Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor, classically associated with the regulation of xenobiotic metabolism in response to environmental toxins. In recent years, transgenic rodent models have implicated AhR in aging and longevity. Moreover, several AhR ligands, such as resveratrol and quercetin, are compounds proven to extend the lifespan of model organisms. In this paper, we first review AhR biology with a focus on aging and highlight several AhR ligands with potential anti-aging properties. We outline how AhR-driven expression of xenobiotic metabolism genes into old age may be a key mechanism through which moderate induction of AhR elicits positive benefits on longevity and healthspan. Furthermore, via integration of publicly available datasets, we show that liver-specific AhR target genes are enriched among genes subject to epigenetic aging. Changes to epigenetic states can profoundly affect transcription factor binding and are a hallmark of the aging process. We suggest that the interplay between AhR and epigenetic aging should be the subject of future research and outline several key gaps in the current literature. Finally, we recommend that a broad range of non-toxic AhR ligands should be investigated for their potential to promote healthspan and longevity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that regulates gene expression and xenobiotic metabolism. Recently, this transcription factor has been associated with aging and longevity (1, 2). Aging research has identified many nutritional and pharmacological compounds that affect the aging process with some that can be considered as anti-aging therapies (3). Interestingly, many of these compounds are AhR ligands, suggesting that activation of the AhR pathway may have fundamental roles in aging/antiaging biology. However, the fact that many of these compounds act through a common mechanism, i.e., AhR, has been underappreciated to date. Moreover, the ability of AhR to bind to DNA is strongly influenced by epigenetic states at its binding sites (4, 5). Changes to epigenetic states are a hallmark of the aging process6 and several AhR ligands with anti-aging properties also affect epigenetic states as we outline below. Therefore, the goals of this paper are to 1) review the role of AhR in aging and longevity, 2) review AhR ligands that show antiaging properties and 3) highlight potentially important epigenetic aging mechanisms that may influence AhR function in the aging process. We argue that an improved understanding of the interplay between AhR modulation, epigenetics and the aging process may inform the development of improved anti-aging supplements or therapies.
The biology of AhR: from regulation of xenobiotic metabolism to several fundamental physiological roles
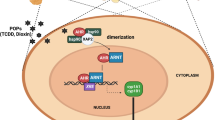
AhR was first identified in the 1970s as the cellular binding protein of the potent toxin tetrachlorodibenzo-p-dioxin (TCDD) (7, 8). AhR is a member of the Basic-Helix/Loop/Helix Per-Arnt-Sim (bHLH/PAS) family of transcription factors, but it is the only ligand-activated member of the family (9). In the cytoplasm, AhR is usually in an inactive state being bound to the molecular chaperone heat shock protein 90 (HSP90), co-chaperone p23, and XAP2 (10) (Figure 1). AhR binding to a ligand or modulator such as TCDD leads to dissociation of the chaperones. AhR then shuttles to the nucleus where it dimerizes with one of the Aryl hydrocarbon receptor nuclear translocators (ARNT1, ARNT2 and ARNT3). The resulting heterodimer can bind to several xenobiotic responsive elements (XREs) depending on the cell type and the modulating ligand. For example, the canonical XRE ([T/C]GCGTG) (Figure 2a) activates the CYP1A1 gene and XRE II (CATG{n6}C[T/A] TG) (Figure 2b) activates CYP1A2 which are the classical AhR detoxification pathways (9, 11). Other XREs include: TCDD-unresponsive, BaP-responsive XRE, which is activated by Benzo(α)pyrene (BaP); TCDD-unresponsive, polyphenol-responsive XRE, which is activated by 3-Methylcholanthrene, BaP and quercetin; Tyrosine hydroxylase AhRE III; Cathepsin D, c-fos, pS2 iXRE and RelB—AhRE, which are all activated by TCDD (11). In response to the binding with the XRE, three mechanisms have been suggested as negative feedback to the AhR activity; 1) the metabolism and excretion of the activating ligand, 2) the induction of Aryl-Hydrocarbon Receptor Repressor (AHRR), which competes with the AhR/ARNT complex in binding to the XRE and 3) by the ubiquitylation and proteasome-mediated degradation of the AhR protein (9, 12).
The role of the transcription factor AhR in drug metabolism
AhR serves as an intracellular mediator of the xenobiotic metabolic pathway as it regulates the transcription of several phase I and phase II drug-metabolizing enzymes (left panel). Upon ligand binding, AhR decouples from molecular chaperones and shuttles to the nucleus where it forms a heterodimer with ARNT, allowing it to bind to XREs to regulate gene transcription. Figure created with https://BioRender.com.
AhR is constitutively expressed in several tissues (Figure 2c), with the highest levels of AHR mRNA detected in the urinary bladder, gall bladder, and lungs. It is also expressed at high levels in the appendix, liver, bone marrow, stomach, esophagus, and placenta. It is expressed in lower levels in different parts of the gastrointestinal tract, spleen, skin, thyroid, brain, and lymph nodes. The exact number of genes regulated by AhR is still unknown and will be tissue dependent. However, the plethora of tissues where AhR is expressed is indicative of its extensive role in normal physiology. In addition to xenobiotic metabolism, AhR is involved in physiological functions such as immunological reactions (13), hematopoietic stem cell (HSC) proliferation (14), reproductive health (15), and normal aging (1).
The role of the transcription factor AhR in normal aging: multiple aging processes with a common AhR connection
Aging is an extremely complex biological phenomenon and part of the challenging nature of aging research is that aging is not a disease but the result of the interplay of numerous factors6. While our understanding of AhR has historically focused on its role in xenobiotic metabolism, here we consider the role of AhR in aging. We focus on two aspects, the effect on lifespan and healthspan. The latter term can be generally defined as the time spent in good health, free from chronic diseases or any age-related disabilities16.
Laboratory rodent studies
Ahr knockout mice (Ahr-/-) have shown that AhR deficiency accelerates the aging process and is associated with a premature aging phenotype. Hirabayashi & Inoue (2009)17 found that Ahr knockout mice had a significantly reduced lifespan in a gene-dosage-dependent manner. Fernandez-Salguero et al. (18) showed that 40–50% of the Ahr-/- mice died or were selectively cannibalized within four days after their birth and those who survived had a slower growth rate. Moreover, both studies found reduced fertility in Ahr-/- mice when they reached adulthood (17, 18).
Ahr knockout mouse models have shown a wide range of age-associated phenotypes. Bravo-Ferrer et al. (2019) (19) demonstrated that Ahr-/- mice up to 24 months old had a significant increase in cardiac hypertrophy, liver fibrosis, splenomegaly, rectal prolapse, kyphosis and decline in motor function when compared to controls. They also showed that AhR deficiency promotes anatomic signs of brain aging including loss of white matter, premature spatial memory impairment and greater astrogliosis in the hippocampus. In particular, they focused on age-associated inflammation (“inflammaging”) and found that AhR deficiency is associated with increased cytokine levels. The study also reported that the brain levels of AhR protein decrease with age in wild-type mice further indicating a link between AhR and aging (19).
Hirabayashi & Inoue (17) also showed that Ahr knockouts had lower antioxidative function in the hematopoietic microenvironment with low oxidative tension, and a reduction in the fraction of the dormant stem cell/progenitor compartment. This reduction increases the differentiation of hematopoietic progenitor cell compartment and leads to early exhaustion (17). Furthermore, their HSCs showed increased levels of reactive oxygen species, increased staining for γ-H2A.X (a sensitive indicator of DNA damage) as well as an increase in Rad50 mRNA (a double-strand break repair protein). This dysregulation of HSCs was supported by Singh et al. (2009) (20) who showed that Ahr knockout results in the altered ability of HSCs to sense appropriate signals in the bone marrow microenvironment leading to hematopoietic disease of imbalance between quiescence and proliferation (14, 20). Singh et al. also examined gene expression changes in SLAM+ cells (surface glycoproteins in the immunoglobulin superfamily CD150 and CD48) in Ahr knockout mice. They found 673 differentially expressed genes, including an upregulation of genes linked with leukemia and abnormal proliferation, and an almost fourfold upregulation mechanistic target of rapamycin (Mtor). Its encoded protein mTOR is considered a regulator of many hallmarks of aging and lifespan and has been extensively investigated in aging research (21, 22). Other age-associated phenotypes connected with Ahr knockouts include the inability to maintain glucose and lipid homeostasis (23), increased levels of cytokines that correlate with inflammaging (19), increased uric acid stones in the urinary bladder (24), development of minor renal insufficiency (25). In contrast, Eckers et al. showed a positive aging sign in Ahr knockout mice, whereby they exhibited a decrease in vascular stiffness (26). Taken together, though, the current literature more often reports reduced lifespan and increased aging signs in Ahr knockout mice.
Caenorhabditis elegans studies
Only a few studies have investigated AhR and aging in C. elegans and these have contradictory findings. Chamoli et al. observed increased ahr-1 expression in worms subjected to dietary restriction (27), an intervention that increases lifespan across several species (28). On the other hand, Brinkmann et al. investigated the resistance of C. elegans to different types of stressors and found that RNAi targeting the ahr-1 gene did not extend the lifespan (29). Eckers et al. (26) found that AhR-deficient C. elegans worms have increased life span and health span, indicated by their increased motility and stress resistance when compared to controls (26). Aarnio et al. showed several physiological disadvantages in ahr-1 mutant C. elegans including slower larval development, fewer eggs laid, a higher number of dead embryos and movement deficits compared with the wild-type (30). However, no differences in longevity in the mutants were reported. Overall, the evidence relating AhR and aging/longevity in C. elegans is inconclusive, in contrast to the more clear-cut findings in mice.
Human studies of AhR and aging
Several human studies have found connections between AhR and aging. The expression of genes in the AhR/ARNT pathway and the AHRR (AhR repressor) was shown to be different between adults 30–50 years old, septuagenarians (70 to 79 years old) and centenarians (100 or more years old). Moreover, centenarians maintained a high expression of AHR, ARNT, CYP1B1 genes and a low expression of AHRR like younger individuals, while septuagenarians showed a significant decrease of AHR, ARNT, CYP1B1 expression and a significant increase of AHRR as compared to young individuals (31, 32). These findings are particularly interesting because they align with the concept of centenarians as “super agers” that have slower aging processes when compared to other older adults.
Exercise is known to have a strongly beneficial influence on human healthy aging, chronic disease and immunity (13, 33). A recent randomized cross-over trial investigating the effects of endurance and resistance exercise on AhR found that both types of exercise can impact AhR levels (34). This trial showed that a single 50-minute session of both types of exercise was able to reduce the gene expression levels of AhR in peripheral blood samples, which could be explained by the increase of the AhR ligand kynurenine that is reported to increase after exercise (35).
Brinkmann et al. (1) pointed to a possible crosstalk between AhR and the mitochondria. Not only does AhR influence mitochondrial function, but some AhR is localized within the mitochondria (the intermembrane space (36) when not bound to any ligands. For example, Hwang et al. (36) showed that when murine hepatoma cells were treated with increasing amounts of TCDD the localization of AhR in the intermembrane space was lost indicating the essential role of the ligand in this process. Brinkmann et al. (1) further suggested that the effect of AhR on the mitochondria is complex and likely varies by tissue, age, and sex, and ligand. Yet, this is potentially an important connection as mitochondrial dysfunction is considered one of the hallmarks of aging (6).
Human genome-wide association studies (GWAS) implicating AhR
GWASs have successfully identified thousands of genetic loci that predispose individuals to certain traits and diseases. Using the GWAS catalogue (https://www.ebi.ac.uk/gwas/) we reviewed all GWAS that identified an association with the human AHR gene. Of the 104 associations from 75 studies, the majority were with bitter non-alcoholic beverage consumption, such as coffee. This is unsurprising as coffee stimulates expression of AHR and is metabolized by AhR-responsive genes such as CYP1A2 (37). Many of the other GWAS hits (Table 1) are associated with phenotypes that are linked with aging, including a decline in kidney function, dyslipidemia and chronic inflammatory disease.
Focusing on the aging process itself, a GWAS conducted by Zhang et al. found an association between genetic variants at the AHR locus and DNA methylation variation in normal aging (38). The epigenome changes extensively during the aging process (6) and, among known epigenetic marks, methylation of cytosine residues in DNA (5-methylcytosine or 5mC) has been the most studied. For example, “epigenetic clocks” use 5mC levels at several CpG positions around the genome to accurately identify the age of individuals (39). In their study Zhang et al. (38) identified CpG sites with statistically significant variation in the rate of change of 5mC among older individuals, and one of these sites was at AHR. More broadly, Farley & Jarman et al. (2) derived a predictive lifespan clock based on CpG density in a selected set of promoters across forty different vertebrate species. They implicated a specific CpG-dense regulatory region at AHR associated with longevity. Taken together, these studies suggest importance of epigenetic states at AHR in aging.
AhR ligands with anti-aging properties
AhR binds to a wide variety of ligands. The canonical ligands in the AhR literature are mostly exogenous toxins including dioxin, 3-methylcholanthrene and benzo[a]pyrene (40, 41). AhR also binds to numerous endogenous ligands (42), indicative of the fact that this transcription factor has more physiological functions beyond mediating the toxicity of environmental pollutants. It also responds to many ligands derived from plants and synthetic chemicals used as pharmaceuticals in clinical practice. Several AhR ligands have anti-aging properties, and this section focuses on the role of AhR ligands in the anti-aging process.
Plant-derived ligands
AhR binds many plant-derived compounds with putative anti-aging properties including: resveratrol, a type of phenol produced by many plants in response to stress or injury; quercetin, a naturally occurring flavonoid, and curcumin, a yellow spice and coloring agent.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) was first isolated from the roots of Veratrum grandiflorum (43). It is present in many plants such as grapes, peanuts, cocoa, berries and soy, and has ROS scavenging properties making it a potent antioxidant (44). In addition to being a known antagonist of AhR (45), resveratrol was shown to activate sirtuin 1 through the NAD+ pathway (46). Several early studies suggested that resveratrol can extend lifespan via stimulating sirtuins which are the same pathways activated by calorie restriction in different species: S.cerevisiae (47), C. elegans (48, 49), and D. melanogaster (50, 51). However, the mechanistic link between resveratrol’s anti-aging effect and sirtuins was subsequently called into doubt, and some initial findings were not reproducible (52, 53). Subsequent meta-analysis (54) and reviews of the resveratrol literature in model organisms (55) concluded that overall resveratrol acts as a life-extending agent, but the underlying mechanism through which this occurs remains unclear. Several clinical trials have found beneficial effects of resveratrol in humans including improvement of skeletal muscle mitochondrial function (56), bone mineral density (57), cerebrovascular function, memory and cognition (58, 59).
Quercetin (3,31,41,5,7-pentahydroxyflavone) is a naturally occurring polyphenol flavonoid, found in fruits and vegetables, including onions, apples, berries, tea, tomatoes and citrus fruits (60). Quercetin is an AhR agonist and directly competes with TCDD as a ligand (61). Quercetin also increases CYP1A1 gene expression (CYP1A1 is regulated by AhR) and interferes with the degradation of 6-formylindolo[3,2-b]carbazole (an endogenous AhR ligand) (62). Quercetin has also been investigated as a senolytic drug, a class of anti-aging drugs that selectively clear senescent cells and have a potential role in cancer treatments (63). Quercetin has been shown to extend lifespan in several animal models. For example, in a C. elegans study, Pietsch et al. showed that quercetin caused a lifespan extension in a dose-dependent manner (64). They reported life span extensions of 11% and 18% in response to 100 and 200 µM of quercetin, respectively. Kampkotter et al. reported similar results with an extension of 15% with 100 µM of quercetin (65). In a mouse model transplanted with senescent cells, administration of the senolytic cocktail of dasatinib plus quercetin reduced the number of naturally occurring senescent cells (66).
Using a D. melanogaster model, Saul et at. showed that quercetin increased mean lifespans up to 15% (67), whereas Proshkina et al. showed no obvious life extension (68).
Curcumin ((1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione)) is an AhR agonist extracted from Curcuma longa (69). It is extensively used as a spice in curry and mustard and gives them a distinctive yellow color. It has antioxidant and anti-inflammatory effects and can prolong the mean lifespan of aging model organisms such as C. elegans (70), D. melanogaster (71), and mouse (72). In C. elegans treated with curcumin, the mean lifespan increased by 39% and the maximum lifespan by 21%. Curcumin extended the life span of two different strains of D. melanogaster. More specifically, curcumin at 100 µM extended the life span of Canton-S female flies by 19% and at 250 µM extended the life span of Ives male flies by 16%. In C57BL/6 mice, those receiving curcumin had an average of 10% longer survival. Curcumin has been shown to have beneficial effects on many age-associated disease states including cancer (73, 74), rheumatoid arthritis (75, 76), cardiovascular disease (77), liver disease (78), and neurodegenerative diseases (79–81).
Pharmaceutical ligands: Metformin
Metformin has antioxidant and anti-inflammatory properties, and it is used mainly for the management of diabetes to improve insulin sensitivity for type 2 diabetes (82). Metformin has attracted interest in the field of aging biology because several studies have shown that it increases healthspan and longevity in animals (83, 84). The drug is currently under testing in the Targeting Aging with Metformin (TAME) Trial to examine whether it has anti-aging effects in humans (85). The exact anti-aging mechanism of metformin is still unknown; however, many are being investigated. In the context of AhR, one study showed that metformin at low doses inhibited mast cells stimulated with the AhR ligand, 5,11-dihydroindolo[3,2-b] carbazole-6-carbaldehyde (FICZ). Furthermore, metformin was shown to inhibit the IgE receptor (FceR1) and AhR-mediated passive cutaneous anaphylaxis, indicating the role of metformin on the AhR pathway (86). Metformin also inhibited endogenous AhR ligand-induced CYP1A1 and CYP1B1 expression in breast cancer cells (87). In summary, metformin has clearly beneficial effects on aging and also modulates AhR activity and/or downstream gene expression, but additional work is needed to identify if it interacts with AhR directly, or acts competitively with other AhR agonists/antagonists.
Xenobiotic metabolism in aging
AhR and its ligands can profoundly affect longevity and healthspan, but the mechanism(s) through which AhR affects the aging process is unclear. Some commentators have suggested that AhR affects overall levels of reactive oxygen species (42) or the function of mitochondria1, both of which may be relevant to an extent. However, an alternative explanation could be that moderate stimulation of AhR helps to maintain the integrity of xenobiotic metabolism into old age. Expression of xenobiotic metabolizing genes and enzymes is elevated in liver tissue of long-lived mouse models such as calorie-restricted mice and long-lived dwarf mice (88–90), while resistance to oxidative toxins is a hallmark of long-lived mouse models (89). Mutation of xenobiotic metabolizing genes in several organisms can affect lifespan (91, 92) and genetic variation in xenobiotic metabolizing genes has been associated with longevity in several human studies (93, 94). There is now substantial and convincing evidence from worm to mouse to human that upregulation of genes involved in xenobiotic detoxification is a common mechanism of increased longevity across phyla (95, 96). Therefore, a deeper understanding of mechanisms governing AhR-driven expression of xenobiotic metabolism genes into old age could increase our understanding of the biology of healthy aging.
Epigenetics and AhR regulation of drug-metabolizing genes in aging
A significant body of work has demonstrated that xenobiotic metabolism genes are under epigenetic control (97, 98) and epigenetic change is a hallmark of the aging process6. Moreover, epigenetic aging has been shown to affect expression of xenobiotic metabolism genes (99). AhR, like other TFs, is strongly influenced by epigenetic states at its target genes (4, 5). However, no studies have yet investigated AhR in the context of epigenetic aging. Therefore, we asked the question, are AhRregulated genes subject to epigenetic aging?
To answer this, we first obtained AhR Chromatin immunoprecipitation (ChIP) data from Fader et al. 2017 that identified areas in the mouse liver genome where AhR binds following activation by TCDD (100). Next, we obtained age-differentially methylated regions (a-DMRs) in the mouse liver genome from Sandoval-Sierra et al., 2020 (101). We then used GREAT (102) to identify the set of genes associated with each set of loci. We then tested for enrichment of liver genes under AhR control among liver genes that undergo epigenetic aging as shown in Table 2. Fisher’s exact test yielded an enrichment p-value < 2.2 × 10−16 and an odds ratio of 4.8. We then ran the 129 gene list in a gene ontology (GO) analysis in PANTHER (103) and DAVID (104). One significant category that we found was “cellular response to chemicals”, which was significantly enriched in both analyses. This category includes a lot of xenobiotic metabolism genes. The enrichment ratios were 1.93-fold (p= 3.172 × 10−5, and FDR= 4.982 × 10−2) and 1.54-fold (p= 4.3 × 10−3, and FDR= 0.2), for PANTHER and DAVID respectively. Restricting the analysis to just genes expressed in the liver obtained similar results (Table 2). For just liver-expressed genes, the GO category “cellular response to chemicals” was significant in PANTHER with 2.48-fold enrichment (p= 1.93 × 10−5, and FDR= 1.60 × 10−2) but not significant in DAVID with 1.23-fold enrichment (p= 2.4 × 10−3, and FDR= 1.1 × 10−1). These findings suggest that genes regulated by AhR that undergo epigenetic change with age are involved in xenobiotic response.
Discussion and Conclusions
In this article, we have shown several lines of intersecting evidence that indicate the importance of AhR to aging biology. As a ligand-dependent TF, the role of AhR ligands in the aging process is also intriguing, while the significant overlap between genes subject to epigenetic aging in the liver and those regulated by AhR suggests a possible interplay between epigenetic aging and AhR. While these findings are of interest, there are gaps in our knowledge of AhR and some instances of apparently conflicting evidence. We will now address several of these issues and suggest the areas where further research would be of immediate benefit.
First, we reviewed evidence that deletion of AhR in rodent models leads to reduced lifespan. Other studies in mice and humans were consistent with this direction of effect, suggesting that up-regulation of AhR is beneficial for longevity and healthspan. However, other commonly used aging models, particularly C. elegans, showed either no aging effect or conflicting evidence for the role of AhR on longevity. A possible explanation is that mammalian AhR is functionally distinct from AhR in invertebrates because the latter does not bind to the canonical ligand TCDD, due to structural differences at the ligand binding domain of the protein (11, 105). Therefore, it is possible that the involvement of AhR in the aging process is somehow related to a vertebrate-specific or perhaps even mammalian-specific function. Currently, insufficient studies have been performed in different species to draw general conclusions. We recommend that additional studies of AhR and aging, in the context of different ligands, are conducted in a broader range of invertebrate and vertebrate species to delineate potential relevant pathways.
Second, there is an apparent inconsistency with respect to the AhR ligands outlined above, with quercetin and curcumin being referred to as AhR agonists and resveratrol being referred to as an AhR antagonist in many studies. How could the apparently opposite properties of these ligands lead to the same outcome of increased longevity and healthspan? To reconcile this apparent conflict, we must first define what it means to be an agonist versus antagonist for a ligand-dependent TF. Typically, agonists bind to the TF and activate it, causing the downstream expression of several gene pathways, while antagonists bind to the TF and prevent it from activating those pathways. Most often, in the case of AhR, the agonist/antagonist distinction is based on whether or not the ligand elicits the downstream activation of CYP1A1 and CYP1A2. Resveratrol downregulates the expression of these xenobiotic metabolism genes, but on the other hand it up-regulates alternative pathways such as paraoxonase 1 (PON1) (106). This activation of alternative pathways while suppressing canonical pathways possibly calls into question the usefulness of the agonist/antagonist distinction in the case of AhR (11). Clearly, additional work is necessary to fully evaluate the complement of genes activated by AhR in response to the binding of different ligands. At present, we are only aware of chromatin immunoprecipitation sequencing (ChIP-seq) studies of AhR binding in mouse liver following administration of TCDD100. Repeating such an experiment for different ligands in both rodent liver and human hepatocytes in culture would yield valuable information on this topic.
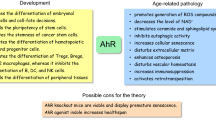
On a related note, it is interesting to highlight the distinction between AhR agonists such as quercetin and curcumin, which have positive effects on longevity and health, as compared to toxic agonists such as TCDD which are clearly detrimental to health. While the inherent toxicity of the ligands themselves plays a role, it has been suggested that moderate activation of AhR is beneficial with respect to aging while over-activation can lead to potential unwanted side effects such as production of free radicals1. Therefore, AhR-activating ligands of moderate potency are preferable as dietary supplements (Figure 3).
Summary of the AhR activation on the aging phenotype
The type and dose of AhR ligands have varying effects on aging phenotype; (a) potent AhR ligands like TCDD produce accelerated aging phenotype and toxic effects, (b) AhR ligands have beneficial anti-aging effects, (c) AhR knockout models lack the beneficial effects of ligands. Figure created with https://BioRender.com.
We have suggested that AhR exerts its effects on the aging process, at least partly, through the up-regulation of xenobiotic metabolism genes. Epigenetics is an essential mediator between the activation of AhR and its ability to bind to its target genes, through the regulation of chromatin state. It is now well established that epigenetic aging is a hallmark of the aging process (6), but less well appreciated is that AhR ligands themselves may affect epigenetic states. Toxins such as Agent Orange (a herbicide mixture used during the Vietnam War that contains dioxin) were found to impact the epigenome of several tissues in veterans (107, 108). In another study, dioxin was found to induce AhR-dependent DNA demethylation of the CYP1A1 promoter in mouse liver, a significant increase in histone H3 lysine 4 trimethylation (H3K4me3) and a significant decrease in histone H4 Lysine 20 trimethylation (H4K20me3) (109).
In addition to the epigenetic effects of these toxic AhR ligands, research has been conducted on the epigenetics of the putative anti-aging compounds that we highlighted above. Resveratrol was reported to effectively reverse epigenetic changes associated with activation of the AhR and its binding to the BRCA1 promoter in breast cancer cells (110). Moreover, a reverse association between serum resveratrol levels and Ras Association Domain Family Member-1a (RASSF1A) methylation was observed, resulting in a higher expression of this tumor suppressor gene (110, 111). Resveratrol has been epigenetically investigated with many other cancers (112). Quercetin has also been reported to have a broad spectrum of cancer-preventive activities, but like resveratrol no aging epigenetic studies investigated its aging effects. It has been shown to inhibit DNA methyltransferase (DNMT) activity in vitro and was associated with the upregulation of both mRNA and protein levels of p16 (a tumor suppressor protein)113 resulting in an inhibition of cell proliferation114. Furthermore, quercetin and curcumin have been shown to enhance BRCA1 expression in Triple Negative Breast Cancer cells (TNBC). The BRCA1 promoter histone H3K9 acetylation was significantly increased with the combined treatment of quercetin and curcumin (115). Moreover, curcumin treatment restored BRCA1 expression through reduction of its promoter methylation level (116). Overall, these studies show the potential epigenetic effects of AhR ligands and these effects could have profound implications for the aging process. Current research on the interaction between these ligands and epigenetic aging are sorely lacking, both with respect to AhR and to aging biology more generally.
Finally, we speculate that other AhR ligands could have anti-aging properties, potentially with greater efficacy than those shown here. For example, Sonowal et al. (117) showed that indoles from commensal bacteria extend healthspan in multiple species (C. elegans, Drosophila melanogaster, and mice) and alter patterns of gene expression in old animals to resemble the young. Interestingly, many indoles including indole-3-acetonitrile (118), 5-hydroxyindole-3-acetic acid (119), and idole-3-acetic acid (120) have been reported to bind to AhR. Snonowal found that AhR-dependent genes represented 36% of indole-regulated genes (117) proposing that indoles may promote healthy aging.
In summary, AhR is intrinsically associated with healthy aging and some of its weaker, natural ligands may have anti-aging properties. We suggest that these anti-aging effects are a result, at least in part, of up-regulation of xenobiotic metabolism into old age. The interplay between epigenetic aging and AhR has not been widely investigated, but we showed through the integration of publicly available datasets that AhR-regulated genes in the liver are over-represented among genes undergoing epigenetic aging. While these intersecting lines of evidence point towards a complex interplay between AhR, its ligands and epigenetics, insufficient research has been carried out in this area. Additional, naturally-occurring dietary AhR ligands should be investigated for their effects on the aging process.
References
Brinkmann, V., Ale-Agha, N., Haendeler, J. & Ventura, N. The Aryl Hydrocarbon Receptor (AhR) in the Aging Process: Another Puzzling Role for This Highly Conserved Transcription Factor. Front Physiol. 2020;10. doi:https://doi.org/10.3389/fphys.2019.01561.
Mayne, B., Berry, O., Davies, C., Farley, J. & Jarman, S. A genomic predictor of lifespan in vertebrates. Scientific Reports. 2019;9:17866. doi:https://doi.org/10.1038/s41598-019-54447-w.
Ros, M. & Carrascosa, J. M. Current nutritional and pharmacological anti-aging interventions. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165612. doi:https://doi.org/10.1016/j.bbadis.2019.165612.
Miura, T., Onodera, R., Terashima, J., Ozawa, S. & Habano, W. β-naphthoflavone-induced upregulation of CYP1B1 expression is mediated by the preferential binding of aryl hydrocarbon receptor to unmethylated xenobiotic responsive elements. Exp Ther Med. 2021;22:1410. doi:https://doi.org/10.3892/etm.2021.10846.
Jiang, J. et al. Role of DNA methylation-related chromatin remodeling in aryl hydrocarbon receptor-dependent regulation of T-2 toxin highly inducible Cytochrome P450 1A4 gene. FASEB J. 2021;35:e21469. doi:https://doi.org/10.1096/fj.202002570RR.
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi:https://doi.org/10.1016/j.cell.2013.05.039.
Nebert, D. W., Robinson, J. R., Niwa, A., Kumaki, K. & Poland, A. P. Genetic expression of aryl hydrocarbon hydroxylase activity in the mouse. J Cell Physiol. 1975;85:393–414. doi:https://doi.org/10.1002/jcp.1040850407.
Poland, A. & Kende, A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: environmental contaminant and molecular probe. Fed Proc. 1976;35:2404–2411.
Bersten, D. C., Sullivan, A. E., Peet, D. J. & Whitelaw, M. L. bHLH-PAS proteins in cancer. Nature Reviews Cancer. 2013;13:827–841. doi:https://doi.org/10.1038/nrc3621.
Kudo, I. et al. The regulation mechanisms of AhR by molecular chaperone complex. The Journal of Biochemistry. 2018;163:223–232. doi:https://doi.org/10.1093/jb/mvx074.
Guyot, E., Chevallier, A., Barouki, R. & Coumoul, X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov Today. 2013;18:479–486. doi:https://doi.org/10.1016/j.drudis.2012.11.014.
McIntosh, B. E., Hogenesch, J. B. & Bradfield, C. A. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. doi:https://doi.org/10.1146/annurev-physiol-021909-135922.
Neavin, D. R., Liu, D., Ray, B. & Weinshilboum, R. M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int J Mol Sci. 2018;19. doi:https://doi.org/10.3390/ijms19123851.
Gasiewicz, T. A., Singh, K. P. & Bennett, J. A. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci. 2014;1310:44–50. doi:https://doi.org/10.1111/nyas.12361.
Hernández-Ochoa, I., Karman, B. N. & Flaws, J. A. The Role of the Aryl Hydrocarbon Receptor in the Female Reproductive System. Biochem Pharmacol. 2009;77:547–559. doi:https://doi.org/10.1016/j.bcp.2008.09.037.
Kaeberlein, M. How healthy is the healthspan concept? GeroScience. 2018;40:361–364. doi:https://doi.org/10.1007/s11357-018-0036-9.
Hirabayashi, Y. & Inoue, T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem Pharmacol. 2009;77:521–535. doi:https://doi.org/10.1016/j.bcp.2008.09.030.
Fernandez-Salguero, P. et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi:https://doi.org/10.1126/science.7732381.
Bravo-Ferrer, I. et al. Lack of the aryl hydrocarbon receptor accelerates aging in mice. The FASEB Journal. 2019;33:12644–12654. doi:https://doi.org/10.1096/fj.201901333R.
Singh, K. P., Casado, F. L., Opanashuk, L. A. & Gasiewicz, T. A. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2009;77:577–587. doi:https://doi.org/10.1016/j.bcp.2008.10.001.
Singh, K. P. et al. Loss of Aryl Hydrocarbon Receptor Promotes Gene Changes Associated with Premature Hematopoietic Stem Cell Exhaustion and Development of a Myeloproliferative Disorder in Aging Mice. Stem Cells and Development. 2013;23:95–106. doi:https://doi.org/10.1089/scd.2013.0346.
Papadopoli, D. et al. mTOR as a central regulator of lifespan and aging. F1000Res. 2019;8. doi:https://doi.org/10.12688/f1000research.17196.1.
Biljes, D. et al. Impaired glucose and lipid metabolism in ageing aryl hydrocarbon receptor deficient mice. EXCLI J. 2015;14:1153–1163. doi:https://doi.org/10.17179/excli2015-638.
Butler, R. et al. Uric acid stones in the urinary bladder of aryl hydrocarbon receptor (AhR) knockout mice. Proc Natl Acad Sci U S A. 2012;109:1122–1126. doi:https://doi.org/10.1073/pnas.1120581109.
Makhloufi, C. et al. Female AhR Knockout Mice Develop a Minor Renal Insufficiency in an Adenine-Diet Model of Chronic Kidney Disease. Int J Mol Sci. 2020;21:E2483. doi:https://doi.org/10.3390/ijms21072483.
Eckers, A. et al. The aryl hydrocarbon receptor promotes aging phenotypes across species. Scientific Reports. 2016;6:19618. doi:https://doi.org/10.1038/srep19618.
Chamoli, M., Singh, A., Malik, Y. & Mukhopadhyay, A. A novel kinase regulates dietary restriction-mediated longevity in Caenorhabditis elegans. Aging Cell. 2014;13:641–655. doi:https://doi.org/10.1111/acel.12218.
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23:56–73. doi:https://doi.org/10.1038/s41580-021-00411-4.
Brinkmann, V., Schiavi, A., Shaik, A., Puchta, D. R. & Ventura, N. Dietary and environmental factors have opposite AhR-dependent effects on C. elegans healthspan. Aging (Albany NY). 2020;13:104–133. doi:https://doi.org/10.18632/aging.202316.
Aarnio, V. et al. Fatty acid composition and gene expression profiles are altered in aryl hydrocarbon receptor-1 mutant Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:318–324. doi:https://doi.org/10.1016/j.cbpc.2009.12.006.
Serna, E., Cespedes, C. & Vina, J. Anti-Aging Physiological Roles of Aryl Hydrocarbon Receptor and Its Dietary Regulators. Int J Mol Sci. 2020;22. doi:https://doi.org/10.3390/ijms22010374.
Kaiser, H. et al. Kynurenine, a Tryptophan Metabolite That Increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxid Med Cell Longev. 2019;2019:9894238. doi:https://doi.org/10.1155/2019/9894238.
Rebelo-Marques, A. et al. Aging Hallmarks: The Benefits of Physical Exercise. Front Endocrinol (Lausanne). 2018;9:258. doi:https://doi.org/10.3389/fendo.2018.00258.
Schenk, A. et al. Acute exercise impacts AhR and PD-1 levels of CD8+ T-cells-Exploratory results from a randomized cross-over trial comparing endurance versus resistance exercise. Eur J Appl Physiol. 2021;121:637–644. doi:https://doi.org/10.1007/s00421-020-04552-w.
Joisten, N. et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc Immunol Rev. 2020;26:24–42.
Hwang, H. J. et al. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicology and Applied Pharmacology. 2016;304:121–132. doi:https://doi.org/10.1016/j.taap.2016.04.005.
Ishikawa, T., Takahashi, S., Morita, K., Okinaga, H. & Teramoto, T. Induction of AhR-mediated gene transcription by coffee. PLoS One. 2014;9:e102152. doi:https://doi.org/10.1371/journal.pone.0102152.
Zhang, Q. et al. Genotype effects contribute to variation in longitudinal methylome patterns in older people. Genome Medicine. 2018;10:75. doi:https://doi.org/10.1186/s13073-018-0585-7.
Field, A. E. et al. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Molecular Cell. 2018;71:882–895. doi:https://doi.org/10.1016/j.molcel.2018.08.008.
Pansoy, A., Ahmed, S., Valen, E., Sandelin, A. & Matthews, J. 3-methylcholanthrene induces differential recruitment of aryl hydrocarbon receptor to human promoters. Toxicol Sci. 2010;117:90–100. doi:https://doi.org/10.1093/toxsci/kfq096.
Shimizu, Y. et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proceedings of the National Academy of Sciences. 2000;97:779–782. doi:https://doi.org/10.1073/pnas.97.2.779.
Kaiser, H., Parker, E. & Hamrick, M. W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp Gerontol. 2020;130:110797. doi:https://doi.org/10.1016/j.exger.2019.110797.
Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. Nippon Kagaku Kaishi. 1939;60:1090–1100.
Burns, J., Yokota, T., Ashihara, H., Lean, M. E. J. & Crozier, A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi:https://doi.org/10.1021/jf0112973.
Revel, A. et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi:https://doi.org/10.1002/jat.916.
Pyo, I. S., Yun, S., Yoon, Y. E., Choi, J.-W. & Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules. 2020;25. doi:https://doi.org/10.3390/molecules25204649.
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi:https://doi.org/10.1038/nature01960.
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi:https://doi.org/10.1038/35065638.
Fischer, N. et al. The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4′-hydroxystilbene and trans-2,4′,5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J Pharm Pharmacol. 2017;69:73–81. doi:https://doi.org/10.1111/jphp.12657.
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi:https://doi.org/10.1038/nature02789.
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences. 2004;101:15998–16003. doi:https://doi.org/10.1073/pnas.0404184101.
Canedo-Santos, J. C. et al. Resveratrol shortens the chronological lifespan of Saccharomyces cerevisiae by a pro-oxidant mechanism. Yeast. 2022;39:193–207. doi:https://doi.org/10.1002/yea.3677.
Ramos-Gomez, M. et al. Resveratrol induces mitochondrial dysfunction and decreases chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. J Bioenerg Biomembr. 2017;49:241–251. doi:https://doi.org/10.1007/s10863-017-9709-9.
Hector, K. L., Lagisz, M. & Nakagawa, S. The effect of resveratrol on longevity across species: a meta-analysis. Biol Lett. 2012;8:790–793. doi:https://doi.org/10.1098/rsbl.2012.0316.
Bhullar, K. S. & Hubbard, B. P. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852:1209–1218. doi:https://doi.org/10.1016/j.bbadis.2015.01.012.
Harper, S. A. et al. Resveratrol and exercise combined to treat functional limitations in late life: A pilot randomized controlled trial. Exp Gerontol. 2021;143:111111. doi:https://doi.org/10.1016/j.exger.2020.111111.
Wong RH, Thaung Zaw JJ, Xian CJ, Howe PR. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J Bone Miner Res. 2020;35(11):2121–2131. doi:https://doi.org/10.1002/jbmr.4115
Huhn, S. et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults — A randomized controlled trial. Neuroimage. 2018;174:177–190. doi:https://doi.org/10.1016/j.neuroimage.2018.03.023.
Thaung Zaw, J. J., Howe, P. R. & Wong, R. H. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: A 24-month randomised, double-blind, placebo-controlled, crossover study. Clin Nutr. 2021;40:820–829. doi:https://doi.org/10.1016/j.clnu.2020.08.025.
Anand David, A. V., Arulmoli, R. & Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev. 2016;10:84–89. doi:https://doi.org/10.4103/0973-7847.194044.
Nguyen, L. P. & Bradfield, C. A. The Search for Endogenous Activators of the Aryl Hydrocarbon Receptor. Chem. Res. Toxicol. 2008;21:102–116. doi:https://doi.org/10.1021/tx7001965.
Mohammadi-Bardbori, A., Bengtsson, J., Rannug, U., Rannug, A. & Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem Res Toxicol. 2012;25:1878–1884. doi:https://doi.org/10.1021/tx300169e.
Wyld, L. et al. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers (Basel). 2020;12. doi:https://doi.org/10.3390/cancers12082134.
Pietsch, K., Saul, N., Menzel, R., Stürzenbaum, S. R. & Steinberg, C. E. W. Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology. 2009;10:565–578. doi:https://doi.org/10.1007/s10522-008-9199-6.
Kampkötter, A. et al. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:314–323. doi:https://doi.org/10.1016/j.cbpb.2007.10.004.
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi:https://doi.org/10.1038/s41591-018-0092-9.
Saul, N., Pietsch, K., Menzel, R. & Steinberg, C. E. W. Quercetin-mediated longevity in Caenorhabditis elegans: is DAF-16 involved? Mech Ageing Dev. 2008;129:611–613. doi:https://doi.org/10.1016/j.mad.2008.07.001.
Proshkina, E. et al. Geroprotective and Radioprotective Activity of Quercetin, (-)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front Pharmacol. 2016;7:505. doi:https://doi.org/10.3389/fphar.2016.00505.
Rinaldi, A. L. et al. Curcumin Activates the Aryl Hydrocarbon Receptor yet Significantly Inhibits (-)-Benzo(a)pyrene-7R-trans-7,8-dihydrodiol Bioactivation in Oral Squamous Cell Carcinoma Cells and Oral Mucosa. Cancer Res. 2002;62:5451–5456.
Liao, V. H.-C. et al. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 2011;132:480–487. doi:https://doi.org/10.1016/j.mad.2011.07.008.
Lee, K.-S. et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13:561–570. doi:https://doi.org/10.1089/rej.2010.1031.
Kitani, K., Osawa, T. & Yokozawa, T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi:https://doi.org/10.1007/s10522-007-9100-z.
Basnet, P. & Skalko-Basnet, N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi:https://doi.org/10.3390/molecules16064567.
McCubrey, J. A. et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY). 2017;9:1477–1536. doi:https://doi.org/10.18632/aging.101250.
Funk, J. L. et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–3464. doi:https://doi.org/10.1002/art.22180.
Chandran, B. & Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. doi:https://doi.org/10.1002/ptr.4639.
Jagtap, S. et al. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr Med Chem. 2009;16:1451–1462. doi:https://doi.org/10.2174/092986709787909578.
El-Agamy, D. S. Comparative effects of curcumin and resveratrol on aflatoxin B(1)-induced liver injury in rats. Arch Toxicol. 2010;84:389–396. doi:https://doi.org/10.1007/s00204-010-0511-2.
Lim, G. P. et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi:https://doi.org/10.1523/JNEUROSCI.21-21-08370.2001.
Monroy, A., Lithgow, G. J. & Alavez, S. Curcumin and neurodegenerative diseases. Biofactors. 2013;39:122–132. doi:https://doi.org/10.1002/biof.1063.
Rainey-Smith, S. R. et al. Curcumin and cognition: a randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br J Nutr. 2016;115:2106–2113. doi:https://doi.org/10.1017/S0007114516001203.
Rojas, L. B. A. & Gomes, M. B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6. doi:https://doi.org/10.1186/1758-5996-5-6.
Anisimov, V. N. et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi:https://doi.org/10.4161/cc.7.17.6625.
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi:https://doi.org/10.1038/ncomms3192.
TAME — Targeting Aging with Metformin. American Federation for Aging Research https://www.afar.org/tame-trial.
Wang, H.-C. & Huang, S.-K. Metformin inhibits IgE- and aryl hydrocarbon receptor-mediated mast cell activation in vitro and in vivo. European Journal of Immunology. 2018;48:1989–1996. doi:https://doi.org/10.1002/eji.201847706.
Do, M. T. et al. Metformin suppresses CYP1A1 and CYP1B1 expression in breast cancer cells by down-regulating aryl hydrocarbon receptor expression. Toxicol Appl Pharmacol. 2014;280:138–148. doi:https://doi.org/10.1016/j.taap.2014.07.021.
Swindell, W. R. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi:https://doi.org/10.1186/1471-2164-8-353.
Steinbaugh, M. J., Sun, L. Y., Bartke, A. & Miller, R. A. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am. J. Physiol. Endocrinol. Metab. 2012;303:E488–495. doi:https://doi.org/10.1152/ajpendo.00110.2012.
Miller, R. A. et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi:https://doi.org/10.1111/acel.12194.
Toba, G. & Aigaki, T. Disruption of the microsomal glutathione S-transferase-like gene reduces life span of Drosophila melanogaster. Gene. 2000;253:179–187. doi:https://doi.org/10.1016/s0378-1119(00)00246-8.
McElwee, J. J., Schuster, E., Blanc, E., Thomas, J. H. & Gems, D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi:https://doi.org/10.1074/jbc.M406207200.
Zhai, G. et al. Eight common genetic variants associated with serum DHEAS levels suggest a key role in ageing mechanisms. PLoS Genet. 2011;7:e1002025. doi:https://doi.org/10.1371/journal.pgen.1002025.
Zeng, Y. et al. Novel loci and pathways significantly associated with longevity. Sci Rep. 2016;6:21243. doi:https://doi.org/10.1038/srep21243.
Shore, D. E. & Ruvkun, G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013;23:409–420. doi:https://doi.org/10.1016/j.tcb.2013.04.007.
Herholz, M. et al. KLF-1 orchestrates a xenobiotic detoxification program essential for longevity of mitochondrial mutants. Nat Commun. 2019;10:3323. doi:https://doi.org/10.1038/s41467-019-11275-w.
Kim, I.-W., Han, N., Burckart, G. J. & Oh, J. M. Epigenetic changes in gene expression for drug-metabolizing enzymes and transporters. Pharmacotherapy. 2014;34:140–150. doi:https://doi.org/10.1002/phar.1362.
Fisel, P., Schaeffeler, E. & Schwab, M. DNA Methylation of ADME Genes. Clin. Pharmacol. Ther. 2016;99:512–527. doi:https://doi.org/10.1002/cpt.343.
Kronfol, M. M. et al. DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. Geroscience. 2020;42:819–832. doi:https://doi.org/10.1007/s11357-020-00181-5.
Fader, K. A. et al. Convergence of Hepcidin Deficiency, Systemic Iron Overloading, Heme Accumulation, and REV-ERBa/β Activation in Aryl Hydrocarbon Receptor-Elicited Hepatotoxicity. Toxicol Appl Pharmacol. 2017;321:1–17. doi:https://doi.org/10.1016/j.taap.2017.02.006.
Sandoval-Sierra, J. V. et al. Body weight and high-fat diet are associated with epigenetic aging in female members of the BXD murine family. Aging Cell. 2020;19:e13207. doi:https://doi.org/10.1111/acel.13207.
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi:https://doi.org/10.1038/nbt.1630.
Thomas, P. D. et al. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Science. 2022;31:8–22. doi:https://doi.org/10.1002/pro.4218.
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;194. doi:https://doi.org/10.1093/nar/gkac194.
Kudo, K., Takeuchi, T., Murakami, Y., Ebina, M. & Kikuchi, H. Characterization of the region of the aryl hydrocarbon receptor required for ligand dependency of transactivation using chimeric receptor between Drosophila and Mus musculus. Biochim Biophys Acta. 2009;1789:477–486. doi:https://doi.org/10.1016/j.bbagrm.2009.06.003.
Gouédard, C., Barouki, R. & Morel, Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler Thromb Vasc Biol. 2004;24:2378–2383. doi:https://doi.org/10.1161/01.ATV.0000146530.24736.ce.
Rytel, M. R. et al. DNA methylation in the adipose tissue and whole blood of Agent Orange-exposed Operation Ranch Hand veterans: a pilot study. Environ Health. 2021;20:43. doi:https://doi.org/10.1186/s12940-021-00717-y.
Kelsey, K. T. et al. Serum dioxin and DNA methylation in the sperm of operation ranch hand veterans exposed to Agent Orange. Environ Health. 2019;18:91. doi:https://doi.org/10.1186/s12940-019-0533-z.
Amenya, H. Z., Tohyama, C. & Ohsako, S. Dioxin induces Ahr-dependent robust DNA demethylation of the Cyp1a1 promoter via Tdg in the mouse liver. Sci Rep. 2016;6:34989. doi:https://doi.org/10.1038/srep34989.
Selmin, O. I., Donovan, M. G., Stillwater, B. J., Neumayer, L. & Romagnolo, D. F. Epigenetic Regulation and Dietary Control of Triple Negative Breast Cancer. Front Nutr. 2020;7:159. doi:https://doi.org/10.3389/fnut.2020.00159.
Zhu, W. et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi:https://doi.org/10.1080/01635581.2012.654926.
Farhan, M. et al. Differential Methylation and Acetylation as the Epigenetic Basis of Resveratrol’s Anticancer Activity. Medicines (Basel). 2019;6:24. doi:https://doi.org/10.3390/medicines6010024.
Liggett, W. H. & Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197–1206. doi:https://doi.org/10.1200/JCO.1998.16.3.1197.
Busch, C. et al. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7:64. doi:https://doi.org/10.1186/s13148-015-0095-z.
Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. doi:https://doi.org/10.1056/NEJM199705153362001
Al-Yousef, N., Shinwari, Z., Al-Shahrani, B., Al-Showimi, M. & Al-Moghrabi, N. Curcumin induces re-expression of BRCA1 and suppression of y synuclein by modulating DNA promoter methylation in breast cancer cell lines. Oncol Rep. 2020;43:827–838. doi:https://doi.org/10.3892/or.2020.7473.
Sonowal, R. et al. Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A. 2017;114:E7506–E7515. doi:https://doi.org/10.1073/pnas.1706464114.
Bjeldanes, L. F., Kim, J. Y., Grose, K. R., Bartholomew, J. C. & Bradfield, C. A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88:9543–9547. doi:https://doi.org/10.1073/pnas.88.21.9543.
Rosser, E. C. et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020;31:837–851.e10. doi:https://doi.org/10.1016/j.cmet.2020.03.003.
Jin, U.-H. et al. Microbiome-Derived Tryptophan Metabolites and Their Aryl Hydrocarbon Receptor-Dependent Agonist and Antagonist Activities. Mol Pharmacol. 2014;85:777–788. doi:https://doi.org/10.1124/mol.113.091165.
Acknowledgment
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Gulf War Illness Research Program under (Award No. W81XWH-20-1-0278). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Additionally, the research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under (Award No. R15AG061649) to JLM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: We report no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Abudahab, S., Price, E.T., Dozmorov, M.G. et al. The Aryl Hydrocarbon Receptor, Epigenetics and the Aging Process. J Nutr Health Aging 27, 291–300 (2023). https://doi.org/10.1007/s12603-023-1908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-023-1908-1