Abstract
Purpose of Review
Post-mastectomy radiotherapy (PMRT) has been shown to improve locoregional control and survival rates when administered to high-risk breast cancer patients. This review provides a compendium of instrumental studies, extant indications, current evidence, and ongoing trials as they pertain to PMRT utilization.
Recent Findings
Increasing evidence supports hypofractionated irradiation as an equivalent, if not superior, alternative to standard fractionation. There is growing interest in PMRT delivery techniques that limit cardiopulmonary radiation exposure. The safety and efficacy profile of proton beam radiotherapy appear similar to conventional irradiation with the potential advantage of reduced cardiac toxicity. Numerous active studies are investigating the impact of PMRT in patients considered to be at intermediate risk of disease recurrence.
Summary
The approach to PMRT should be personalized based on individual risk factors and tumor biology. As radiotherapy techniques and genomic testing continue to improve, there may be a role for PMRT de-escalation in particular subpopulations with breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of post-mastectomy radiotherapy (PMRT) has undergone a considerable paradigm shift over several decades. Adjuvant radiotherapy (RT) targets microscopic disease that may remain within the chest wall, residual breast parenchyma, or regional lymph nodes, therein reducing rates of locoregional recurrence. Results of randomized trials and meta-analyses have suggested improved disease-free survival and overall survival associated with PMRT delivery [1, 2•, 3,4,5,6,7]. This article will review the indications for, areas of active investigation, and future directions of PMRT in the modern era.
Landmark Studies
The Danish Breast Cancer Cooperative Group (DBCG) 82b and 82c Randomized Trials are two of the first landmark studies to show significant benefit across several endpoints of PMRT in high-risk breast cancer, including overall survival (OS). DBCG 82b evaluated the benefit of PMRT in premenopausal women receiving adjuvant systemic chemotherapy, and DBCG 82c evaluated the benefit of PMRT in postmenopausal women receiving adjuvant tamoxifen. In DBCG 82b, 1708 premenopausal women with high-risk stage II–III breast cancer who had undergone mastectomy with axillary lymph node dissection (ALND) were assigned to receive either eight cycles of cyclophosphamide, methotrexate, and fluorouracil (CMF) plus chest radiation or nine cycles of CMF alone. Women were followed over ten years to assess locoregional recurrence (LRR), distant metastases, disease-free survival (DFS), and OS. PMRT showed a significant benefit in rates of LRR, DFS, and OS. While distant metastasis occurred more frequently in the RT group, when all sites of recurrence were evaluated as the probability of DFS, PMRT plus CMF patients had significantly better DFS than patients treated with CMF alone [3]. Similarly, DBCG 82c followed 1375 postmenopausal women with high-risk breast cancer status post-mastectomy with ALND over 10 years to assess the benefit of randomly assigned adjuvant tamoxifen plus PMRT compared to tamoxifen alone. Again, LRR, DFS, and OS showed a significant benefit of PMRT with systemic therapy over systemic therapy alone, though distant metastasis occurred more frequently in the RT group [4]. In both studies, OS after 10 years favored PMRT plus CMF or tamoxifen over systemic therapy alone. Neither study included short-term and long-term complications (such as lymphedema, cardiotoxicity, or lung fibrosis) in their primary analyses. Notably, systemic therapy in both trials was given for a shorter duration than what is currently standard practice [3, 4].
The British Columbia Randomized Trial further evaluated the survival impact of locoregional RT with mastectomy and adjuvant chemotherapy, across a 20-year follow-up period. The study included 318 premenopausal women with node-positive breast cancer treated with mastectomy, axillary node dissection, and adjuvant chemotherapy, which were randomized to receive PMRT or chemotherapy alone. At the 15-year follow-up interim, results showed that RT was significantly associated with reductions in breast cancer mortality and recurrence, but improvement in OS was not statistically significant. However, at the 20-year analysis, the study found that PMRT with adjuvant chemotherapy did offer long-term survival benefit, in addition to delays in breast cancer-related events. The relative magnitude of benefit was similar for patients with one to three positive axillary lymph nodes and those with four or more positive nodes. Ultimately, it was determined that in selected high-risk patients with breast cancer, routine use of adjuvant RT in addition to adjuvant chemotherapy is indicated as it substantially reduces mortality [5].
Finally, in 2014 the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a meta-analysis of 22 randomized trials including a total of 8135 women which evaluated the effect of RT after mastectomy and axillary dissection. They found that for women with ALND and no positive nodes, RT had no significant effect on LRR, overall recurrence, or breast cancer mortality. However, for women found to have 1–3 positive nodes on ALND, as well as those with 4 or more positive nodes, PMRT offered statistically significant benefit in all three endpoints, even when systemic therapy (chemotherapy or endocrine therapy) was given [1, 7]. Many of the trials included 20-year follow-up periods, providing information about the long-term benefits of RT for women with various levels of lymph node involvement. While advances in screening and treatment of breast cancer have reduced the absolute risks of breast cancer recurrence and mortality since many of these trials were conducted, thus reducing the absolute benefit of PMRT, over the same period RT techniques have also improved and have increased the proportional benefits of PMRT compared to those reported in the trials.
Intermediate-Risk Breast Cancer
Since the landmark historical trials that demonstrated benefit to PMRT, there have been consistent improvements in breast cancer detection, pathologic evaluation of lymph nodes, surgical and RT techniques, and systemic treatment advances that have all contributed to improved outcomes for breast cancer patients. This has led to the re-evaluation of PMRT in certain node-negative patients and those with 1–3 positive lymph nodes. Without randomized evidence specific to these populations to guide recommendations, we are left with the many retrospective series published on T1-2N0, T3N0, and T1-2N1 patients to inform PMRT decisions.
Retrospective series of patients with T1-2N0 breast cancer treated with mastectomy have reported low overall rates of LRR with most estimates of 10-year LRR around 4–8% [8,9,10,11]. However, many studies have consistently identified factors associated with increased risks of LRR to be young age (generally < 40 or < 50), larger tumor size (≥ 2 cm), high grade, lymphovascular invasion, triple-negative receptor status, and close or positive margins. For patients with multiple risk factors, the 10-year LRR estimates are frequently > 10% and sometimes up to 40% with the majority of these recurrences on the chest wall [8,9,10,11,12]. Therefore, while PMRT is not routinely recommended for these patients, in select cases with multiple risk factors for increased LRR it may be considered.
Patients with node-negative disease and tumors ≥ 5 cm (pT3N0) represented just 3.5% of those treated on the National Surgical Adjuvant Breast and Bowel Project clinical trials B-13, B-14, B-19, B-20, and B-23 who underwent mastectomy without PMRT [13]. In this retrospective analysis, the 10-year LRR rate was 10% on trials starting 1981–1991 [13]. A separate retrospective analysis of more contemporary patients treated from 2000 to 2016 noted 8-year LRR rates of 2% with PMRT and 8.7% without PMRT (although not statistically significant) [14].
Patients with node-positive disease are known to have higher rates of LRR. The EBCTCG meta-analysis of trials starting between 1964 and 1986 reported a 10-year LRR of 20.3% in patients with 1–3 positive lymph nodes that did not receive PMRT [1]. More modern series for patients with 1–3 positive lymph nodes treated with mastectomy and without PMRT note 10-year LRR estimates of < 10% and typically < 5% [15,16,17,18,19]. Similar to the node-negative setting, younger age, lymphovascular invasion, larger tumor size, and high grade were associated with increased risk of LRR in addition to > 1 nodal positivity and extracapsular extension [15,16,17,18,19]. Of note, some series reported similar rates of LRR between those patients who received PMRT and those who did not, but those selected for PMRT in these non-randomized series were more likely to have multiple high-risk features noted above and therefore were at higher baseline risk [15, 18, 19].
The type of pathologic axillary evaluation also merits consideration in deciding on PMRT with the increasing utilization of the less morbid SLNB over ALND after the publication of the ACOSOG Z0011, AMAROS, and OTOASOR trials [20,21,22]. Further, the AMAROS and OTOASOR trials demonstrated similarly low axillary recurrence rates in pathologic node-positive patients following sentinel lymph node biopsy (SLNB) treated with either completion ALND or regional nodal irradiation, with significantly less lymphedema noted in the patients managed with SLNB and RT [20, 21]. Importantly approximately 30–40% of patients with a positive sentinel lymph node had additional lymph nodes involved on ALND with 10–20% ultimately having 4 or more total positive lymph nodes [20,21,22]. This risk of additional axillary disease should be considered when deciding on PMRT in a patient for whom completion ALND is not planned. A recently published screening trial sought to prospectively compare lymphedema development among SLNB alone, ALND alone, SLNB with RT, and ALND with RT, and found that the 5-year cumulative incidence rates were, respectively, 8.0%, 24.9%, 10.7%, and 30.1%. The study results demonstrated that while RT slightly increased lymphedema risk, the more salient risk factor associated with lymphedema development was ALND [23].
Indications
Two major practice guidelines exist to guide the selection of patients who may benefit from PMRT. First, the National Comprehensive Cancer Network (NCCN) Guidelines Version 7 2021 offers indications for RT following total mastectomy with surgical axillary staging, based on tumor size, node status, and margins. The recommendations are the same for mastectomy with or without reconstruction, as well as skin-sparing vs. standard mastectomy [24].
In patients with negative axillary nodes, tumor ≤ 5 cm, and negative margins ≥ 1 mm, PMRT is generally not recommended, although it may be considered in patients with multiple high-risk recurrence factors, such as central/medial tumors or tumors ≥ 2 cm with < 10 axillary nodes removed and either grade 3, ER-negative, or lymphovascular invasion. In all patients with tumor > 5 cm or margins < 1 mm, RT to the chest wall should be considered. Additionally, for those patients with tumors > 5 cm and/or patients with 1–3 positive axillary nodes, physicians should consider the addition of RT to the supraclavicular/infraclavicular regions, internal mammary nodes, and any part of the axillary bed at risk. RT to the chest wall and the supraclavicular/infraclavicular regions, internal mammary nodes, and any part of the axillary bed at risk are indicated in patients with 4 or more positive axillary nodes. For any patient with positive margins, the NCCN guidelines recommend re-excision to negative margins if possible; if not feasible, RT to the chest wall and supraclavicular/infraclavicular regions, internal mammary nodes, and any part of the axillary bed at risk are strongly recommended.
The second major guideline comes from a collaboration between the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology. Their joint clinical practice guideline (updated 9/18/2016) focused on three clinical treatment scenarios [25].
For patients with T1-2 tumors with 1–3 positive axillary lymph nodes who undergo ALND, the decision to recommend PMRT should be based on multidisciplinary clinical judgement. There is strong evidence that PMRT reduces the risk of LRR, any disease recurrence, and breast cancer mortality for this subset of patients, but in patients with low risk of LRR, the additional benefit of PMRT is outweighed by its potential toxicities. Therefore, physicians must assess individual patient risk (such as patient characteristics, comorbidities, tumor burden, and cancer biology as highlighted earlier) as well as the patient's own estimation of sufficient benefit in order to make a final treatment recommendation.
For patients with T1-2 tumors with a positive sentinel node biopsy who do not undergo completion ALND, physicians must weigh the potential toxicities of PMRT against those of ALND in making their recommendation [23]. PMRT should be used as long as sufficient evidence exists to justify its use without knowing that additional nodes are involved. If a physician would not recommend PMRT if the patient had undergone simultaneous ALND and no additional nodal metastases were found, then that patient should undergo ALND rather than PMRT alone.
In patients with clinical stage I or II cancers who have persistent axillary nodal disease after neoadjuvant systemic therapy, PMRT should be administered. In those patients with clinically negative nodes who receive neoadjuvant systemic therapy or those with a complete pathologic response in the axilla to neoadjuvant systemic therapy, there is insufficient evidence to recommend administration or omission of PMRT, though this is being investigated in the recently completed NSABP B-51/RTOG 1304 trial [26]. These patients seem to have low rates of LRR, but it is not clear whether some of these patients may still benefit from PMRT.
Finally, the joint practice guideline recommended that regional nodal irradiation should include both the internal mammary and supraclavicular–axillary apical nodes, in addition to the chest wall/reconstructed breast. While certain subgroups of patients may derive limited benefit of treatment to either or both of these areas while incurring additional toxicity, there is insufficient data at present to determine which patients should undergo irradiation of only one or neither of these areas.
Active Investigation and Future Directions
Hypofractionation
Following publication of the British START A and B trials, the paradigmatic fractionation schedule in the post-mastectomy setting was brought into question [27, 28]. At present, standard fractionated (SF) irradiation is conventionally delivered to the chest wall in 25 to 28 fractions at 1.8 to 2 Gy per day with or without regional nodal irradiation [29, 30••, 31, 32]. Alternatively, hypofractionated (HF) irradiation is a shorter-course approach consisting of higher doses per fraction, typically greater than 2.0 Gy per day, resulting in fewer total treatments [32,33,34].
While the results of the START A and B studies supported HF use after breast-conserving surgery (BCS), the proportion of participants who underwent mastectomy was only 8–15% and questions regarding the safety and efficacy of HF irradiation after mastectomy remain for some [27,28,29, 30••, 34]. A randomized phase III trial published in 2019 sought to determine 5-year LRR in post-mastectomy patients without reconstruction assigned to HF irradiation (43.5 Gy in 15 fractions) compared to SF irradiation receipt (50 Gy in 25 fractions). No significant differences were demonstrated in LRR, DFS, or OS. Furthermore, toxicity profiles were equivalent between cohorts with the exception of fewer cases of grade 3 acute skin toxicity in the HF cohort [30••]. The prospect of shortened PMRT duration could increase accessibility and compliance while reducing costs and resource expenditure [29].
Breast Reconstruction
The optimal timing and type of breast reconstruction for irradiated patients remain an area of active study. Autologous flap reconstruction and implant-based breast reconstruction (IBBR) are both susceptible to contracture, suboptimal cosmesis, and reconstructive failure in the setting of RT; however, the concatenation of IBBR and PMRT has been associated with higher morbidity rates and lower patient-reported satisfaction [35,36,37,38, 39•]. A recent network meta-analysis found autologous reconstruction prior to PMRT correlated with improved rates of reconstruction failure and surgical site infections [39•]. Nonetheless, the preferred approach to reconstruction timing remains inconclusive, and collective evidence shows practice patterns vary considerably by institution [38, 39•, 40]. Furthermore, immediate reconstruction poses a salient challenge to radiation oncologists related to treatment planning and technical considerations.
There are multiple randomized trials actively investigating the trade-offs of PMRT dose and fractionation on reconstruction complication rates and oncologic outcomes. Participants with planned chest wall reconstruction enrolled in the RT CHARM study (Alliance A221505; ClinicalTrials.gov identifier: NCT03414970) are designated to receive either SF irradiation over 5–6 weeks or HF irradiation over 3–4 weeks. Likewise, the FABREC trial (ClinicalTrials.gov identifier: NCT03422003) randomly assigns patients undergoing immediate IBBR to receive conventional or HF radiotherapy. Findings of these and other studies will conceivably address the ambiguity surrounding PMRT management of the reconstructed breast.
Tumor Biology
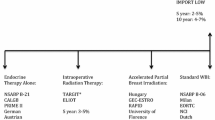
Genomically guided RT in breast oncology incorporates tumor biology to facilitate personalized cancer treatment strategies [41]. There is speculation that risk stratification based on biological factors could identify subgroups of patients who may safely forgo PMRT. The primary outcome of the TAILOR RT trial (ClinicalTrials.gov identifier: NCT03488693) is recurrence-free interval in patients with intermediate-risk breast cancer randomized to either regional RT or no RT. Patients with hormone-positive, HER2-negative, pT3N0 disease or pT1-2N1 disease, with an Oncotype DX recurrence score ≤ 25 treated with BCS or mastectomy, are eligible to enroll. The SUPREMO trial randomly assigned women treated with mastectomy for intermediate-risk breast cancer (pT2N0 grade III or with lymphovascular invasion, pT3N0, or pT1-2N1) to receive PMRT or no chest wall irradiation [42•]. Long-term data from these two studies will provide further insight into the impact of PMRT on the treatment of intermediate-risk breast cancers.
Alternately, the question has been raised whether PMRT should be administered to select patients with node-negative T1-2 disease and adverse prognostic features. PMRT is not routinely recommended for early-stage breast cancer without nodal involvement as cumulative LRR is relatively low. The impact of irradiation in the presence of multiple high-risk factors, such as lymphovascular invasion, high histological grade, young age, or premenopausal status, is a subject of ongoing research [34, 43,44,45]. Considering the heterogeneous nature of breast cancer the integration of biological parameters into PMRT decision-making could potentially enhance clinical outcomes while eliminating overtreatment.
Special Considerations
Inflammatory Breast Cancer
The aggressive biologic properties and tendency toward rapid progression have made the management of inflammatory breast cancer (IBC) a therapeutic challenge. The contemporary trimodality therapy (neoadjuvant chemotherapy, modified radical mastectomy, and PMRT) approach to non-metastatic IBC confers a survival advantage when compared to receipt of one or two treatment modalities [46,47,48]. RT delivery in the setting of IBC varies across institutions with a multiformity of PMRT techniques and doses described in the literature [49,50,51,52]. Examples of regimen modifications include dose intensification with or without acceleration, twice-daily treatments, overlapping field junctions, and tissue-equivalent bolus to broaden skin coverage [52, 53]. Retrospective reviews have described a benefit to chest-wall radiation dose escalation from 60 to 66 Gy for IBC patients with close or positive margins, patients younger than 45 years, and patients with a poor response to systemic therapy [49, 53]. The co-administration of a radiosensitizing agent while undergoing PMRT is emerging as a novel strategy to treat IBC [54,55,56].
PMRT Technique
Among the various PMRT techniques, three-dimensional conformal RT with photon tangents is the most frequently used due to widespread availability, low cost, and technical ease in setup, planning, and reproducibility [34]. While virtual wedges and field-in-field techniques allow for adequate dose distribution to the chest wall with relative sparing of the heart and lungs in most patients, there may be clinical scenarios where additional beam modulation or arc therapy is beneficial (i.e., extreme curvature of the chest wall, close proximity of the heart to the chest wall, deep target internal mammary nodes, gross residual disease). A general trade-off with these other photon techniques is that there is almost always a larger volume of normal tissue receiving low doses of RT that may increase secondary cancer risk [57].
Proton particle therapy with sharp dose falloff and finite range due to the physical properties of the beam provides a theoretical benefit with this technique to spare the deeper heart and lungs from RT and is an ongoing area of investigation [58]. A recent prospective phase 2 clinical trial of 69 patients who received regional nodal irradiation including the internal mammary nodes demonstrated low doses to the deep normal tissues with a mean heart dose of 0.5 Gy and ipsilateral lung V20Gy of 14.5% (relative biological effectiveness) with an acceptably low 5-year LRR of 1.5% [59••]. Acute toxicity was comparable to that seen with photon tangents but there was a higher rate (7%) of grade 1 rib fracture noted, perhaps owing to the increase in effective dose associated with the end of the proton beam, which generally falls within the ribs and intercostal space as the dose is pulled away from the deeper heart and lungs. This potential trade-off in toxicity warrants continued investigation [59••]. The accruing RadComp trial is a pragmatic, randomized Phase 3 photon versus proton clinical trial with primary aim to assess reduction in major coronary events with the use of protons and secondary aim to assess non-inferiority of proton versus photon RT in reducing the risk of breast cancer recurrence and will offer further evidence for the potential benefits of proton RT [60].
The use of bolus with PMRT to generate a brisk desquamation skin reaction, a prior dogmatically pursued goal, has also recently been questioned, and the practice is waning with significant decreases in LRR rates with modern therapies [61]. In fact, a large retrospective series from Canada noted low rates of 10-year LRR overall (upper bound of 2.7% 95% confidence interval) in patients treated with modern systemic therapies and PMRT [62]. Further, the 10-year LRR with bolus was 1.9% and was 0.9% without bolus and the use bolus was not associated with better local control or breast cancer mortality in multivariate analysis controlling for clinicopathologic factors [62]. However, while routine use of bolus is likely unnecessary, common indications for bolus to achieve adequate skin dose are direct skin involvement, positive margins, or IBC.
Conclusion
Trends in PMRT application and utilization are dynamic and continue to evolve with the ever-changing landscape that is breast cancer treatment. Consensus guidelines from NCCN and ASTRO/ASCO are helpful in the context of ever-expanding data evaluating the efficacy of PMRT, but the benefits of PMRT in certain subgroups of patients remain to be defined. Ongoing clinical trials will help refine future guidelines, including guidance about dose and fractionation schedules, and genomically guided, risk-stratified recommendations represent an emerging area of treatment personalization anticipated in the coming years.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials [published correction appears in Lancet. 2014;384(9957):1848]. Lancet. 2014;383(9935):2127–2135. https://doi.org/10.1016/S0140-6736(14)60488-8.
• Poortmans PM, Weltens C, Fortpied C, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial [published correction appears in Lancet Oncol. 2021;22(1):e5]. Lancet Oncol. 2020;21(12):1602–1610. https://doi.org/10.1016/S1470-2045(20)30472-1. This paper presented the 15-year results from a randomized trial evaluating the effects of internal mammary and medial supraclavicular (IM-MS) irradiation in stage I-III breast cancer on overall survival. Although rates of breast cancer recurrence and mortality were lower in the IM-MS treatment group, there was no difference in overall survival.
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–55. https://doi.org/10.1056/NEJM199710023371401.
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–8. https://doi.org/10.1016/S0140-6736(98)09201-0.
Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–26. https://doi.org/10.1093/jnci/djh297.
Whelan TJ, Julian J, Wright J, Jadad AR, Levine M. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18(6):1220–9. https://doi.org/10.1200/JCO.2000.18.6.1220.
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials. Lancet. 2000;355(9217):1757–1770. https://doi.org/10.1016/s0140-6736(00)02263-7.
Abi-Raad R, Boutrus R, Wang R, et al. Patterns and risk factors of locoregional recurrence in T1–T2 node negative breast cancer patients treated with mastectomy: implications for postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(3):e151–7. https://doi.org/10.1016/j.ijrobp.2011.01.015.
Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62(4):1035–9. https://doi.org/10.1016/j.ijrobp.2004.12.014.
Mamtani A, Patil S, Stempel MM, Morrow M. Are there patients with T1 to T2, lymph node-negative breast cancer who are “high-risk” for locoregional disease recurrence? Cancer. 2017;123(14):2626–33. https://doi.org/10.1002/cncr.30658.
Truong PT, Lesperance M, Culhaci A, Kader HA, Speers CH, Olivotto IA. Patient subsets with T1–T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys. 2005;62(1):175–82. https://doi.org/10.1016/j.ijrobp.2004.09.013.
Wang J, Shi M, Ling R, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100(2):200–4. https://doi.org/10.1016/j.radonc.2011.07.007.
Taghian AG, Jeong JH, Mamounas EP, et al. Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2006;24(24):3927–32. https://doi.org/10.1200/JCO.2006.06.9054.
Kim K, Jung J, Kim H, et al. Postmastectomy Radiation Therapy for Node-Negative Breast Cancer of 5cm or Larger Tumors: A Multicenter Retrospective Analysis (KROG 20–03) [published online ahead of print, 2021 Aug 25]. Cancer Res Treat. 2021; https://doi.org/10.4143/crt.2021.933
Tendulkar RD, Rehman S, Shukla ME, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1–3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83(5):e577–81. https://doi.org/10.1016/j.ijrobp.2012.01.076.
Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1–T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1337–47. https://doi.org/10.1016/j.ijrobp.2004.08.009.
Sharma R, Bedrosian I, Lucci A, et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010;17(11):2899–908. https://doi.org/10.1245/s10434-010-1089-x.
McBride A, Allen P, Woodward W, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1–3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89(2):392–8. https://doi.org/10.1016/j.ijrobp.2014.02.013.
Moo TA, McMillan R, Lee M, et al. Selection criteria for postmastectomy radiotherapy in t1–t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20(10):3169–74. https://doi.org/10.1245/s10434-013-3117-0.
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10. https://doi.org/10.1016/S1470-2045(14)70460-7.
Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017;43(4):672–9. https://doi.org/10.1016/j.ejso.2016.12.011.
Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264(3):413–20. https://doi.org/10.1097/SLA.0000000000001863.
Naoum GE, Roberts S, Brunelle CL, et al. Quantifying the Impact of Axillary Surgery and Nodal Irradiation on Breast Cancer-Related Lymphedema and Local Tumor Control: Long-Term Results From a Prospective Screening Trial. J Clin Oncol. 2020;38(29):3430–8. https://doi.org/10.1200/JCO.20.00459.
Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 8.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Sep 13.
Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol. 2016;34(36):4431–42. https://doi.org/10.1200/JCO.2016.69.1188.
Mamounas EP, Bandos H, White JR, et al. NRG Oncology/NSABP B-51/RTOG 1304: Phase III trial to determine if chest wall and regional nodal radiotherapy (CWRNRT) post mastectomy (Mx) or the addition of RNRT to whole breast RT post breast-conserving surgery (BCS) reduces invasive breast cancer recurrence-free interval (IBCR-FI) in patients (pts) with pathologically positive axillary (PPAx) nodes who are ypN0 after neoadjuvant chemotherapy (NC). J Clin Oncol. 2019;37(14 suppl):TPS600.
START Trialists' Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–341. https://doi.org/10.1016/S1470-2045(08)70077-9.
START Trialists' Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. https://doi.org/10.1016/S0140-6736(08)60348-7.
Khan AJ, Poppe MM, Goyal S, et al. Hypofractionated Postmastectomy Radiation Therapy Is Safe and Effective: First Results From a Prospective Phase II Trial. J Clin Oncol. 2017;35(18):2037–43. https://doi.org/10.1200/JCO.2016.70.7158.
•• Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–360. https://doi.org/10.1016/S1470-2045(18)30813-1. This study was the first randomized trial comparing hypofractionated versus conventional fractionated radiotherapy in the postmastectomy setting. There were no significant differences in 5-year locoregional recurrences or late toxicities between the 3-week hypofractionated regimen and the standard 5-week schedule.
Haussmann J, Corradini S, Nestle-Kraemling C, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol. 2020;15(1):71. Published 2020 Mar 30. https://doi.org/10.1186/s13014-020-01501-x.
Sayan M, Yehia ZA, Ohri N, Haffty BG. Hypofractionated Postmastectomy Radiation Therapy. Adv Radiat Oncol. 2020;6(1):100618. Published 2020 Nov 21. doi:https://doi.org/10.1016/j.adro.2020.11.003.
Poppe MM, Yehia ZA, Baker C, et al. 5-Year Update of a Multi-Institution, Prospective Phase 2 Hypofractionated Postmastectomy Radiation Therapy Trial. Int J Radiat Oncol Biol Phys. 2020;107(4):694–700. https://doi.org/10.1016/j.ijrobp.2020.03.020.
Torres MA, Horst KC, Freedman GM. Postmastectomy and Regional Nodal Radiation for Breast Cancer. J Clin Oncol. 2020;38(20):2299–309. https://doi.org/10.1200/JCO.19.02908.
Jagsi R, Li Y, Morrow M, et al. Patient-reported Quality of Life and Satisfaction With Cosmetic Outcomes After Breast Conservation and Mastectomy With and Without Reconstruction: Results of a Survey of Breast Cancer Survivors. Ann Surg. 2015;261(6):1198–206. https://doi.org/10.1097/SLA.0000000000000908.
Jagsi R, Momoh AO, Qi J, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst. 2018;110(2):157–65. https://doi.org/10.1093/jnci/djx148.
Manyam BV, Shah C, Woody NM, et al. Long-Term Outcomes After Autologous or Tissue Expander/Implant-Based Breast Reconstruction and Postmastectomy Radiation for Breast Cancer. Pract Radiat Oncol. 2019;9(6):e497–505. https://doi.org/10.1016/j.prro.2019.06.008.
Shumway DA, Momoh AO, Sabel MS, Jagsi R. Integration of Breast Reconstruction and Postmastectomy Radiotherapy. J Clin Oncol. 2020;38(20):2329–40. https://doi.org/10.1200/JCO.19.02850.
• O'Donnell JPM, Murphy D, Ryan ÉJ, et al. Optimal reconstructive strategies in the setting of post-mastectomy radiotherapy - A systematic review and network meta-analysis [published online ahead of print, 2021 Jul 9]. Eur J Surg Oncol. 2021;S0748–7983(21)00620-X. https://doi.org/10.1016/j.ejso.2021.07.001. This review was a network meta-analysis evaluating the impact of PMRT on the type and timing of breast reconstruction. Immediate autologous flap-based reconstruction was associated with reduced failure rates and surgical site infections.
Berbers J, van Baardwijk A, Houben R, et al. “Reconstruction: before or after postmastectomy radiotherapy?” A systematic review of the literature. Eur J Cancer. 2014;50(16):2752–62. https://doi.org/10.1016/j.ejca.2014.07.023.
Liveringhouse CL, Washington IR, Diaz R, et al. Genomically Guided Breast Radiation Therapy: A Review of the Current Data and Future Directions. Adv Radiat Oncol. 2021;6(4):100731. Published 2021 May 28. https://doi.org/10.1016/j.adro.2021.100731.
• Velikova G, Williams LJ, Willis S, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial [published correction appears in Lancet Oncol. 2018 Dec;19(12):e668]. Lancet Oncol. 2018;19(11):1516-1529. https://doi.org/10.1016/S1470-2045(18)30515-1. This substudy of the randomized controlled SUPREMO trial sought to examine the effect of chest wall radiotherapy on self-reported quality of life outcomes in intermediate-risk breast cancer patients. The 2-year results showed PMRT was associated with worse local symptoms, but the clinical significance was relatively low and symptoms improved with time.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. https://doi.org/10.1016/S0140-6736(05)67887-7.
Frandsen JE, Cannon G, Kokeny KE, et al. Is radiation indicated for young women with early stage, node-negative breast cancer after mastectomy? A multi-institution, retrospective review. Breast J. 2018;24(1):7–11. https://doi.org/10.1111/tbj.12827.
Montero A, Ciérvide R, García-Aranda M, Rubio C. Postmastectomy radiation therapy in early breast cancer: Utility or futility? Crit Rev Oncol Hematol. 2020;147:102887. https://doi.org/10.1016/j.critrevonc.2020.102887.
Rosso KJ, Tadros AB, Weiss A, et al. Improved Locoregional Control in a Contemporary Cohort of Nonmetastatic Inflammatory Breast Cancer Patients Undergoing Surgery. Ann Surg Oncol. 2017;24(10):2981–8. https://doi.org/10.1245/s10434-017-5952-x.
Rueth NM, Lin HY, Bedrosian I, et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. J Clin Oncol. 2014;32(19):2018–24. https://doi.org/10.1200/JCO.2014.55.1978.
van Uden DJP, van Maaren MC, Bult P, et al. Pathologic complete response and overall survival in breast cancer subtypes in stage III inflammatory breast cancer. Breast Cancer Res Treat. 2019;176(1):217–26. https://doi.org/10.1007/s10549-019-05219-7.
Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72(2):474–84. https://doi.org/10.1016/j.ijrobp.2008.01.039.
Damast S, Ho AY, Montgomery L, et al. Locoregional outcomes of inflammatory breast cancer patients treated with standard fractionation radiation and daily skin bolus in the taxane era. Int J Radiat Oncol Biol Phys. 2010;77(4):1105–12. https://doi.org/10.1016/j.ijrobp.2009.06.042.
Rehman S, Reddy CA, Tendulkar RD. Modern outcomes of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):619–24. https://doi.org/10.1016/j.ijrobp.2012.01.030.
Brown L, Harmsen W, Blanchard M, et al. Once-daily radiation therapy for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2014;89(5):997–1003. https://doi.org/10.1016/j.ijrobp.2014.01.054.
Stecklein SR, Rosso KJ, Nuanjing J, et al. Excellent Locoregional Control in Inflammatory Breast Cancer With a Personalized Radiation Therapy Approach. Pract Radiat Oncol. 2019;9(6):402–9. https://doi.org/10.1016/j.prro.2019.05.011.
Woodward WA, Debeb BG, Xu W, Buchholz TA. Overcoming radiation resistance in inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2840–5. https://doi.org/10.1002/cncr.25173.
Woodward WA, Fang P, Arriaga L, et al. A phase 2 study of capecitabine and concomitant radiation in women with advanced breast cancer [published correction appears in Int J Radiat Oncol Biol Phys. 2018 Jun 1;101(2):497]. Int J Radiat Oncol Biol Phys. 2017;99(4):777–783. https://doi.org/10.1016/j.ijrobp.2017.04.030.
Michmerhuizen AR, Pesch AM, Moubadder L, et al. PARP1 inhibition radiosensitizes models of inflammatory breast cancer to ionizing radiation. Mol Cancer Ther. 2019;18(11):2063–73. https://doi.org/10.1158/1535-7163.MCT-19-0520.
Paganetti H, Botas P, Sharp GC, Winey B. Adaptive proton therapy. Phys Med Biol. 2021;66(22). https://doi.org/10.1088/1361-6560/ac344f.
Mutter RW, Choi JI, Jimenez RB, et al. Proton Therapy for Breast Cancer: A Consensus Statement From the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int J Radiat Oncol Biol Phys. 2021;111(2):337–59. https://doi.org/10.1016/j.ijrobp.2021.05.110.
•• Jimenez RB, Hickey S, DePauw N, et al. Phase II Study of Proton Beam Radiation Therapy for Patients With Breast Cancer Requiring Regional Nodal Irradiation. J Clin Oncol. 2019;37(30):2778–2785. https://doi.org/10.1200/JCO.18.02366. This paper was the first prospective trial assessing the safety and efficacy proton beam radiation therapy for locally advanced breast cancer. The 5-year rates of disease control were comparable between proton therapy and conventional radiation. The results provided the framework for the phase III RadCOMP Consortium Trial.
Bekelman JE, Lu H, Pugh S, et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open. 2019;9(10):e025556. https://doi.org/10.1136/bmjopen-2018-025556.
Yap ML, Tieu M, Sappiatzer J, et al. Outcomes in Patients Treated with Post-mastectomy Chest Wall Radiotherapy without the Routine Use of Bolus. Clin Oncol (R Coll Radiol). 2018;30(7):427–32. https://doi.org/10.1016/j.clon.2018.03.005.
Nichol A, Narinesingh D, Raman S, et al. The Effect of Bolus on Local Control for Patients Treated With Mastectomy and Radiation Therapy. Int J Radiat Oncol Biol Phys. 2021;110(5):1360–9. https://doi.org/10.1016/j.ijrobp.2021.01.019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Local-Regional Evaluation and Therapy
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thompson, J.L., Allen, S.G., Pesavento, C. et al. Post-Mastectomy Radiation Therapy: Applications and Advancements. Curr Breast Cancer Rep 14, 75–82 (2022). https://doi.org/10.1007/s12609-022-00449-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-022-00449-z