Abstract
Purpose
Venous thromboembolism (VTE) is a common complication of critical illness. Sex- or gender-based analyses are rarely conducted and their effect on outcomes is unknown. We assessed for an effect modification of thromboprophylaxis (dalteparin or unfractionated heparin [UFH]) by sex on thrombotic (deep venous thrombosis [DVT], pulmonary embolism [PE], VTE) and mortality outcomes in a secondary analysis of the Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT).
Methods
We conducted unadjusted analyses using Cox proportional hazards analysis, stratified by centre and admission diagnostic category, including sex, treatment, and an interaction term. Additionally, we performed adjusted analyses and assessed the credibility of our findings.
Results
Critically ill female (n = 1,614) and male (n = 2,113) participants experienced similar rates of DVT, proximal DVT, PE, any VTE, ICU death, and hospital death. In unadjusted analyses, we did not find significant differences in treatment effect favouring males (vs females) treated with dalteparin (vs UFH) for proximal leg DVT, any DVT, or any PE, but found a statistically significant effect (moderate certainty) favouring dalteparin in males for any VTE (males: hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.52 to 0.96 vs females: HR, 1.16; 95% CI, 0.81 to 1.68; P = 0.04). This effect remained after adjustment for baseline characteristics (males: HR, 0.70; 95% CI, 0.52 to 0.96 vs females: HR, 1.17; 95% CI, 0.81 to 1.68; P = 0.04) and weight (males: HR, 0.70; 95% CI, 0.52 to 0.96 vs females: HR, 1.20; 95% CI, 0.83 to 1.73; P = 0.03). We did not identify a significant effect modification by sex on mortality.
Conclusions
We found an effect modification by sex of thromboprophylaxis on VTE in critically ill patients that requires confirmation. Our findings highlight the need for sex- and gender-based analyses in acute care research.
Résumé
Objectif
La maladie thromboembolique veineuse (MTEV) est une complication fréquente au cours des maladies critiques. Des analyses basées sur le sexe ou le genre sont rarement effectuées et leur effet sur les critères d’évaluation est inconnu. Nous avons évalué une modification de l’effet de la thromboprophylaxie (daltéparine ou héparine non fractionnée [HNF]) selon le sexe sur la maladie thrombotique (thrombose veineuse profonde [TVP], embolie pulmonaire [EP], MTEV) et sur les critères de mortalité au cours d’une analyse secondaire de l’étude PROTECT (essai de prophylaxie de la thromboembolie en soins critiques).
Méthode
Nous avons réalisé des analyses non ajustées au moyen d’une analyse des risques proportionnels de Cox, stratifiées par site et catégorie diagnostique à l’admission, incluant le sexe, le traitement et un terme d’interaction. Nous avons aussi réalisé des analyses ajustées et avons évalué la crédibilité de nos constatations.
Résultats
Les participant·es dans un état critique de sexe féminin (n = 1 614) et masculin (n = 2 113) ont présenté des taux semblables de TVP, EP, et MTEV de tout type, de décès en soins intensifs et de décès en milieu hospitalier. Nous n’avons pas trouvé de différences significatives dans les analyses non ajustées en faveur des hommes (par rapport aux femmes) traités par la daltéparine (par rapport à l’HNF) pour la TVP de la cuisse, la TVP de tout type, ou tout type d’EP; en revanche, nous avons trouvé un effet statistiquement significatif (certitude modérée) en faveur de la daltéparine pour la MTEV de tout type (hommes : rapport de risque [RR], 0,71; intervalle de confiance [IC] à 95 %, 0,52 à 0,96 par rapport aux femmes : RR, 1,16; IC 95 %, 0,81 à 1,68; P = 0,04). Cet effet a persisté après ajustement pour les caractéristiques à l’inclusion (hommes : RR, 0,70; IC 95 %, 0,52 à 0,96 par rapport aux femmes : RR, 1,17; IC 95 %, 0,81 à 1,68; P = 0,04) et le poids (hommes : RR, 0,70; IC 95 %, 0,52 à 0,96 par rapport aux femmes : RR, 1,20; IC 95 %, 0,83 à 1,73; P = 0,03). Nous n’avons pas identifié de modification significative de l’effet en fonction du sexe sur la mortalité.
Conclusion
Nous avons trouvé une modification de l’effet en fonction du sexe sur la thromboprophylaxie sur la MTEV chez les patient·es en état critique; cette constatation nécessite une confirmation. Nos constatations soulignent le besoin d’analyses en fonction du sexe et du genre dans la recherche sur les soins aigus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in hospitalized and critically ill patients. Patients in intensive care units (ICUs) are at increased risk of VTE due to predisposing comorbidities, invasive procedures, immobilization, and use of sedative and paralytic medications.1 The incidence of DVT during critical illness varies based on study design with estimates of 5.1% to 5.8% shown in a large international randomized controlled trial (RCT) that utilized biweekly surveillance ultrasound to detect DVTs in medical-surgical ICUs.2 Except in COVID-19 patients, DVT is responsible for most cases of PE—a potentially life-threatening event that has an approximate 30% mortality rate in the ICU.3,4 For acutely ill hospitalized medical patients, the 9th American College of Chest Physicians (ACCP) Antithrombotic Therapy and Prevention of Thrombosis Guideline recommends thromboprophylaxis with either low-molecular-weight heparin (LMWH), low-dose unfractionated heparin (UFH) administered two or three times daily, or fondaparinux (Grade 1B).5,6 For critically ill patients, the ACCP guideline suggests LMWH or low-dose UFH thromboprophylaxis (Grade 2C), except for patients who are bleeding or are at high risk for major bleeding, in which case mechanical thromboprophylaxis with graduated compression stockings and/or intermittent pneumatic compression devices is recommended until the risk of bleeding is attenuated (Grade 2C).5 Meta-analyses conducted in hospitalized patients7 and critically ill patients8 have found that the risk of DVT is similar for patients treated with UFH and LMWH.
Although sex (biologic attributes) and gender (socially constructed roles) influence health and wellbeing in several ways,9 few studies have examined how sex and gender impact the treatment effect of various interventions on outcomes in the ICU.10 Despite well-documented gaps in the representation of women participants in clinical research and females in basic science research,9,11,12 most studies have failed to address whether inherent differences may exist in biology, dosing, metabolic pathways, and responses to treatments between male and female research participants. Sex- and gender-based analyses recognize that a standardized approach to treatment of males and females (alternatively, men and women) as research participants may have unintended consequences. For example, the pharmacokinetics and pharmacodynamics of medications may differ between sexes, resulting in differential treatment effects and adverse event profiles.13 Remarkably, eight of the ten prescription pharmaceuticals that were withdrawn from the USA market between 1997 and 2001 caused harm to women disproportionately compared with men.14
The 2016 Sex and Gender Equity in Research (SAGER) guidelines suggested a comprehensive procedure for reporting of sex and gender information in trials.15 Although most trials report the proportion of females and males enrolled, few trials report treatment effect by sex or gender.15 Not considering sex and gender in research assumes similar treatment effects for all participants and may underestimate the frequency and magnitude of beneficial effects or adverse events. In the Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT) (ClinicalTrials.gov number, NCT00182143), we found that dalteparin was not superior to UFH in decreasing incident proximal DVT or any DVT. There was no significant difference in any VTE or VTE or death, but a significant difference in PE favouring dalteparin compared with UFH (24/1,873, 1.3% vs 43/1,873, 2.3%; HR, 0.51; 95% confidence interval [CI], 0.30 to 0.88; P = 0.01).2 We assessed for an effect modification of thromboprophylactic dose anticoagulation (thromboprophylactic dose dalteparin or UFHs) by sex on thromboembolic events (DVT, PE, VTE) and mortality in the ICU and hospital in a secondary analysis of the PROTECT trial.2
Methods
Ethics review was not required for this secondary analysis of data from the PROTECT trial.
This trial evaluated the superiority of a LMWH, dalteparin, over UFH2 among patients recruited in 67 ICUs in Canada, Australia, Brazil, Saudi Arabia, the UK, and the USA. Patients enrolled in PROTECT were at least 18 yr of age, weighed at least 45 kg, and were expected to remain in the ICU for at least three days. Exclusion criteria in PROTECT included major trauma, neurosurgery, orthopedic surgery, need for therapeutic anticoagulation, heparin administration in the ICU for at least three days, contraindication to heparin or blood products, pregnancy, life-support limitation, or enrollment in a related trial. Investigators randomized 3,764 patients to receive either subcutaneous dalteparin 5,000 IU once daily plus placebo (administered daily) or UFH (5,000 IU twice daily) for the duration of their ICU stay. Randomization was stratified by centre and admission diagnostic category (medical vs surgical) but not by sex. Research pharmacists at participating sites prepared identical syringes of either dalteparin once daily plus placebo once daily (for parallel group twice-daily injections) or of UFH twice daily for subcutaneous injection. Patients, family members, all clinicians, research personnel, ultrasonographers, and outcome adjudicators were blinded to treatment assignment.
The primary outcome was new proximal leg DVT defined as a lack of compressibility using ultrasound evaluation at 1-cm intervals at any of six sites: common femoral vein, proximal femoral vein, middle femoral vein, distal superficial femoral vein, popliteal veins, or the venous trifurcation. Proximal DVT was considered incident or “new” if detected three or more days after randomization.16 Conversely, DVTs that were diagnosed on the first screening ultrasonography were classified as prevalent DVTs. Patients with prevalent DVTs were included in the main analysis but did not contribute to the primary outcome of incident DVT in PROTECT.16 Surveillance compression ultrasound examinations were performed within two days after ICU admission, twice weekly during the ICU stay, and as clinically indicated. Patients were followed until the time of hospital discharge or death.16 Secondary outcomes included any DVT, PE, VTE (combination of DVT and PE), death, a composite of either VTE or death, major bleeding, and heparin-induced thrombocytopenia. The authors classified PE as definite (characteristic intraluminal filling defect on computed tomography of the chest, a high-probability ventilation–perfusion scan, or autopsy finding), probable (high clinical suspicion and either no test results or nondiagnostic results on noninvasive testing), possible (clinical suspicion and nondiagnostic results on noninvasive testing), or absent (negative or normal test results without reference to pretest probability).16 Outcomes were reviewed independently by two blinded adjudicators, except for PE, which was reviewed by four blinded adjudicators.16
Statistical analysis
We report continuous variables as mean and standard deviation (SD) and binary variables as frequency and percentage. In PROTECT, we collected information regarding participant apparent sex (male or female) as opposed to gender (man, woman, or other). Consequently, we analyzed thromboembolic and mortality outcomes based on sex and treatment (dalteparin, UFH).
To examine the effect of sex on outcome, we performed Cox proportional hazards analysis with sex and treatment as the independent variables and including centre and admission diagnostic category (medical vs surgical) as stratification variables as per PROTECT design. To assess for effect modification of treatment (dalteparin, UFH prophylaxis) by sex on thrombosis and mortality outcomes, we conducted Cox proportional hazards analysis, stratified by centre and admission diagnostic category including sex, treatment, and the interaction between them as independent variables. We report summary estimates of effect using the hazard ratio (HR) with 95% CIs.
Subsequently, we performed adjusted analyses wherein we added independent variables including personal history of VTE, family history of VTE, end-stage renal disease, baseline inotrope/vasopressor use, and APACHE II17 score to the Cox proportional hazards analysis described above. In an additional analysis, we examined the effect of weight as a covariate on thrombotic events. We conducted Cox proportional hazards analysis, stratified by centre and admission diagnostic category and included sex, treatment, weight (< 90 kg vs ≥ 90 kg) and the interaction between treatment assignment and sex as independent variables. All analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). We used the Instrument for Assessing the Credibility of Effect Modification Analyses (ICEMAN) criteria for RCTs (five core questions) to assess the credibility of our sex subgroup analyses.18 With this instrument, each core question contains four response options (definitely reduced credibility, probably reduced credibility, probably increased credibility, and definitely increased credibility) and “probably no” or “unclear” if insufficient information exists to accurately appraise and an optional question to assess additional considerations (e.g., sensitivity analysis, or dose response) that can reduce or increase credibility. The instrument provides an overall credibility assessment on a visual analogue scale (very low, low, moderate, high) credibility that roughly correspond to probabilities of < 25%, 25–50%, 51–75%, and > 75%, respectively, that an effect modification truly exists.18
Results
Baseline characteristics
Of the 3,746 patients included in the PROTECT analysis, we included 3,727 (1,614 females and 2,113 males) with a documented sex. Sex was not collected for 19 trial patients who did not fully meet eligibility criteria but were included in the intention-to-treat analysis. For this analysis, we excluded these patients.
Aligned with the SAGER recommendations, we present the demographic characteristics of participants by sex and treatment arm in Table 1. Female (vs male) trial participants had a slightly higher mean (SD) body mass index (BMI; 28.8 [8.6] kg·m–2 vs 27.9 [6.9] kg·m–2; P < 0.001). Females were more likely to be admitted with a medical diagnosis, have a personal or familial history of DVT, and have sepsis. Compared with females, male participants were more likely to have a history of malignancy and a cardiovascular or gastrointestinal admitting diagnosis. Although similar in frequency overall, on study day one, females were more likely to receive vasopressors and males were more likely to have end-stage renal disease and receive dialysis.
Overall thrombosis and mortality outcomes by sex
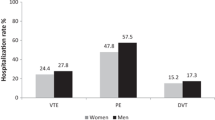
Disregarding treatment assignment, female and male PROTECT participants experienced similar rates of any DVT (129/1,614, 8.0% vs 170/2,113, 8.0%; P = 0.38) and proximal DVT (96/1,614, 5.9% vs 109/2,113, 5.2%; P = 0.91). Additionally, females had similar rates of PE (possible, probable, or definite) (22/1,614, 1.4% vs 45/2,113, 2.1%; P = 0.07) or definite/probable PE (21/1,614, 1.3% vs 41/2,113, 1.9%; P = 0.15) compared with males. Females also had similar VTE (143/1,614, 8.9% vs 197/2,113, 9.3%; P = 0.16) and ICU mortality (269/1,614, 16.7% vs 318/2,113, 15.0%; P = 0.80) and hospital mortality rates (374/1,614, 23.2% vs 497/2,113, 23.5%; P = 0.24) compared with males (Table 2). Disregarding treatment assignment, adjusted analyses supported similar thromboembolic and mortality outcome event rates between female and male participants.
Analyses assessing for treatment by sex interaction
Unadjusted analyses did not support an interaction of treatment by sex for proximal leg DVT, any DVT, and any PE with dalteparin compared with UFH. An interaction between treatment effect and sex was statistically significant for any VTE (males: HR, 0.71; 95% CI, 0.52 to 0.96 vs females: HR, 1.16; 95% CI, 0.81 to 1.68; P = 0.04). (Table 3, Figure) We did not identify an effect modification of treatment by sex on either ICU or hospital mortality.
Similarly, adjusted analyses did not support an interaction of treatment by sex for proximal DVT (males: HR, 0.74; 95% CI, 0.49 to 1.11 vs females: HR, 1.19; 95% CI, 0.76 to 1.85; P = 0.12 for the interaction), any DVT (males: HR, 0.79; 95% CI, 0.57 to 1.10 vs females: HR, 1.13; 95% CI, 0.77 to 1.66; P = 0.17 for the interaction) or any PE (males: HR, 0.39; 95% CI, 0.20 to 0.77 vs females: HR, 0.87; 95% CI, 0.33 to 2.30; P = 0.195 for the interaction). Nevertheless, adjusted analyses supported a statistically significant interaction of treatment by sex, with a significantly lower HR for any VTE in male compared with female trial participants (males: HR, 0.70; 95% CI, 0.52 to 0.96 vs females: HR, 1.17; 95% CI, 0.81 to 1.68; P = 0.04).
The statistically significant interaction of treatment by sex, with a lower HR for any VTE in male vs female PROTECT participants remained robust in an additional analysis that also included weight (HR, 0.70; 95% CI, 0.52 to 0.96 vs HR, 1.20; 95% CI, 0.83 to 1.73; P = 0.03).
Credibility of effect modification analyses
We evaluated our analyses to have moderate credibility (likely effect modification) using ICEMAN criteria.18 Consequently, we summarize our findings using separate effects for subgroups and note remaining uncertainty.
Discussion
In this secondary analysis of a multinational thromboprophylaxis trial,2 we found that, although critically ill female and male trial participants experienced similar rates of DVT, proximal DVT, PE, VTE, and ICU and hospital mortality, there were no significant differences in treatment effect by sex on outcomes (proximal leg DVT, any DVT, and PE). We found an interaction between treatment effect and sex that was statistically significant for any VTE. As such, we did not identify a significant interaction of sex by treatment for the different subtypes of VTE, only for the composite classification of any VTE as a whole. The significantly lower HR for any VTE favouring male participants treated with dalteparin was supported by adjusted and additional analyses. We did not identify an effect modification of treatment by sex on either ICU or hospital mortality. These findings are hypothesis-generating and highlight the need for sex-based analyses, and gender-based analyses where data permit, in research.
The impact of risk factors, including sex, on incident VTE is not fully understood in the outpatient setting. The COMMAND VTE Registry enrolled 3,027 patients (29 centres) with symptomatic VTE and compared clinical characteristics and outcomes between men (1,169/3,207; 39%) and women (1,858/3,207; 61%). Compared with women, men were significantly younger, more often had had prior VTE, and less often had transient VTE risk factors but had similar three-year incidence risks for recurrent VTE and major bleeding.19 In an analysis of three large studies, Scheres et al. found sex-specific differences across databases from three large studies in the distribution of the presenting VTE location with PE being more often the location of presentation for women compared with men.20 The relationship between sex and incident VTE has not been clearly characterized and may vary based on whether sex-specific risk factors are considered during analysis.21,22,23,24 Male sex is associated with an increased risk of recurrent VTE.25 A meta-analysis of seven outpatient studies (n = 20,534) found that men were more likely to develop proximal DVT (popliteal, femoral, or iliac veins) and women were more likely to develop distal DVT (calf veins).26 The latter finding may relate to the higher prevalence of lower leg varicose veins in women compared with men. The differences in the risk of recurrent thrombosis in males compared with females has led to the development and validation of a widely used scoring system that using sex-specific criteria for assigning risk of recurrent thrombosis, and, subsequently, for determining the duration of anticoagulation therapy.27,28
In a national sample of hospitalized patients (n = 107,896) receiving treatment for VTE, Marshall and coworkers identified a significantly higher incidence of proximal DVT in men but no sex-based differences in PE, hospital mortality, or length of stay.29 Nevertheless, after controlling for age, race, insurance status, hospital division, and medical comorbidities, these findings were no longer statistically significant, and the authors concluded that VTE-related outcomes did not differ significantly based on sex. The risk of recurrent VTE has been reported to be two to threefold higher in men compared with women in observational studies,30,31 but in RCTs the magnitude of risk is less pronounced with relative risk estimates of approximately 1.5.32,33,34,35,36,37 Given the small magnitude of increased recurrence risk in men in RCTs, and the fact that many confidence intervals crossed unity,25 it is not surprising that VTE-related outcomes did not differ significantly based on sex. Although male sex has been widely associated with an increased risk of recurrent VTE, the risk for provoked VTE after ICU admission may depend upon the population, study design, and analytic approach and the risk of recurrent VTE is lower.
Our findings contrast with sex- and gender-based analyses of cardiology treatment trials. Although no thromboprophylaxis trials have examined modification of treatment effects by sex, several cardiology trials have noted greater benefit of therapeutic LMWH vs UFH in women. A meta-analysis of two large trials that compared enoxaparin vs UFH (Efficacy and Safety of Subcutaneous Enoxaparin in Non–Q-Wave Coronary Events [ESSENCE] and Thrombolysis in Myocardial Infarction [TIMI] II-B)38,39 for unstable angina/non–Q-wave myocardial infarction (MI) found that enoxaparin was more effective than iv dose-adjusted UFH in reducing the risk of death, MI, or revascularization, and that the benefit on the composite outcome was greater in women.40 Similarly, in the Fragmin During Instability in Coronary Artery Disease (FRISC) trial, dalteparin (Fragmin) was shown to reduce the risk of death and MI compared with placebo in patients with acute coronary syndrome (ACS) with women experiencing both a larger absolute and relative reduction of the composite primary endpoint, death and nonfatal MI, compared with men.41 Lastly, in the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-TIMI 25 (ExTRACT-TIMI 25) trial, which randomized patients with ST-segment elevation MI and planned fibrinolysis to either enoxaparin or UFH, women had a similar relative benefit and greater absolute benefit when treated with enoxaparin vs UFH compared with men, despite women presenting with higher baseline risk and increased short-term mortality.42,43 We postulate that the sex differences noted in our study are not likely solely a medication effect. A post hoc analysis of the TIMI II-A trial44 showed that the anti-Xa pharmacokinetic and pharmacodynamic profiles of enoxaparin-treated men and women who presented with non-ST segment elevation ACS were not affected by age, sex, or BMI; however, both profiles were affected by renal insufficiency, especially with creatinine clearance ≤ 40 mL·min–1.44,45 In our study, slightly more males than females had a renal condition at baseline (2.0% vs 1.4%); in these patients, dalteparin clearance could have been lower in male participants resulting in higher plasma concentrations and fewer VTEs. It is also conceivable that a drug-host interaction exists and that dalteparin may be a more effective thromboprophylaxis agent in critically ill males or alternatively UFH may be less effective in males. Aligned with this, women, who typically have higher average body fat and lower weight, plasma volume, and blood flow than men,46 treated with therapeutic dose intravenous UFH typically achieve a higher maximum concentration (Cmax), are predisposed to a higher risk of bleeding, and have lower average heparin requirements to reach target therapeutic activated partial thromboplastin clotting times.47,48 Similarly, women have higher anti-Xa activity during the acute phase of treatment and experience more minor bleeding events with LMWH.43 Little is known about the pharmacokinetics and pharmacodynamics of fixed dose, prophylactic LMWH, and UFH in females. This may be an important consideration with fixed dose prophylaxis as female patients typically have a higher average BMI as was observed in our study. Finally, differences in the patient populations and illness severity may also explain the differences between our findings and those of cardiology trials. Baseline risk factors (e.g., sex, BMI) may be less important determinants of VTE in heterogeneous critically ill patients with concomitant organ failure, infection, or inflammation compared with cardiology outpatients presenting with ACS.
Our study has several strengths. First, to our knowledge, it is the first study of critically ill patients to assess whether a treatment effect by sex in thromboprophylaxis effectiveness exists as opposed to a difference in baseline risk of VTE between sexes. Second, it directly addresses the call by the National Institutes of Health Research, the Canadian Institutes of Health Research, and others to report sex-disaggregated outcomes data.49,50,51,52 Sex- and gender-based analyses enhance scientific rigor, examine reproducibility, and directly evaluate the generalizability of research findings to all study participants that can ultimately inform patient care and health policy.53 Third, we conducted an additional analysis using weight as a covariate to reflect the clinical practice of weight-based dosing. Our study also has weaknesses. We conducted apparent sex-based analyses as data regarding participant gender was not collected. As an analysis of a single large trial, our database may be underpowered to identify differences in thromboembolic outcomes that occur at low frequency (incident DVT, PE, and mortality) and consequently we were only able to discern differences for the outcome of any VTE. We cannot exclude that the significantly lower HR for any VTE favouring dalteparin in males may be spurious or represent a statistically significant but not clinically significant finding. Conversely, our findings may reflect the critically ill population studied. We did not adjust P values for multiple testing. Although, robustly conducted primary subgroup sex-based analysis can provide high quality data to assess for sex differences in outcomes, these findings are hypothesis-generating and require confirmation or refutation in other thromboprophylaxis trials of critically ill patients or a meta-regression. They also highlight the importance of and need for sex- and gender-based analyses in acute care research.
References
Moser KM, LeMoine JR, Nachtwey FJ, Spragg RG. Deep venous thrombosis and pulmonary embolism. Frequency in a respiratory intensive care unit. JAMA 1981; 246: 1422–4.
PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Cook D, Meade M, et al. Dalteparin versus unfractionated heparin in critically ill patients. New Engl J Med 2011: 364: 1305–14. https://doi.org/10.1056/nejmoa1014475
Geerts W, Cook D, Selby R, Etchells E. Venous thromboembolism and its prevention in critical care. J Crit Care 2002; 17: 95–104. https://doi.org/10.1053/jcrc.2002.33941
Cook DJ, Donadini MP. Pulmonary embolism in medical-surgical critically ill patients. Hematol Oncol Clin North Am 2010; 24: 677–82. https://doi.org/10.1016/j.hoc.2010.05.002
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e195S–e226S. https://doi.org/10.1378/chest.11-2296
Schünemann H, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018; 2: 3198–225. https://doi.org/10.1182/bloodadvances.2018022954
Al Yami MS, Silva MA, Donovan JL, Kanaan AO. Venous thromboembolism prophylaxis in medically ill patients: a mixed treatment comparison meta-analysis. J Thromb Thrombolysis 2018; 45: 36–47. https://doi.org/10.1007/s11239-017-1562-5
Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci 2016; 31: 1828–37. https://doi.org/10.3346/jkms.2016.31.11.1828
Coen S, Banister E (Eds.). What a Difference Sex and Gender Make: A Gender, Sex and Health Research Casebook. Ottawa: Canadian Institutes of Health Research; 2012.
Canadian Institutes of Health Research. How to integrate sex and gender into research. Available from URL: http://www.cihr-irsc.gc.ca/e/50836.html (accessed July 2022).
Kim ESH, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol 2009; 29: 279–83. https://doi.org/10.1161/atvbaha.108.179796
Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007; 132: S26–45. https://doi.org/10.1016/j.pain.2007.10.014
U.S. Food and Drug Administration. Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist), 2013. Available from URL: https://www.fda.gov/files/drugs/published/Drug-Safety-Communication--Risk-of-next-morning-impairment-after-use-of-insomnia-drugs--FDA-requires-lower-recommended-doses-for-certain-drugs-containing-zolpidem-%28Ambien--Ambien-CR--Edluar--and-Zolpimist%29.pdf (accessed July 2022).
U.S. Government Accountability Office. Drug safety: most drugs withdrawn in recent years had greater health risks for women, 2001. Available from URL: http://www.gao.gov/products/GAO-01-286R (accessed July 2022).
Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 2016; 1: 2. https://doi.org/10.1186/s41073-016-0007-6
Cook D, Meade M, Guyatt G, et al. PROphylaxis for thromboembolism in critical care trial protocol and analysis plan. J Crit Care 2011; 26: e1–9. https://doi.org/10.1016/j.jcrc.2011.02.010
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29.
Schandelmaier S, Briel M, Varadhan R, et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020; 192: E901–6.
Yoshikawa Y, Yamashita Y, Morimoto T, et al. Sex differences in clinical characteristics and outcomes of patients with venous thromboembolism. Circ J 2019; 83: 1581–9. https://doi.org/10.1253/circj.cj-19-0229
Scheres LJ, Brekelmans MP, Beenen LFM, Büller HRB, Cannegieter SC, Middeldorp S. Sex-specific differences in the presenting location of a first venous thromboembolism. J Thromb Haemost 2017; 15: 1344–50. https://doi.org/10.1111/jth.13712
White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost 2005; 93: 298–305. https://doi.org/10.1160/th04-08-0506
Tormene D, Ferri V, Carraro S, Simioni P. Gender and the risk of venous thromboembolism. Semin Thromb Hemost 2011; 37: 193–8. https://doi.org/10.1055/s-0031-1273083
Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation 2014; 129: 51–6. 15. https://doi.org/10.1161/circulationaha.113.004768
Guistozzi M, Valerio L, Agnelli G, et al. Sex-specific differences in the presentation, clinical course, and quality of life of patients with acute venous thromboembolism according to baseline risk factors. Insights from the PREFER in VTE. Eur J of Intern Med 2021; 88: 43–51. https://doi.org/10.1016/j.ejim.2021.03.014
McRae S, Tran H, Schulman S, Ginsberg J, Kearon C. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet 2006; 368: 371–8. https://doi.org/10.1016/s0140-6736(06)69110-1
Trinchero A, Scheres LJ, Prochaska JH, et al. Sex-specific differences in the distal versus proximal presenting location of acute deep vein thrombosis. Thromb Res 2018; 172: 74–9. https://doi.org/10.1016/j.thromres.2018.10.025
Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008: 179: 417–26. https://doi.org/10.1503/cmaj.080493
Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356: j1065. https://doi.org/10.1136/bmj.j1065
Marshall AL, Bartley AC, Ashrani AA, et al. Sex-based disparities in venous thromboembolism outcomes: a national inpatient sample (NIS)-based analysis. Vasc Med 2017; 22: 121–7. https://doi.org/10.1177/1358863x17693103
Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. N Engl J Med 2004; 350: 2558–63. https://doi.org/10.1056/nejmoa032959
Baglin T, Luddington R, Brown K, Baglin C. High risk of recurrent venous thromboembolism in men. J Thromb Haemost 2004; 2: 2152–5. https://doi.org/10.1111/j.1538-7836.2004.01050.x
Levine MN, Hirsh J, Gent M, et al. Optimal duration of anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost 1995; 74: 606–11.
Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332: 1661–5. https://doi.org/10.1056/nejm199506223322501
Schulman S, Grangvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336: 393–8. https://doi.org/10.1056/nejm199702063360601
Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348: 1425–34. https://doi.org/10.1056/nejmoa035029
Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation after a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340: 901–7. https://doi.org/10.1056/nejm199903253401201
Schulman S, Wåhlander K, Lundström T, Clason SB, Eriksson H, THRIVE III Investigators. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 2003; 349: 1713–21. https://doi.org/10.1056/nejmoa030104
Cohen M, Demers C, Gurfinkel EP, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med 1997; 337: 447–52. https://doi.org/10.1056/nejm199708143370702
Roberts R, Rogers WJ, Mueller HS, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B study. Circulation 1991; 83: 422–37. https://doi.org/10.1161/01.cir.83.2.422
Antman EM, Cohen M, Radley D, et al. Assessment of the treatment effect of enoxaparin for unstable angina/non–Q-wave myocardial infarction: TIMI 11B-ESSENCE meta-analysis. Circulation 1999; 100: 1602–8. https://doi.org/10.1161/01.cir.100.15.1602
Fragmin during Instability in Coronary Artery Disease (FRISC) Study Group. Low-molecular-weight heparin during instability in coronary artery disease. Lancet 1996; 347: 561–8. https://doi.org/10.1016/S0140-6736(96)91270-2
Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354: 1477–88. https://doi.org/10.1056/nejmoa060898
Mega JL, Morrow DA, Ostör E, et al. Outcomes and optimal antithrombotic therapy in women undergoing fibrinolysis for ST-elevation myocardial. Circulation 2007; 115: 2822–28. https://doi.org/10.1161/circulationaha.106.679548
Rogers WJ, Baim DS, Gore JM, et al. Comparison of immediate invasive, delayed invasive, and conservative strategies after tissue-type plasminogen activator. Results of the thrombolysis in myocardial infarction (TIMI) Phase II-A trial. Circulation 1990; 81: 1457–76. https://doi.org/10.1161/01.cir.81.5.1457
Becker RC Spencer F, Gibson M, et al. Influence of patient characteristics and renal function on factor Xa inhibition pharmacokinetics and pharmacodynamics after enoxaparin administration in non-ST-segment elevation acute coronary syndromes. Am Heart J 2002; 143: 753–9. https://doi.org/10.1067/mhj.2002.120774
Tamargo J, Rosano G, Walther T, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017; 3: 163–82. https://doi.org/10.1093/ehjcvp/pvw042
Campbell NR, Hull RD, Brant R, Hogan DB, Pineo GF, Raskob GE. Different effects of heparin in males and females. Clin Invest Med 1998; 21: 71–8.
Jick H, Slone D, Borda I, Shapiro S. Efficacy and toxicity of heparin in relation to age and sex. N Engl J Med 1968; 279: 284–6. https://doi.org/10.1056/nejm196808082790603
National Institutes of Health. Sex and gender. Available from URL: http://orwh.od.nih.gov/research/sex-gender (accessed July 2022).
Canadian Institutes of Health Research. Meet the methods series: methods for prospectively and retrospectively incorporating gender-related variables in clinical research. Available from URL: https://cihr-irsc.gc.ca/e/52608.html (accessed July 2022).
Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA 2016; 316: 1863–4. https://doi.org/10.1001/jama.2016.16405
Prins MH, Smits KM, Smits LJ. Methodologic ramifications of paying attention to sex and gender differences in clinical research. Gend Med 2007, 4: S106–10. https://doi.org/10.1016/s1550-8579(07)80051-9
Johnson JL, Greaves L, Repta R. Better science with sex and gender: facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health 2009; 8: 14. https://doi.org/10.1186/1475-9276-8-14
Author contributions
Karen E. A. Burns, Diane Heels-Ansdell, and Deborah J. Cook contributed to study design. Diane Heels-Ansdell and Lehana Thabane contributed to data analysis and presentation. All authors contributed to data interpretation and manuscript preparation.
Acknowledgements
We would like to thank the patient and families who provided consent to participate in PROTECT. In addition, we would like to thank Dr. Aimee J. Sarti for her review of our manuscript for the Grants and Manuscripts Committee of the Canadian Critical Care Trials Group. We also wish to acknowledge the investigators and research coordinators around the world who participated in this trial. PROTECT was supported by the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. The list of PROTECT collaborators is provided in the Data Online Supplement to the original trial report (2).
Disclosures
Mark A. Crowther declares that in the last 24 months he has provided educational materials and/or presented on behalf Bayer, Pfizer, and CSL Behring, and he has served in an advisory capacity to Hemostasis Reference Laboratories and Syneos Health. The other authors declare that they have no financial or nonfinancial conflicts of interest to declare.
Funding statement
This study was not funded. The original PROTECT trial was funded by the Canadian Institutes of Health Research and the Australian and New Zealand College of Anesthetists Research Foundation. Prophylactic dalteparin was provided by Pfizer Inc. and Esai Co., Ltd. None of these agencies played a role in the design, conduct, analysis, interpretation, or write-up of the original trial or this study. K. Burns was supported in this work by the Physician Services Incorporated of Ontario Mid-Career Research Award. D. Cook and S. Kahn were supported by Tier-1 Research Chairs from the Canadian Institutes of Health Research. M. Crowther was supported by the Leo Chair in Thrombosis of McMaster University. S. Kahn is an investigator of the CanVECTOR Network, which holds grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT142654) and from the Fonds de recherche du Québec – Santé (File # 309911).
Data availability statement
The data will be made available upon written request to Drs Cook and Burns.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/ Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Consortia
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the study group PROTECT Investigators, the Canadian Critical Care Trials Group, and the Australian and New Zealand Intensive Care Society Clinical Trials Group are placed in Acknowledgement section.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burns, K.E.A., Heels-Ansdell, D., Thabane, L. et al. Sex differences in thromboprophylaxis of the critically ill: a secondary analysis of a randomized trial. Can J Anesth/J Can Anesth 70, 1008–1018 (2023). https://doi.org/10.1007/s12630-023-02457-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02457-8