Abstract
Purpose of review
With ever more sophisticated imaging modalities and screening programs, the incidence of small, non-palpable breast cancers is increasing. This poses a unique challenge to surgeons who seek to obtain negative margins while maintaining a cosmetically acceptable breast. Herein, we review the current localization techniques available and discuss the latest advancements.
Recent findings
While wire localization remains the historical gold standard for non-palpable breast lesion localization, many new invasive and non-invasive techniques have been utilized in recent years. These techniques can be performed by the surgeon alone or in conjunction with a radiologist partner. Multiple new techniques employ the insertion or deposit of a radioactive device or substance to identify the lesion. Positive margin status, clinician and patient comfort, and procedural time and ease have been serially evaluated as means to judge differences between localization techniques. However, the literature measuring these variables is heterogeneous with respect to definition across techniques making direct comparisons difficult. Further, the recent widespread adoption of “no tumor” at ink as a negative margin has revolutionized and standardized what constitutes a negative margin in invasive breast cancer. As a result, some of the previously reported benefits to certain localization techniques may not be as relevant today.
Summary
Localization techniques for non-palpable breast lesions are evolving. Trends away from wire-guided localization to radioactive-implanted sources to surgeon-directed, ultrasound-guided or non-radioactive implantable devices are occurring. Unfortunately, current literature is heterogenous with respect to type of localization technique and outcomes reported, making direct comparison between the multiple localization techniques difficult. As such, the main differences among techniques remain with volume of resection, source radioactivity, institutional resources, and surgeon and radiologist preference. The future will likely see implementation of technology that has the benefits of current techniques but without the associated limitations of tracking radioactive sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Halsted’s description of the radical mastectomy in 1882 became the mainstay of surgical treatment for breast cancer, changing only minimally for the next 80 years. Surgeons performed radical mastectomy for malignancy regardless of size. It was a “one-surgery-fits-all” dogma. This approach began to change dramatically with the introduction of the “quadrantectomy” (lumpectomy) in the late 1960s [1]. Skeptics of this less-is-more technique were largely quieted upon finding equivalent survival among women who underwent breast-conserving surgery (BCS) and those who underwent radical mastectomy at the 20-year mark [2]. This fostered the paradigm shift towards today’s use of BCS for patients with small breast cancers and no other contraindications. Today, the majority of newly diagnosed breast cancers are not palpable due to heightened emphasis on screening, advanced imaging modalities, increased patient awareness, and the increased use of neoadjuvant chemotherapy for down-staging breast cancers improving patient eligibility for BCS [3,4,5]. These trends have perpetuated surgeons’ interest in the best localization technique [6].
The goals of BCS include resection of the tumor with negative margins and cosmetic preservation of the breast. Historically, localization techniques have been judged on accuracy, ease of process, patient and surgeon convenience, volume of resection, and rates of positive margins. While many of these remain relevant today, the recent widespread adoption of “no tumor” at ink for invasive breast cancers and 2 mm for pure DCIS as acceptable negative margins has condensed the discussion and heterogeneity of margin status with respect to BCS [7, 8].
Historical Localization Techniques
Non-invasive techniques such as radiopaque markers or ink markings on the skin, and orthogonal mammography localization as the only means to direct the surgeon to the point of interest have largely become of historical interest only. These non-invasive localization techniques were plagued by inaccuracy and large excision volumes. In addition, considerable extrapolation of measurement was necessary to account for positional breast changes occurring between the localization position and the patient’s supine position on the operating table.
Contemporary Localization Options
Modern day localization options are classified according to their invasive or non-invasive approach. Herein, we briefly review available options, associated data, advantages and disadvantages, and novel techniques.
Invasive
Wire-Guided Needle Localization
First described in 1965 by Dodd, preoperative localization by means of a fine wire placed under image guidance has been the gold standard for localization of non-palpable breast lesions since that time [9]. A number of small modifications, such as adding a hooked tip to avoid wire migration and a reinforced portion to better identify the area of interest, have been made over the last 50 years, but the fundamental process and intent remain the same [10]. Patients are seen in the radiology department on the day of surgery where a guidewire with a thick and thin portion is placed into the lesion under either mammographic, US, or MRI guidance after infiltration of local anesthesia into the breast. While this localization technique has the advantage of versatility and can be used for any lesion under any imaging modality, there remain significant disadvantages. Most notably is the logistical component coupling the surgery and radiology schedules as most wires are placed the morning of the day of surgery which can result in operative delays. One recent small study did show feasibility of placing a long wire and affixing it to the skin either the night before or in a location away from the operating room; however, this is not the current practice in most hospitals [11]. The point of skin entry of the wire is at the discretion of the radiologist, which can result in an insertion site that is distant to the optimal surgical incision site as determined by the surgeon. The wire protrudes from the patient’s skin from the time of placement to the time of excision providing multiple opportunities for wire displacement or migration and patient distress and discomfort [12,13,14]. Flexible and soft wires reduce the risk for needlestick injury to staff, but are harder to place. Nonetheless, the technical skills required for wire-guided needle localization (WGL) serve as the foundation for skills needed for mastery of all other localization techniques.
Injectables
Additional invasive procedures require either the injection of a chemical substance marking the area of interest or the insertion of a radiolabeled device.
-
1.
Carbon Suspension: An inexpensive technique first reported by Svane in 1983, carbon suspension localization used preoperative injection of an aqueous suspension of carbon particles. This injection leaves a distinct trail from the lesion to the skin [15]. Small case series have shown this technique to be accurate and rapid to perform, with no risk of initial tissue or systemic reaction. The injected carbon does not disseminate into the breast nor is there reported interference with pathological examination of the excised lesion [16, 17]. This technique can be performed under stereotactic or US guidance [18]. While safe to perform, residual carbon injectate within the breast produces a giant cell reaction and, as a result, can simulate malignancy on follow-up imaging.
-
2.
Toluidine/Methylene Blue Dye: Similar in concept to the carbon suspension technique, this method utilizes blue dyes instead of inert carbon. Either preparation is injected immediately preoperatively [19, 20]. Methylene blue has a cost advantage over toluidine blue, but one study showed a smaller diffusion radius with toluidine [21]. Because of diffusion of the dyes within the breast tissue, timing of the injection in relation to the surgical excision must be carefully timed to allow for specific localization of the offending lesion. Too much time lapse between injection and incision can result in inaccurate localization. Also, importantly, both carry a risk of allergic reaction. Carbon suspension and blue dye localization are infrequently used as localization techniques.
-
3.
Cryo-assisted Localization (CAL): In CAL, a cryo-probe is inserted into the lesion under US guidance after the patient has been anesthetized, usually in the OR suite. The probe is then used to freeze the lesion, creating a palpable iceball that includes a margin of healthy tissue. The surgeon can then use the probe or the palpable iceball for guidance [22]. Unfortunately, the freezing process creates significant morphological changes to the tissue, making pathological review more difficult and immunohistochemical staining unreliable which can be problematic for assessment of malignant lesions [23]. Additionally, CAL has led to longer operative times as it requires the patient to be anesthetized and the lesion to be frozen intraoperatively prior to commencing the surgical resection.
-
4.
Hematoma Ultrasound-Guided/Sonographic Hematoma-Guided Localization (HUG/SHG): HUG/SHG is based on the fact that core needle and vacuum-assisted biopsy of breast lesions leave a sonographically visible hematoma. To enhance visualization, an additional 2–5 mL of the patient’s own blood can be injected into the lesion preoperatively. The surgeon then uses US guidance intraoperatively to guide excision of the hematoma-marked lesion(s). Some studies show HUG/SHG to be superior to wire localization in obtaining adequate margins and lower resection volumes, while others found them to be equivalent [24,25,26,27]. HUG/SHG require US visualization of the hematoma, an ultrasound-proficient surgeon, and timely surgery scheduled within 4–6 weeks to avoid hematoma reabsorption. However, this technique often avoids the additional preoperative scheduling and procedural conflicts seen when localization requires radiologist participation.
-
5.
Radioguided occult lesion localization (ROLL): Introduced in 1998 by Luini et al., ROLL involves intratumoral injection of a macroaggregate or nanocolloid human albumin labeled with 99Technetium (Tc-99) combined with approximately 0.2 mL of a non-ionic solution under sonographic or stereotactic guidance [28]. This can be done the day before or morning of surgery. Post-injection, accuracy can be confirmed by scintigraphy. Intraoperatively, a handheld gamma probe is used to guide resection. While study results are mixed, they show at least no difference between ROLL and WGL with regard to positive margins [29,30,31,32,33,34]. A recent meta-analysis showed that ROLL numerically had slightly lower positive margin rates and lower re-intervention rates, though neither was statistically significant [34]. Benefits to this technique are that it can be successfully combined with injections of differently labeled Tc-99 for sentinel node biopsy (SNB),Further, the procedure is similar to that for SNB and familiar to surgeons, allows more flexibility in OR scheduling, and eliminates the need for a wire extending from a patient’s chest [35,36,37]. Drawbacks to this technique include reliance on accurate injection by the radiologist, “shine-through phenomenon” with upper outer quadrant lesions making SNB difficult, and possibly institutional nuclear medicine regulations regarding personnel credentialed to inject Tc-99.
-
6.
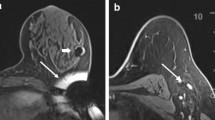
Radioactive Seed Localization (RSL): Introduced by Cox et al., RSL was a modification to the ROLL procedure utilizing a small, radio-opaque, 125Iodine (125I)-labelled titanium seed initially used in brachytherapy for prostate cancer. The 4 mm × 0.8 mm, 0.125–0.25 mCi 125I seed is placed under sonographic or stereotactic guidance into the center of the lesion (Fig. 1). With this technique, the radiation exposure from the seed is considered to be nominal. Obviously, the longer a seed is left in place, the larger dose seed is used, or the more seeds placed, the higher the radiation exposure to the surrounding breast tissue. With a single 0.125 mCi 125I seed placed 5 days prior to excision, breast tissue 2 cm from the seed itself will receive less than 2.8 cGy radiation [38]. In some instances where the lesion/calcifications are extensive or multifocal, multiple seeds can be safely placed to bracket the area of concern. Follow-up imaging can confirm seed placement and allow for surgical planning based on determination of the relationship between seed and biopsy clip. Since 125I has a half-life of 59.6 days, it can theoretically be placed up to 12 months before surgery. However, given variations in restrictions imposed by state nuclear regulatory agencies, most states restrict placement of these seeds to no more than a few days prior to the surgical procedure.
Intraoperatively, the surgeon uses a handheld gamma probe to locate the seed prior to incision and the incision can be placed anywhere on the breast suitable for the most cosmetic incision. The probe permits re-orientation during the procedure-guiding resection and then the same probe can be adjusted to the Tc-99 setting allowing completion of the SNB.
While the safety of the 125I seed is well documented, its use does require nuclear regulatory approval, staff training, and protocols for seed handling and disposal [38,39,40,41,42]. Establishing a radioactive seed localization program can take up to 9 months but is feasible even in a small community hospital setting [43]. Complications are rare and include seed migration, displacement in the OR, and failure to deploy seed by radiology [40]. Lost seeds need to be reported to the US Nuclear Regulatory Commission within 30 days and may be cited on the institution’s nuclear medicine license. As with other techniques, the data is not consistent when comparing RSL to ROLL or WGL, but shows at least no difference in the ability to achieve a negative margin resection and data is mixed on resection volumes though some studies suggest RSL and ROLL may achieve oncologic resection with smaller excision volumes when compared to WGL [34, 44,45,46,47,48,49].
The advantages of RSL include the ability to uncouple surgery and radiology schedules with placement of the seed days in advance, an easy to learn technique using familiar equipment, and the opportunity to make remote skin incisions while performing constant re-orientation intraoperatively with respect to the location of the seed. Disadvantages to RSL primarily stem from the radioactive nature of the seed which requires a multidisciplinary team including support from the institution radiation safety officer and pathology, nuclear regulatory approval, annual training on processes, and protocols for seed handling and disposal [38, 40,41,42,43]. Finally, while RSL-guided breast excision is an intuitive procedure and may be performed in many academic and private centers, transitioning from RSL to wire-guided excision where wire entry sites may be remote from the lesion to be removed can present a unique learning challenge for surgical trainees trained to do RSL but who are expected to do WGL when entering independent practice.
Non-invasive
Intraoperative Ultrasound Guidance
Similar to HUG/SHG, intraoperative US-guided localization can be done in the operative suite. Intraoperative Ultrasound Guidance (IOUS) can be performed to target the non-palpable lesion. In addition, in settings where the lesion is not sonographically visible, intratumoral markers can be placed to facilitate ultrasound guidance. Currently, several commercially available, sonographic detectable markers of variable lengths exist to facilitate this process [50]. Once the lesion is identified preoperatively, skin marks are placed to aid in resection trajectory and incision placement. A sterile probe is used to continuously monitor as the specimen is resected. This also allows for real-time monitoring of the resection cavity, ex vivo evaluation of the specimen, and evaluation of the need for additional margins. IOUS guidance can be successfully used for both invasive cancers and DCIS [51]. Multiple small studies and one meta-analysis have shown mixed results for IOUS compared to wire-guided localization with regard to positive margins and resection volumes, but most suggest they are at least comparable with respect to outcome [51,52,53,54,55,56].
IOUS has the advantages of allowing surgeons to be self-reliant, avoiding scheduling issues for preoperative localization through radiology, is least intrusive to the patient, is the most efficient use of OR time as it avoids post-excision specimen radiographs, and allows intraoperative assessment of margins. Furthermore, surgeons can also bill for the localization portion of the procedure. IOUS is limited by the fact that the lesion and/or the markers must be sonographically visible and that the surgeon has to be proficient in US skills.
Novel Techniques
-
1.
Radar Reflector: In December 2014, the FDA approved Cianna Medical’s SAVI SCOUT® Radar Localization System. Similar to WGL and RSL, this technique uses image guidance (US/mammography) for placement of a 12-mm-long reflector that includes two antennae, an IR light receptor, and a transistor switch (Fig. 2). Post-procedure imaging can be used for confirmation of placement and operative planning. Intraoperatively, a detector handpiece connected to a console emitting IR light and radar waves is used to guide dissection based on audible and visual feedback, similar to ROLL and RSL [57].
Exploratory studies have demonstrated this technique to be easy, feasible, safe, and effective [57,58,59]. These trials did identify a potential interaction with electrocautery disabling the reflector or loss of reflector signal when the distance between the probe and reflector was greater than 4.5 cm; however, the manufacturer has made subsequent design modifications rectifying these early challenges. Recent data finds this localization technique resulted in clear margins in 136/153 patients, with re-operation being required in only 22/153 [59]. Similar to RSL, radar reflectors can be placed in advance of surgery. As the reflector lacks radioactivity, the FDA approves placement of the reflector up to 30 days preoperatively. The biggest advantage over RSL, however, is the lack of nuclear medicine regulations as the reflector is not radioactive. Potential disadvantages of this new system include a handpiece that is not the same for SNB necessitating two separate systems for breast localization and SNB and a potential signal loss or non-detection at the skin with extreme distances between the reflector and handpiece though detection occurs during dissection when the handpiece nears the 4–5 cm range from the radar.
-
2.
Magnetic Occult Lesion Localization: Endomagnetics, Cambridge, UK, has recently published a feasibility study using injection of a magnetic tracer (Sienna + ®) and placement of a non-ferromagnetic marker coil under US guidance, followed by skin marking directly overlying the lesion. This was combined with injection of a radioisotope and blue dye for SNB in the magnetic sentinel node and occult lesion localization in breast cancer (MagSNOLL) Trial [57]. Intraoperative guidance was done with the use of a handheld magnetometer (SentiMAG®), similar to the RSL technique. Intraoperative specimen radiograph was obtained to confirm the presence of the marker coil and adequate surgical margins. Results of the feasibility study showed successful surgical excision in 20/20 (100%) patients with non-palpable breast cancers, while 2/20 (10%) required surgical re-excision for DCIS at the margins. This study demonstrates feasibility, with further trials currently enrolling. Similar to the electromagnetic reflector, this technique offers the benefit of RSL without the nuclear regulatory restrictions.
Conclusion
A growing number of women with non-palpable tumors are eligible for BCS requiring an adequate means of localization in order to excise the tumor with negative margins and optimize cosmesis by minimizing the volume of healthy breast tissue removed. Each localization technique has advantages and disadvantages, as summarized in Table 1; however, none is clearly superior to another [34, 58]. Further, it is more likely the characteristics of the tumor and the experience of the surgeon not the localization technique that will ultimately impact margin status [59]. Ideally, surgeons are familiar with all localization options available and facile with more than one. After considering risks, benefits, alternatives, and institutional restrictions, surgeons can determine the best localization option for their patients. It is possible the future may see relaxed nuclear regulatory restrictions for use of radioactive localization techniques or a switch to non-radioactive options allowing more surgeons more options.
References
Veronesi U. Conservative treatment of breast cancer: a trial in progress at the cancer Institute of Milan. World J Surg. 1977;1(3):324–6.
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32.
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Outlaw ED, Strom EA, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103(4):689–95.
Huang EH, Strom EA, Perkins GH, Oh JL, Chen AM, Meric-Bernstam F, et al. Comparison of risk of local-regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int J Radiat Oncol Biol Phys. 2006;66(2):352–7.
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–34.
Jakub JW. The search continues for the ideal method to localize nonpalpable breast lesions. Ann Surg Oncol. 2016;23(6):1799–800.
Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21(3):704–16.
Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol. 2016;6(5):287–95.
Dodd G, Fry K, Delany W. Pre-operative localization of occult carcinoma of the breast. Nealon TF Management of the patient with cancer Philadelphia: Saunders. 1965:88–113.
Hall FM, Frank HA. Preoperative localization of nonpalpable breast lesions. Am J Roentgenol. 1979;132(1):101–5.
Rodhouse C, Soliman I, Cruse M, Kastrenakes J, Augustine CJ, Ludy A, et al. Localization methods for excisional biopsy in women with nonpalpable mammographic abnormalities. Clin Breast Cancer. 2017;17(1):18–22.
Davis PS, Wechsler RJ, Feig SA, March DE. Migration of breast biopsy localization wire. AJR Am J Roentgenol. 1988;150(4):787–8.
Grassi R, Romano S, Massimo M, Maglione M, Cusati B, Violini M. Unusual migration in abdomen of a wire for surgical localization of breast lesions. Acta Radiol. 2004;45(3):254–8.
Seifi A, Axelrod H, Nascimento T, Salam Z, Karimi S, Avestimehr S, et al. Migration of guidewire after surgical breast biopsy: an unusual case report. Cardiovasc Intervent Radiol. 2009;32(5):1087–90.
Svane G. A stereotaxic technique for preoperative marking of non-palpable breast lesions. Acta Radiol Diagn (Stockh). 1983;24(2):145–51.
Mazy S, Galant C, Berliere M, Mazy G. Localization of non-palpable breast lesions with black carbon powder (experience of the Catholic University of Louvain). J Radiol. 2001;82(2):161–4.
Rose A, Collins JP, Neerhut P, Bishop CV, Mann GB. Carbon localisation of impalpable breast lesions. Breast. 2003;12(4):264–9.
Ko K, Han BK, Jang KM, Choe YH, Shin JH, Yang JH, et al. The value of ultrasound-guided tattooing localization of nonpalpable breast lesions. Korean J Radiol. 2007;8(4):295–301.
Egan JF, Sayler CB, Goodman MJ. A technique for localizing occult breast lesions. CA Cancer J Clin. 1976;26(1):32–7.
Nasrinossadat A, Ladan F, Fereshte E, Asieh O, Reza C, Akramossadat S, et al. Marking non-palpable breast masses with injected methylene blue dye, an easy, safe and low cost method for developing countries and resource-limited areas. Asian Pac J Cancer Prev. 2011;12(5):1189–92.
Czarnecki DJ, Feider HK, Splittgerber GF. Toluidine blue dye as a breast localization marker. AJR Am J Roentgenol. 1989;153(2):261–3.
Tafra L, Fine R, Whitworth P, Berry M, Woods J, Ekbom G, et al. Prospective randomized study comparing cryo-assisted and needle-wire localization of ultrasound-visible breast tumors. Am J Surg. 2006;192(4):462–70.
Sahoo S, Talwalkar SS, Martin AW, Chagpar AB. Pathologic evaluation of cryoprobe-assisted lumpectomy for breast cancer. Am J Clin Pathol. 2007;128(2):239–44.
Larrieux G, Cupp JA, Liao J, Scott-Conner CE, Weigel RJ. Effect of introducing hematoma ultrasound-guided lumpectomy in a surgical practice. J Am Coll Surg. 2012;215(2):237–43.
Layeequr Rahman R, Crawford S, Larkin A, Quinlan R. Superiority of sonographic hematoma guided resection of mammogram only visible breast cancer: wire localization should be an exception--not the rule. Ann Surg Oncol. 2007;14(8):2228–32.
Layeequr Rahman R, Iuanow E, Crawford S, Quinlan R. Sonographic hematoma-guided vs wire-localized lumpectomy for breast cancer: a comparison of margins and volume of resection. Arch Surg. 2007;142(4):343–6.
Thompson M, Henry-Tillman R, Margulies A, Thostenson J, Bryant-Smith G, Fincher R, et al. Hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol. 2007;14(1):148–56.
Luini A, Zurrida S, Galimberti V, Paganelli G. Radioguided surgery of occult breast lesions. Eur J Cancer. 1998;34(1):204–5.
Medina-Franco H, Abarca-Perez L, Garcia-Alvarez MN, Ulloa-Gomez JL, Romero-Trejo C, Sepulveda-Mendez J. Radioguided occult lesion localization (ROLL) versus wire-guided lumpectomy for non-palpable breast lesions: a randomized prospective evaluation. J Surg Oncol. 2008;97(2):108–11.
Moreno M, Wiltgen JE, Bodanese B, Schmitt RL, Gutfilen B, da Fonseca LM. Radioguided breast surgery for occult lesion localization—correlation between two methods. J Exp Clin Cancer Res. 2008;27:29.
Rampaul RS, Bagnall M, Burrell H, Pinder SE, Evans AJ, Macmillan RD. Randomized clinical trial comparing radioisotope occult lesion localization and wire-guided excision for biopsy of occult breast lesions. Br J Surg. 2004;91(12):1575–7.
Dua SM, Gray RJ, Keshtgar M. Strategies for localisation of impalpable breast lesions. Breast. 2011;20(3):246–53.
Nadeem R, Chagla LS, Harris O, Desmond S, Thind R, Titterrell C, et al. Occult breast lesions: a comparison between radioguided occult lesion localisation (ROLL) vs. wire-guided lumpectomy (WGL). Breast. 2005;14(4):283–9.
Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;12:CD009206.
Follacchio GA, Monteleone F, Anibaldi P, De Vincentis G, Iacobelli S, Merola R, et al. A modified sentinel node and occult lesion localization (SNOLL) technique in non-palpable breast cancer: a pilot study. J Exp Clin Cancer Res. 2015;34:113.
Monti S, Galimberti V, Trifiro G, De Cicco C, Peradze N, Brenelli F, et al. Occult breast lesion localization plus sentinel node biopsy (SNOLL): experience with 959 patients at the European Institute of Oncology. Ann Surg Oncol. 2007;14(10):2928–31.
Thind CR, Tan S, Desmond S, Harris O, Ramesh HS, Chagla L, et al. SNOLL. Sentinel node and occult (impalpable) lesion localization in breast cancer. Clin Radiol. 2011;66(9):833–9.
Pavlicek W, Walton HA, Karstaedt PJ, Gray RJ. Radiation safety with use of I-125 seeds for localization of nonpalpable breast lesions. Acad Radiol. 2006;13(7):909–15.
Dauer LT, Thornton C, Miodownik D, Boylan D, Holahan B, King V, et al. Radioactive seed localization with 125I for nonpalpable lesions prior to breast lumpectomy and/or excisional biopsy: methodology, safety, and experience of initial year. Health Phys. 2013;105(4):356–65.
Goudreau SH, Joseph JP, Seiler SJ. Preoperative radioactive seed localization for nonpalpable breast lesions: technique, pitfalls, and solutions. Radiographics. 2015;35(5):1319–34.
Graham RP, Jakub JW, Brunette JJ, Reynolds C. Handling of radioactive seed localization breast specimens in the pathology laboratory. Am J Surg Pathol. 2012;36(11):1718–23.
Sung JS, King V, Thornton CM, Brooks JD, Fry CW, El-Tamer M, et al. Safety and efficacy of radioactive seed localization with I-125 prior to lumpectomy and/or excisional biopsy. Eur J Radiol. 2013;82(9):1453–7.
Jakub J, Gray R. Starting a radioactive seed localization program. Ann Surg Oncol. 2015;22(10):3197–202.
Lovrics PJ, Cornacchi SD, Vora R, Goldsmith CH, Kahnamoui K. Systematic review of radioguided surgery for non-palpable breast cancer. Eur J Surg Oncol. 2011;37(5):388–97.
Murphy JO, Moo TA, King TA, Van Zee KJ, Villegas KA, Stempel M, et al. Radioactive seed localization compared to wire localization in breast-conserving surgery: initial 6-month experience. Ann Surg Oncol. 2013;20(13):4121–7.
van der Noordaa ME, Pengel KE, Groen E, van Werkhoven E, Rutgers EJ, Loo CE, et al. The use of radioactive iodine-125 seed localization in patients with non-palpable breast cancer: a comparison with the radioguided occult lesion localization with 99m technetium. Eur J Surg Oncol. 2015;41(4):553–8.
Janssen NN, Nijkamp J, Alderliesten T, Loo CE, Rutgers EJ, Sonke JJ, et al. Radioactive seed localization in breast cancer treatment. Br J Surg. 2016;103(1):70–80.
Diego EJ, Soran A, McGuire KP, Costellic C, Johnson RR, Bonaventura M, et al. Localizing high-risk lesions for excisional breast biopsy: a comparison between radioactive seed localization and wire localization. Ann Surg Oncol. 2014;21(10):3268–72.
Dryden MJ, Dogan BE, Fox P, Wang C, Black DM, Hunt K, et al. Imaging factors that influence surgical margins after preoperative 125I radioactive seed localization of breast lesions: comparison with wire localization. AJR Am J Roentgenol. 2016;206(5):1112–8.
Carmon M, Olsha O, Gekhtman D, Nikitin I, Cohen Y, Messing M, et al. Detectability of hygroscopic clips used in breast cancer surgery. J Ultrasound Med. 2017;36(2):401–8.
James TA, Harlow S, Sheehey-Jones J, Hart M, Gaspari C, Stanley M, et al. Intraoperative ultrasound versus mammographic needle localization for ductal carcinoma in situ. Ann Surg Oncol. 2009;16(5):1164–9.
Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systematic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–46.
Haloua MH, Volders JH, Krekel NM, Lopes Cardozo AM, de Roos WK, de Widt-Levert LM, et al. Intraoperative ultrasound guidance in breast-conserving surgery improves cosmetic outcomes and patient satisfaction: results of a multicenter randomized controlled trial (COBALT). Ann Surg Oncol. 2016;23(1):30–7.
Harlow SP, Krag DN, Ames SE, Weaver DL. Intraoperative ultrasound localization to guide surgical excision of nonpalpable breast carcinoma. J Am Coll Surg. 1999;189(3):241–6.
Rahusen FD, Bremers AJ, Fabry HF, van Amerongen AH, Boom RP, Meijer S. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol. 2002;9(10):994–8.
Rubio IT, Esgueva-Colmenarejo A, Espinosa-Bravo M, Salazar JP, Miranda I, Peg V. Intraoperative ultrasound-guided lumpectomy versus mammographic wire localization for breast cancer patients after neoadjuvant treatment. Ann Surg Oncol. 2016;23(1):38–43.
Ahmed M, Anninga B, Goyal S, Young P, Pankhurst QA, Douek M, et al. Magnetic sentinel node and occult lesion localization in breast cancer (MagSNOLL trial). Br J Surg. 2015;102(6):646–52.
Landercasper J, Attai D, Atisha D, Beitsch P, Bosserman L, Boughey J, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Annals of surgical oncology. 2015;22(10):3174–83.
Bennett C. Use of radioactive seed localization for nonpalpable breast cancer. LWW; 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jinny Gunn and Sarah McLaughlin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Local-Regional Evaluation and Therapy
Rights and permissions
About this article
Cite this article
Gunn, J., McLaughlin, S. Current Trends in Localization Techniques for Non-palpable Breast Lesions: Making the Invisible Visible. Curr Breast Cancer Rep 9, 165–171 (2017). https://doi.org/10.1007/s12609-017-0244-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-017-0244-9