion of the treating anesthesiologist. Heparin reversal with protamine was standardized using the previously validated pharmacokinetic PRODOSE algorithmic mathematical model.10 Because the study was a pilot investigation, it was concluded when 20 patients were recruited to each group.
Blood sampling and analysis
Blood sampling was performed via the indwelling radial arterial catheter or directly from the circuit while on CPB. Samples were collected in 0.109 M sodium citrate tubes following induction of anesthesia, at 30 min and 60 min of CPB and three minutes after heparin reversal with protamine. They were immediately transferred to be centrifuged and stored for batch testing to maintain consistency. Once thawed, samples were analyzed for fibrinopeptide A (FPA), fibrinopeptide B (FPB), D-dimer, and thrombin-antithrombin (TAT). Fibrinopeptide A and FPB (MyBioSource, Inc., San Diego, California, USA) and TAT (Abcam, Cambridge, UK) were quantified using enzyme linked immunosorbent assays while D-dimer was measured using the latex agglutination method (Werfen, Bedford, USA). Postoperative bleeding was assessed using the anesthetic and intensive care unit electronic patient record system MetaVision (iMDsoft, Dusseldorf, Germany).
Primary and secondary outcomes
The primary outcomes for the study were total blood loss, measured by chest tube drainage, and blood product requirements, measured by the number of transfused allogenic packed red blood cells, fresh frozen plasma, platelets and cryoprecipitate transfused after separation from CPB until intensive care unit discharge. Postoperative transfusion was guided by our institutional transfusion protocol, based on laboratory hemoglobin < 80 g·L−1 and/or a platelet count of < 100 × 109 L−1 and viscoelastic testing with ROTEM (Werfen, Barcelona, Spain) based on EXTEM A5, FIBTEM A5, EXTEM CT, and HEPTEM CT, which is similar to other transfusion algorithms in the UK. Secondary outcomes included analysis of the quantities of heparin administered on CPB and statistical differences in plasma concentrations of FPA, FPB, D-dimer, and TAT at predefined intervals during CPB and after administration of protamine.
Statistical analysis
A power calculation was not possible because of the exploratory nature of the study and the absence of any previously published similar data. It was therefore not possible to know the standardized effect size prior to conducting this pilot. We anticipated the main trial to be designed with 90% power and a two-sided significance of 5% and a small to medium effect size. In this case, the recommended pilot size was 15–25 per treatment arm11 and the group size of 20 was agreed after seeking statistician advice. Statistical analysis was performed using MDCalc (Ostend, Belgium) comprising Kolmogorov–Smirnov and Shapiro–Wilk tests, alongside histogram evaluation and values of skewness and kurtosis to assess distribution. Chi squared tests were used for group comparison of categorical data while normally distributed continuous variables were analyzed using Student t test or analysis of variance. The Kruskal–Wallis test was used for nonparametric continuous variables.
Results
Study population
Patient baseline characteristics were comparable among the three groups (Table 1). There were no significant differences in age, gender, body surface area, EuroSCORE II, and creatinine. The average duration of CPB and aortic cross clamp time were 99, 106, and 122 min and 72, 74, and 91 min respectively for the 300, 400, and 500 U·kg−1 heparin groups. None of these differences were statistically significant.
Primary endpoints
Blood loss and transfusion
There were no clinical or statistically significant differences in mean total blood loss between the 300 U·kg−1, 400 U·kg−1, and 500 U·kg−1 heparin groups (see Figure). We excluded one patient in the 500 U·kg−1 heparin group who lost 1,240 mL blood in the 24-hr postoperative period and who was re-explored in the operating room with a surgical cause of bleeding identified. Further analysis of transfusion records showed no significant differences in transfusion of red cells, fresh frozen plasma, platelets or cryoprecipitate in the first 24-hr postoperative period. None of the patients required further doses of protamine after admission to the intensive care unit. The results are summarized in Table 2.
Secondary endpoints
Heparin/protamine dosing and activated clotting time
Significantly greater doses of heparin were given before CPB in the largest heparin dosing group, which resulted in a reduced requirement for additional boluses of heparin on CPB (Table 3). A larger total quantity of heparin was administered throughout the operation in the 500 U·kg−1 group compared with the 400 U·kg−1 and 300 U·kg−1 heparin groups. The increasing doses of heparin were reflected in prolonged ACT measurements immediately after heparin administration and at 30 and 60 min of CPB. In analogy to the heparin doses, the increase in the protamine doses given after separation from CPB was statistically significant. The protamine/heparin ratio, however, was similar between the 300 U·kg−1, 400 U·kg−1, and 500 U·kg−1 groups. The results are summarized in Table 3.
Coagulation parameters
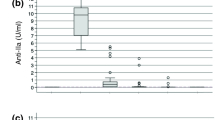
Baseline heparin activity, measured with anti Xa, was similar in all three groups. At 30 min of CPB, heparin activity was significantly higher in the 500 U·kg−1 group and evened out by 60 min of CPB and after protamine. There were no statistically significant differences between D-dimer, FPA, and FPB measurements at any measurement points. Although there was no statistically significant difference in TAT, FPA, and FPB measurements at baseline, there was a decrease in TAT at 30 and 60 min of CPB and a significant difference after cessation of CPB and protamine administration in the 500 U·kg−1 group. The results are summarized in Table 4. Thrombin-antithrombin after protamine was significantly lower in the 500 U·kg−1 group than in the other subgroups.
Discussion
Several studies have previously reported activation of the coagulation system during CPB.12,13 In this study, we investigated the relationship between different pre-CPB heparin doses and markers of early coagulation activation, postoperative blood loss and transfusion rates. Contact with foreign surfaces results in contact factor activation, triggering the intrinsic pathway and a resultant consumptive coagulopathy beginning at initiation of perfusion.14,15 We hypothesized that a larger heparin bolus could therefore minimize the initial insult, thereby limiting the degree of early clotting factor activation and depletion of substrates seen during CPB. Our pilot study was not able to show a difference in transfusion rates between the various predetermined initial heparin doses. We did, however, see a difference in TAT after protamine administration.
The dose of heparin and ACT threshold required to commence CPB differs greatly among centres. The ACT is influenced not only by plasma heparin levels in blood but also by factors such as platelet dysfunction, thrombocytopenia, fibrinogen levels, reduced kidney function, inflammation via factor VIII levels, and concomitant drugs such as aprotinin.5 Earlier studies have established a threshold range of 400–480 sec to account for the interindividual variability dose-response, ongoing heparin metabolism, and margin of safety.16 In spite of this, some institutions routinely and safely employ ACT targets as low as 300 sec prior to CPB.17 Furthermore, the evidence on the safety of maintaining higher than conventional ACT values (i.e., greater than 600 sec) for CPB18,19,20 remains contentious. Our study found that heparin doses of 300, 400, and 500 U·kg−1 resulted in an average pre-CPB ACT of greater than 500, 600, and 700 sec, respectively. While administering a larger initial dose of heparin significantly reduced the requirement for additional doses of heparin, it did not reduce the cumulative dose of heparin overall. A heparin dose rather than an ACT target was chosen to reflect the clinical applicability of the project and to account for the difference in patients’ individual heparin response.21
There was no difference in baseline heparin activity between the groups. Anti Xa levels were significantly higher only at 30 min of CPB even though the difference in ACT was significant at all three points during CPB. We chose not to use heparin activity (such as anti Xa or measures of thrombin suppression) as our heparin target to keep the study as relevant to clinical practice as possible. There were two reasons for that. Firstly, in our practice, the draw to result time for anti Xa is in excess of one hour, so this measure is impractical. Secondly, most centres use bedside ACT over laboratory tests to ascertain adequate anticoagulation for CPB.
Fibrinopeptide A is a 16-amino-acid peptide generated following thrombin cleavage of the fibrinogen alpha chain. It quantifies thrombin activity at the first stage of fibrin polymerization and represents the main determinant of fibrin polymerization. Initially, FPA is generated more quickly than the similar cleavage product of the fibrinogen beta chain (FPB). As FPA generation increases, so does FPB; therefore, higher FPB values represent polymer generation. The relative balance of fibrin polymerization (through FPA and FPA generation) and the inactivation of thrombin (through formation of TAT complexes) dictates clot structure as a consequence of thrombin availability. High thrombin results in thin, numerous, highly branched clots whereas low thrombin results in clots with large pores and fewer thicker, less-branched fibres.
The measurement of FPA and FPB and D-dimers is more sensitive than measuring fibrinogen levels alone and allows a differential assessment of residual fibrinogen polymerization and fibrinolytic activity22,23 while anticoagulated with heparin. Therefore, raised FPA and FPB plasma concentrations reflect cumulative fibrin activation by thrombin.22 Our results indicate that the three groups were well matched at baseline and that there were no statistically significant differences between them at 30 min and 60 min of CPB and after protamine administration.
The rate of FPA release influences the rate of FPB cleavage.24 The increase in FPA levels during CPB has been described previously and the lack of return to pre-CPB levels over time has been explained with potentially inadequate heparinization.25 The fact that FPA shows similar behaviour in all three groups makes this unlikely. This also throws into question our hypothesis that greater heparin doses suppress early onset of coagulation during CPB more effectively than lower doses.
D-dimer is one of multiple cross-linked fibrin polymers generated through the action of factor XIII. They are a specific subset of the numerous fibrin degradation products present from fibrinolysis but are considered the most sensitive marker for full fibrin polymerization. D-dimer levels were similar between the three groups at all four measuring points, albeit increasing after heparin neutralization with protamine.23 The administration of protamine showed greater effect on the D-dimer levels than on FPA and FPB levels.
Thrombin is inactivated to TAT and its plasma concentration reflects the amount of thrombin generation during CPB. There is a reduction in antithrombin plasma level at the end of CPB.26 The elevation of TAT levels represents an imbalance towards excessive thrombin production, which is increased within five minutes of commencing CPB and peaks after protamine administration.27 This rate of increase is also regularly observed in prothrombotic states or in the advent of thrombotic events.27,28 In our study, there was increased thrombin suppression with increasing doses of heparin at both 30 and 60 min of CPB, reflected by lower TAT levels. This relationship was statistically significant after protamine administration, reflecting reduced thrombin generation in larger heparin dosing after heparin neutralization. This trend is evident from the first measurement on CPB, suggesting that the generation of TAT complex is independent of surgery duration and can be attenuated by increased heparin doses.
Cleavage of fibrinogen to fibrin monomers by thrombin releases FPA and FPB29 and subsequently degrades fibrin by plasmin, resulting in the release of D-dimer. Our study suggests that, despite decreasing TAT levels during and after CPB, there were no significant dose-dependent differences in FPA, FPB, or D-dimer levels at 30 and 60 min of CPB and after protamine administration. There is a marked difference in half-lives between these parameters: TAT has a half-life of ten minutes,30 D-dimer 15 hr,31 and FPA five minutes.32 It is therefore also likely that there is a different ratio between the enzyme thrombin and its substrate fibrinogen at the end of CPB, and this may be the underlying reason for the differential effects on FPA, FPB, D-dimer, and TAT levels. Interestingly, there is no data on the half-life of FPB.
High doses of protamine are known to downregulate thrombin production.33 We have dosed protamine guided by a pharmacokinetic algorithm with no difference in the protamine/heparin ratio between the three groups. The level of heparin reversal was the same in all patients, as evidenced by the similar anti Xa levels and ACT measurements after protamine. Based on this, we are confident that the difference in TAT between the groups is a heparin effect and not caused by the different doses of protamine.
Although the protamine doses were, for obvious reasons, significantly different between the groups, there was no difference in the amount of protamine given for each unit of heparin i.e., the protamine/heparin ratio. As previously described by several groups, a protamine/heparin ratio of roughly 0.6 is safe to reverse heparin after separation from CPB10,34,35 and was achieved here in equal measure in all three groups, making it unlikely that any differences in coagulation could be attributed to differences in protamine dosing.
The clinical significance of these subtle differences in biochemical markers of coagulation appears to be negligible in our cohort. There was no difference in overall blood loss or in the amount of transfusion of blood or blood products after protamine administration. The transfusion endpoints were chosen to reflect the clinical relevance of this pilot study and the applicability of the intervention to power a future larger randomized control trial. High doses of heparin, however, might become obsolete in future as heparin-bonded and newer third-generation heparin-polymer-bonded CPB circuits might lead to improved clinical outcomes and biocompatibility in patients undergoing cardiac surgery, making them a preferable option over standard nonheparin-bonded circuits.36
Limitations
It should be noted that findings from our study need to be interpreted in the context of a pilot project. Firstly, we were unable to adequately analyze statistical power required prior to commencement because preexisting data were scarce. Our inclusion criteria aimed to select a homogeneous group of patients with a lower bleeding risk, which may have minimized the magnitude of the intervention on outcomes. The impact of larger heparin doses in cohorts with high bleeding risk (i.e., patients with long CPB times, pre-existing coagulopathy or congenital heart disease) where the suppression of microtriggers of coagulation might be beneficial, remains unknown.
The anesthesiologist was not blinded to the dose of heparin given, while the surgeon and the intensive care team were. This potential bias was mitigated by 1) standardizing the amount of protamine given by using a pharmacologic algorithm to determine the dose and 2) strictly adhering to our institutional transfusion protocol.
As this was a pilot study, participants were also not randomized, and the intervention was instead influenced by the treating anesthesiologist’s usual heparin dosing preference. The potential for selection bias is evident by those having relatively longer predicted surgeries being given the largest heparin doses. There is a potential to skew outcomes given that length of surgery is known to be associated with perioperative bleeding. It is probable that—if at all possible—standardizing duration of surgery could further reduce bleeding rates in the larger heparin dosing groups, and this magnitude of effect could become significant. Nevertheless, it is evident from our plasma biomarker trends and from the postoperative blood loss data that there are no significant differences among the three heparin groups during the early (30 and 60 min) and immediately post-CPB period or in postoperative bleeding in the population included in the study at our institution.
We have not considered measuring any harmful side effects related to over anticoagulation in this pilot study as there are numerous institutions around the world using high doses, such as 500 U·kg−1 that were used in this pilot study.6 These centres have not reported higher morbidity or mortality than others.
We did collect all data necessary to assess the feasibility of designing and running a large randomized, controlled trial based on this pilot. As our results do not justify a larger trial, we are not reporting on these in the interest of maintaining a compendious manuscript.
Conclusion
Our pilot study shows that in low-risk cardiac surgery patients an increased upfront heparin dose is associated with subtle biochemically detectable changes in coagulation parameters at our institution. While it appears to be safe to administer such doses, they do not confer any clinically significant benefits in postoperative bleeding or rate of transfusion in this population.
References
Shore-Lesserson L, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg 2018; 105: 650–62. https://doi.org/10.1016/j.athoracsur.2017.09.061
Lax M, Pesonen E, Hiippala S, Schramko A, Lassila R, Raivio P. Heparin dose and point-of-care measurements of hemostasis in cardiac surgery-results of a randomized controlled trial. J Cardiothorac Vasc Anesth 2020; 34: 2362–8. https://doi.org/10.1053/j.jvca.2019.12.050
Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002; 21: 232–44. https://doi.org/10.1016/s1010-7940(01)01099-5
Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci 2010; 47: 197–212. https://doi.org/10.3109/10408363.2010.549291
Falter F, MacDonald S, Matthews C, Kemna E, Cañameres J, Besser M. Evaluation of point-of-care ACT coagulometers and anti-Xa activity during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2020; 34: 2921–7. https://doi.org/10.1053/j.jvca.2020.06.027
Miles LF, Coulson TG, Galhardo C, Falter F. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg 2017; 125: 1871–7. https://doi.org/10.1213/ane.0000000000002052
Despotis GJ, Joist JH, Hogue CW Jr, et al. More effective suppression of hemostatic system activation in patients undergoing cardiac surgery by heparin dosing based on heparin blood concentrations rather than ACT. Thromb Haemost 1996; 76: 902–8.
Okita Y, Takamoto S, Ando M, et al. Coagulation and fibrinolysis system in aortic surgery under deep hypothermic circulatory arrest with aprotinin: the importance of adequate heparinization. Circulation 1997; 96: 376–81.
Hirsh J, Anand SS, Halperin JL, Fuster V, American Heart Association. AHA scientific statement: guide to anticoagulant therapy: heparin: a statement for healthcare professionals from the American Heart Association. Arterioscler Thromb Vasc Biol 2001; 21: E9. https://doi.org/10.1161/hq0701.093520
Miles LF, Burt C, Arrowsmith J, et al. Optimal protamine dosing after cardiopulmonary bypass: the PRODOSE adaptive randomised controlled trial. PLoS Med 2021; 18: e1003658. https://doi.org/10.1371/journal.pmed.1003658
Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016; 25: 1057–73. https://doi.org/10.1177/0962280215588241
Boisclair MD, Lane DA, Philippou H, Sheikh S, Hunt B. Thrombin production, inactivation and expression during open heart surgery measured by assays for activation fragments including a new ELISA for prothrombin fragment F1 + 2. Thromb Haemost 1993; 70: 253–8.
Brister SJ, Ofosu FA, Buchanan MR. Thrombin generation during cardiac surgery: is heparin the ideal anticoagulant? Thromb Haemost 1993; 70: 259–62.
Apte G, Börke J, Rothe H, Liefeith K, Nguyen TH. Modulation of platelet-surface activation: current state and future perspectives. ACS Appl Bio Mater 2020; 3: 5574–89. https://doi.org/10.1021/acsabm.0c00822
Koster A, Yeter R, Buz S, et al. Assessment of hemostatic activation during cardiopulmonary bypass for coronary artery bypass grafting with bivalirudin: results of a pilot study. J Thorac Cardiovasc Surg 2005; 129: 1391–4. https://doi.org/10.1016/j.jtcvs.2004.09.016
Lander H, Zammert M, FitzGerald D. Anticoagulation management during cross-clamping and bypass. Best Pract Res Clin Anaesthesiol 2016; 30: 359–70. https://doi.org/10.1016/j.bpa.2016.07.002
Garvin S, Fitzgerald D, Muehlschlegel JD, et al. Heparin dose response is independent of preoperative antithrombin activity in patients undergoing coronary artery bypass graft surgery using low heparin concentrations. Anesth Analg 2010; 111: 856–61. https://doi.org/10.1213/ane.0b013e3181ce1ffa
Shuhaibar MN, Hargrove M, Millat MH, O'Donnell A, Aherne T. How much heparin do we really need to go on pump? A rethink of current practices. Eur J Cardiothorac Surg 2004; 26: 947–50. https://doi.org/10.1016/j.ejcts.2004.07.009
Bull BS, Korpman RA, Huse WM, Briggs BD. Heparin therapy during extracorporeal circulation. I. Problems inherent in existing heparin protocols. J Thorac Cardiovasc Surg 1975; 69: 674–84.
Palmer K, Ridgway T, Al-Rawi O, Poullis M. Heparin therapy during extracorporeal circulation: deriving an optimal activated clotting time during cardiopulmonary bypass for isolated coronary artery bypass grafting. J Extra Corpor Technol 2012; 44: 145–50.
Delavenne X, Ollier E, Chollet S, et al. Pharmacokinetic/pharmacodynamic model for unfractionated heparin dosing during cardiopulmonary bypass. Br J Anaesth 2017; 118: 705–12. https://doi.org/10.1093/bja/aex044
Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113: 1319–33. https://doi.org/10.1213/ane.0b013e3182354b7e
Chandler WL, Velan T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coagul Fibrinolysis 2004; 15: 583–91. https://doi.org/10.1097/00001721-200410000-00009
Nossel HL, Ti M, Kaplan KL, Spanondis K, Soland T, Butler VP Jr. The generation of fibrinopeptide A in clinical blood samples: evidence for thrombin activity. J Clin Invest 1976; 58: 1136–44. https://doi.org/10.1172/jci108566
Davies GC, Sobel M, Salzman EW. Elevated plasma fibrinopeptide A and thromboxane B2 levels during cardiopulmonary bypass. Circulation 1980; 61: 808–14. https://doi.org/10.1161/01.cir.61.4.808
Muedra V, Bonanad S, Gómez M, Villalonga V, Sánchez F, Llopis JE. Relationships between antithrombin activity, anticoagulant efficacy of heparin therapy and perioperative variables in patients undergoing cardiac surgery requiring cardiopulmonary bypass. Perfusion 2011; 26: 487–95. https://doi.org/10.1177/0267659111412999
Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101: 4355–62. https://doi.org/10.1182/blood-2002-08-2400
Sato H, Yamamoto K, Kakinuma A, Nakata Y, Sawamura S. Accelerated activation of the coagulation pathway during cardiopulmonary bypass in aortic replacement surgery: a prospective observational study. J Cardiothorac Surg 2015; 10: 84. https://doi.org/10.1186/s13019-015-0295-9
Riedel T, Suttnar J, Brynda E, Houska M, Medved L, Dyr JE. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood 2011; 117: 1700–6. https://doi.org/10.1182/blood-2010-08-300301
Rimpo K, Tanaka A, Ukai M, Ishikawa Y, Hirabayashi M, Shoyama T. Thrombin-antithrombin complex measurement using a point-of-care testing device for diagnosis of disseminated intravascular coagulation in dogs. PLoS One 2018; 13: e0205511. https://doi.org/10.1371/journal.pone.0205511
Ruhl H, Berens C, Winterhagen A, Müller J, Oldenburg J, Potzsch B. Label-free kinetic studies of hemostasis-related biomarkers including D-dimer using autologous serum transfusion. PLoS One 2015; 10: e0145012. https://doi.org/10.1371/journal.pone.0145012
Eisenberg PR, Lucore C, Kaufman L, Sobel BE, Jaffe AS, Rich S. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation 1990; 82: 841–7. https://doi.org/10.1161/01.cir.82.3.841
Ainle FN, Preston RJ, Jenkins PV, et al. Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood 2009; 114: 1658–65. https://doi.org/10.1182/blood-2009-05-222109
Meesters MI, Veerhoek D, de Jong JR, Boer C. A Pharmacokinetic model for protamine dosing after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2016; 30: 1190–5. https://doi.org/10.1053/j.jvca.2016.04.021
Goedhart AL, Gerritse BM, Rettig TC, et al. A 0.6-protamine/heparin ratio in cardiac surgery is associated with decreased transfusion of blood products. Interact Cardiovasc Thorac Surg 2020; 31: 391–7. https://doi.org/10.1093/icvts/ivaa109
Mahmood S, Bilal H, Zaman M, Tang A. Is a fully heparin-bonded cardiopulmonary bypass circuit superior to a standard cardiopulmonary bypass circuit? Interact Cardiovasc Thorac Surg 2012; 14: 406–14. https://doi.org/10.1093/icvts/ivr124
Author contributions
Thar Nyan Lwin and Rahul Mudannayake contributed to data curation, investigation, and writing the original draft and edited versions. Stephen MacDonald contributed to laboratory and data analysis, writing the original draft, and reviewing and editing the manuscript. Joseph Arrowsmith and Christiana Burt contributed to data curation and investigation. Martin Besser contributed to supervision, formal analysis, and reviewed and edited the manuscript. Florian Falter contributed to conceptualization, data curation, investigation, formal analysis, supervision, and reviewed and edited the manuscript.
Acknowledgements
We thank Dr Martin Law form the MRC Cambridge Biostatistics Unit for reviewing the statistical calculations.
Disclosures
Martin Besser receives honoraria, educational support and grants from Sanofi, Novartis, Griffols, Novartis, GBT, Amgen. He is a member of advisory boards for Novartis, GBT, Forma, Amgen, Hemeo, Octapharma. Florian Falter receives honoraria from Abbott Point of Care and Werfen. He is a member of advisory boards for Abbott Diabetics, Abbott Point of Care and Werfen. No other authors have any disclosures to make.
Funding statement
No funding was received.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lwin, T.N., Mudannayake, R., MacDonald, S. et al. Assessing the impact of different heparin dosing regimens for cardiopulmonary bypass on anticoagulation: the HepDOSE pilot study. Can J Anesth/J Can Anesth 71, 234–243 (2024). https://doi.org/10.1007/s12630-023-02645-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02645-6