Abstract
Glucagon-like peptide-2 (GLP-2) plays a major role in repairing impaired intestinal mucosa, but its mechanism in the improvement of intestinal barrier function during the aging process remains unclear. In this study, 26-month-old male Sprague-Dawley rats were randomized to control group and GLP-2 group treated with a dose of 250 μg•kg-1•d-1 by intraperitoneal injection. After 14 days of treatment, intestinal mucosal morphometric changes were observed by light microscopy and transmission electron microscopy (TEM). Small intestinal permeability was evaluated by fluorescein isothiocyanate (FITC)-labeled dextran. The mRNA and protein expression of Zonula Occludens-1 (ZO-1), occludin, claudin-1 and the GLP-2 receptor (GLP-2R) were detected by Real-time PCR and Western blot. Our results showed that GLP-2 administration significantly improved the age-related atrophy of intestinal mucosa and villi and increased small intestinal permeability. The mRNA and protein expression of ZO-1and occludin in ileum were up regulated in the GLP-2-treated old rats. In addition, the serum GLP-2 levels were negatively correlated with small intestinal permeability measured by FITC-dextran levels (r=-0.610, P<0.01). Taking all these data together, it is concluded that GLP-2 improved small intestinal epithelial barrier function in aged rats mainly by facilitating intestinal mucosa growth, alleviating the increased small intestinal permeability and increasing ZO-1 and occludin expression. Our observations provide evidence for the clinical significance of GLP-2 in preventing the intestinal epithelial barrier dysfunction during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The functions of various tissues and organs are degraded in the course of aging, including intestinal barrier dysfunction. Changes in the intestinal mucosal barrier function, nutritional absorption, mucosal immunity, and intestinal microorganism patterns are more commonly seen in geriatric patients (1). Impaired blood flow, ischemic changes, chronic psychological stress and increased use of NSAIDs naturally contribute to an impaired epithelial barrier in elderly patients (2). Integrity of intestinal epithelial cells serves as the largest mucosal barrier between the internal host and external environment. Intestinal permeability indicates the mucosal barrier integrity and describes the paracellular leakiness of the intestinal lining. Increased intestinal permeability is one of the important signs of impaired intestinal barrier function. Previous studies have reported that intestinal permeability increases with age in rodents and old baboons, suggesting an age-related declination of the intestinal barrier function (3-5).
Ageing is accompanied by a chronic state of low-grade inflammatory status. The intestinal environment is substantially changed with ageing, which in turn contributes to the disturbances in the inflammatory status of the elderly [6]. Disturbances of the gut barrier have long been associated with local as well as systemic diseases (7). Age-related intestinal barrier dysfunction and differences in the gut microbiota composition are related to the progression of inflammatory aging and age-related diseases (8). It is generally perceived that the intestinal mucosal barrier becomes leaky in the elderly and contributes to the occurrence of age-related diseases, such as Parkinson’s disease, Alzheimer’s disease, atherosclerosis, and diabetes (9, 10). Current understanding regarding the effects of aging on the physical and immunological properties of this important epithelial barrier is limited. Our previous studies have showed that age-related intestinal barrier dysfunction may be associated with mucosal atrophy, damages to tight junction (TJ) structure, and remodeling of intestinal epithelial tight junction proteins (11). Studies on the mechanism and protective measures of intestinal barrier dysfunction may provide information to future clinical treatments to improve the quality of life of geriatric patients.
Glucagon-like peptide-2 (GLP-2), a gut hormone that promotes growth and function of the intestinal epithelium, is derived from the L cells of the distal ileum and colon in response to proximal enteric neuronal signaling and luminal nutrients. The intestinal growth-promoting effects of GLP-2 result in significant enhancements in both small intestinal weight and crypt-villus height in mice (12). Extensive studies have demonstrated that GLP-2 played a major role in the repair of impaired intestinal mucosa by promoting intestinal growth, attenuating apoptosis in the intestinal mucosal epithelial cells, and reducing intestinal permeability (13-15). GLP-2 primarily functions as a trophic regulator of mucosal function in the small intestine. Recent studies demonstrated that GLP-2 administration increased the crypt cell proliferation rate, suppressed apoptosis, and decreased the level of inflammatory cytokines in rat models of inflammatory bowel disease, necrotizing enterocolitis, and neonatal piglets (16, 17). However, the protective effects and underlying mechanisms of GLP-2 on age-related intestinal barrier dysfunction still remains unclear.
On the basis of previous research studies, we hypothesized that GLP-2 works as an intestinal specific growth factor, maintaining mucosal health and reducing intestinal permeability in naturally aged rat model intestines. Intestinal mucosal morphometry, intestinal permeability, intestinal epithelial TJ structure and TJ proteins expression, and GLP-2 receptor (GLP-2R) expression were examined in this study to clarify the protective effects of GLP-2 on the epithelial barrier of aged intestines.
Materials and methods
Animals and materials
3-month-old and 26-month-old male Sprague-Dawley rats were provided by Animal Science Laboratory, Fudan University. The study protocol was approved by the local institutional review board at the authors’ affiliated institution and animal study was carried out in accordance with the established institutional and state guidelines regarding use of experimental animals. All rats were grouped after one week of adaptive feeding. A total of 24 rats were assigned to four groups: (1) Young rats (3-month-old, n=6); (2) Young+GLP-2 rats (3-month-old rats treated with GLP-2, n=6); (3) Old rats (26-month-old, n=6); (4) Old+GLP-2 rats (26-month-old rats treated with GLP-2, n=6). Young+GLP-2 rats and Old+GLP-2 rats were given GLP-2 (Mimotopes, China) at a dose of 250 μg•kg-1•d-1 by intraperitoneal injection, 2 times/day, for 14 consecutive days. The dose of GLP-2 was decided based on our preliminary experiments. On the fifteenth day, the rats were fasted for 12 hours and then anesthetized intraperitoneally with 10% chloral hydrate before sampling and analysis.
All chemical reagents, with the exception of those specifically noted in the following sections, were obtained from Sigma-Aldrich (St Louis, MO, USA). Antibodies were obtained from Abcam (Abcam, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). Materials used throughout the study were of high grade quality.
Small Intestinal permeability measurement
The small intestinal permeability was measured by intraluminal injection of fluorescently labeled macromolecules. The rats were fixed in supine position after being anesthetized. The abdominal wall was incised to expose the abdominal cavity. A 20-cm segment of small intestine was separated below the ligament of Treitz. Both ends of the intestinal tract were clamped with a bulldog clamp wrapped with a rubber sleeve. One milliliter of phosphate-buffered solution (PBS) containing 25 mg of fluorescein isothiocyanate-labeled dextran (FITCdextran, SIGMA, USA) was injected into the intestinal canal. Thirty minutes later, 100 μL of blood was extracted from the portal vein. The blood sample was immediately dissolved in 1.9 mL of 50 mM Tris solution (Invitrogen, USA) and centrifuged at 3000 g, 4°C for 7 min. The supernatant was collected and placed in a specialized 96-well plate of a spectrofluorometer to measure the FITC-dextran concentration. The excitation wave length of the spectrofluorometer was set at 480 nm, and the emission wave length was set at 520 nm. The level of FITCdextran was calculated according to a standard curve (in units of μg/mL).
Intestinal morphology and histology
The rats were euthanized, and their intestines were removed and then washed by PBS. A 1-cm section of the distal ileum was harvested and fixed in 4% paraformaldehyde, and then embedded in paraffin. The resulting 4-μm sections were stained with hematoxylin and eosin (HE). Each stained section was examined under light microscopy (Olympus IX51, Tokyo, Japan). The intestinal villus height and surface area were measured in ten villi for each section by Image-Pro Plus software (Image-Pro Plus, Media Cybernetics, Inc., Rockville, MD, USA), and the mean was calculated.
Transmission electron microscopy (TEM)
The proximal ileal specimens were fixed for at least 4 h in Karnovsky solution (2.5 % glutaraldehyde and 2 % paraformaldehyde in 0.1 M sodium-cacodylate buffer, pH 7.0). The fixed samples were washed three times for 10 min with a 0.1 M sodium-cacodylate buffer (pH 7.0) followed by a second fixation in 1% cacodylate-buffered osmium tetroxide solution for 1 hour. After dehydration in an increasing series of alcohol solution, the samples were embedded in Epon-812 resin and cut into thin sections and observed using a JEM-1200 transmission electron microscope (Jeol Ltd., Tokyo, Japan).
Real-time polymerase chain reaction
Total RNA from ileal mucosal tissue was extracted using Trizol reagent according to the manufacturer’s instructions. cDNA was synthesized by reverse transcription using the Prime ScriptTM reagent kit (TaKaRa, Japan). Quantitative real-time PCR was carried out using the SYBR fluorescence (TaKaRa, Japan) on an ABI 7500 SDS RT-PCR system (Applied Biosystems, Foster City, CA, USA). The reaction conditions were as follows: 40 cycles of two-stage PCR consisting of pre denaturation at 95 °C for 30 s and subsequently amplification cycle (95 °C for 5 s, 60 °C for 20 s, 72 °C for 30 s). GAPDH was used as a reference gene. The relative expression of each protein mRNA was calculated using the 2-ΔΔCt method. The expression of ZO-1, occludin, claudin-1 and GLP-2 receptor (GLP-2R) was assayed.
All primers were synthesized by Shanghai BioSune Biotech Co. Ltd. The primer sequences used for quantitative PCR were as follows:
-
ZO- 1 [ N M _ 0 0 1 1 0 6 2 6 6 . 1 ] , f o r w a r d 5’ - CCCGAAACTGATGCTATGGAT -3’ and reverse 5’- GCCTTGGAATGTATGTGGAGA -3’ (146 bp)
-
o c c l u d i n [ N M _ 0 3 1 3 2 9 . 2 ] , f o r w a r d 5’ - TACGGCTACGGTTACGGCTAT -3’ and reverse 5’- ATCACCAAGGAAGCGATGAAG -3’ (101 bp)
-
c l a u d i n - 1 [NM_03 1 6 9 9 . 2 ] , f o r w a r d 5’ - ATCGGCTCTATCGTCAGCACT -3’ and reverse 5’- GACATCCACAGTCCCTCGTAG -3’ (104 bp)
-
G L P - 2 R [ N M _ 0 2 1 8 4 8 . 1 ] , f o r w a r d 5’ - ACCTGTTCGCTTCGTTCATC -3’ and reverse 5’- ATCCATCCACTCTCATCATCG -3’ (103 bp)
-
GAPDH, forward 5’- AACGACCCCTTCATTGAC -3’ and reverse 5’- TCCACGACATACTCAGCAC -3’ (191 bp)
Western blot analysis
The ileal tissue was scraped and added to radio immunoprecipitation assay (RIPA) lysis buffer. Proteins were collected to determine the concentration. Gel electrophoresis was conducted with 40 μg of protein per well. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA). After blocking with 5% skim milk proteins for 2 h at room temperature, membranes were probed with polyclonal rabbit anti-rat antibodies that were targeted against ZO-1 (1:250; Beyotime, China), occludin (1:1000; Abcam, USA), claudin-1 (1:1000; Abcam, USA), GLP-2R (1:200; Abcam, USA), and β-actin (1:1000; Santa Cruz, USA) for overnight at 4 °C. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:1000; Beyotime, China) at room temperature for 1 hour. After washing twice with TBST, membrane-bound antibodies were visualized using an enhanced chemiluminescent kit (Beyotime, China) according to the manufacturer’s instructions. The housekeeping gene β-actin was used as an internal control. The integral optical density (IOD) of each band was measured using Image software (Pro 3DS 6.0, National Institutes of Health, USA).
Enzyme-linked immunosorbent assay
The serum GLP-2 level was analyzed using a rat GLP-2 ELISA assay kit (Xinqidi, Wuhan, China) according to the manufacturer’s instructions. The optical density (OD) was measured at wavelengths of 450 and 630 nm.
Statistical analysis
Data are presented as mean ±SD. Statistical analyses were performed using SPSS 17.0. One-way analysis of variance (ANOVA) was used to compare data between different groups with post-hoc analysis by the least significant difference (LSD). A P-value <0.05 was considered to be statistically significant.
Results
GLP-2 alleviated morphometric changes in ileal mucosa and increased the density of tight junctions in old rats
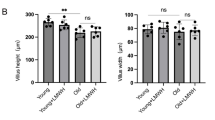
The ileal mucosal morphology was observed by using light microscope. In the young rats, complete intestinal villus structure was observed and the intestinal villi were arranged regularly. In contrast, the ileal mucosal layer was atrophied, mucosal thickness and glands were reduced, and the intestinal villi were scattered and narrowed in the old rats. GLP-2 promoted intestinal villi and gland growth in both young and old rats. More obviously, GLP-2 treatment improved agerelated changes of intestinal mucosal morphology in the old rats, including reduced mucosal atrophy, increased villus height and villus number, and a more orderly arrangement of intestinal villi (Fig.1A).
The villus height, width and superficial area were measured by the image analysis software. As shown in Fig.1B, ileal villus height, width, and surface area were significantly decreased in the old rats (P<0.01) compared with the young rats. GLP-2 treatment increased villus height, width and surface area (P<0.01).
Transmission electron microscopy was used to observe the ultra-structure of intestinal epithelial TJs in rats. The results showed that the TJs between epithelial cells were intact and compact, the desmosomes exhibited high density in the young and young+GLP-2 rats. There were increased vacuoles in the epithelial cells, damaged TJ structure with loosened connections, widened gaps and decreased density, and incomplete desmosome structure in the old rats. GLP-2 treatment improved age-related changes of TJ structure (Fig. 1C).
GLP-2 decreased small intestinal permeability and increased serum GLP-2 level in old rats
The small intestinal permeability was measured by FITCdextran level. As showed in Fig. 2A, the FITC-dextran level was markedly higher in the Old group than in the Young group (12.845±1.064 μg/mL vs. 3.565±0.246 μg/mL, P<0.05). The FITC-dextran level was slightly lower in the Young+GLP-2 group than in the Young group, but showed no statistically significant difference (P>0.05). However, a significantly lower value was found in the Old+GLP-2 group compared with the Old group (6.928±0.628 μg/mL vs. 12.845±1.064 μg/mL, P<0.01).
The serum GLP-2 level was obviously lower in the Old group than in the Young group (P<0.01). GLP-2 treatment increased the GLP-2 level was in both young rats (1.801±0.305 ng/mL vs. 0.995±0.139 ng/mL, P<0.01) and old rats (0.905±0.147 ng/mL vs. 0.544±0.060 ng/mL, P<0.01) (Fig. 2B). The difference of GLP-2 levels in young rats before and after GLP-2 administration was higher than that in old rats (0.806 ng/mL vs. 0.361 ng/mL).
The result suggested a negative correlation between the GLP-2 and FITC-dextran levels (r=-0.610, P<0.01; Fig. 2C).
GLP-2 up regulated expression of ZO-1 and occludin in old rats
The mRNA expression of TJ proteins (ZO-1, occludin, and claudin-1) in ileum were analyzed by qRT-PCR and the protein expressions were determined by Western blot. Compared to the Young group, the mRNA and protein expressions of ZO-1, occludin and claudin-1 were markedly decreased in old rats (Fig. 3, Fig. 4). GLP-2 treatment resulted in increased mRNA and protein expressions of ZO-1 and occludin in both young rats and old rats (P<0.01; Fig. 3A-B, Fig. 4A-B). Claudin-1 expression was increased slightly in the old rats after GLP-2 treatment; however, the increase was not significant (P>0.05;Fig. 3C, 4C).
Ileal GLP-2R expression decreased in old rats but not associated with increased small intestinal permeability
Compared to the Young group, the mRNA and protein expressions of GLP-2R were remarkably decreased in the Old group (Fig. 5). There was no significant increase in the GLP- 2R mRNA or protein expression after GLP-2 administration in neither young rats nor old rats (P>0.05).
We also observed the relationships between small intestinal permeability and TJs or GLP-2R expression. As showed in Fig 6, there was a significantly negative correlation between FITCdextran levels and protein expression of ZO-1, occludin and claudin-1(P<0.05). However, the protein expression of GLP-2R was not associated with FITC-dextran levels (P>0.05).
Discussion
Results of our previous study (11) and this study showed obvious intestinal mucosal atrophy in the ileal tissue and damage to the TJ structure in naturally aged rats. These results were consistent with the previously reported results regarding age-related histological changes in the rat small intestine. The current study further examined the changes in the small intestinal permeability and serum GLP-2 levels in 26-monthold aged rats to provide a clearer picture of the mechanism of intestinal barrier dysfunction with age.
Extensive evidence has demonstrated that various digestive diseases are associated with intestinal barrier dysfunction and increase in the intestinal permeability (18, 19). In the older population, intestinal atrophy and the increase in the intestinal permeability were closely linked with age-related diseases (1, 20, 21). Firstly, many facts, including the decreased peristalsis capability and gland-secreted intestinal juice and digestive enzymes, result in digestion and absorption dysfunction. Secondly, the changes in gut microbiota composition and increased gram-negative bacteria often lead to over release of lipopolysaccharide (LPS). Impaired intestinal barrier and increased intestinal permeability leads to easy absorption of LPS into blood, resulting in endotoxemia and systemic lowgrade inflammation.
In this study, we found that the small intestinal permeability was significantly increased in 26-month-old rats, which was consistent with the previous study reports that have described age-related epithelial permeability changes in the rat intestine (4, 22-24). Intestinal permeability reflects the barrier function of gut mucosa, which relies on a number of structural elements, most prominently the epithelial TJ proteins (25). A pivotal contributing factor to the geriatric gastrointestinal dysfunction was increased intestinal permeability via age-associated remodeling of intestinal epithelial TJs (26, 27). We analyzed the expression of TJ proteins (ZO-1, occludin, and claudin-1) in the rat ileum. As expected, ZO-1, occludin, and claudin-1 mRNA and protein expression were remarkably decreased in 26-month old rats together with TJ ultra-structural change.
GLP-2 is a newly discovered intestinal epithelial-specific growth factor. Previous studies have demonstrated that GLP-2 increases intestinal digestion, absorptive and barrier functions under a wide variety of conditions. Therefore, GLP-2 is considered as a potential therapeutic method in patients with intestinal dysfunction (15, 28). For the first time, we investigated the protective effect of GLP-2 on intestinal barrier dysfunction induced by aging. In this study, we found that the serum levels of GLP-2 in old rats were decreased and GLP-2 level was negatively correlated with the small intestinal permeability (FITC-dextran level). Continuous GLP-2 treatment for 14 consecutive days promoted the growth of intestinal villi, reduced the intestinal mucosa atrophy, and alleviated the tight junction ultra-structure in 26-month-old rats. In addition, GLP-2 treatment reduced small intestinal permeability and increased ZO-1 and occludin expressions in the ileum of old rats. These results demonstrated beneficial effects of GLP-2 in preventing or repairing age-related intestinal barrier impairment. These results are consistent with previous studies, where administration of exogenous GLP-2 promoted intestinal mucosal growth and repair in animal models of enteritis, burns, and ischemia-reperfusion (29-31).
The secretion and metabolism of GLP-2 are influenced by many factors, especially nutrient ingestion and DDP-IV activity (15, 28). We also confirmed that the serum GLP-2 levels were negatively correlated with age in adults with or without Diabetes (data not show). However, the specific reasons and mechanisms of GLP-2 secretion decrease during the process of ageing are still unknown. In the present study, after administration of GLP-2 young rats exhibited more than two fold of increasing serum GLP-2 level as compared with old rats. This difference couldn’t be simply explained by different weight of old and young rats, taking into account that the dose of GLP-2 used in our experiments was calculated according to the weight of rats. This difference of circulating GLP-2 levels between young and old rats could be explained by different degradation mechanisms of GLP-2 in ageing. This way will be further explored by using resistant GLP-2 analogues or inhibiting GLP-2 degrading enzymes.
GLP-2 activities were mediated by glucagon-like peptide-2 receptor (GLP-2R), which is highly selective for GLP- 2. However, expression of GLP-2R was almost exclusively restricted to the intestinal tract and its mRNA transcript levels are extremely rare (32). The definition of GLP-2R and the precise sites of GLP-2R expression within the gastrointestinal tracts of different animals and humans are still controversial. Some researchers believe the distribution of GLP-2R in the intestinal endocrine cells, whereas others suggest that GLP- 2R was not confined to particular cells or cell lines due to the possible existence of subtypes (33, 34). We subsequently detected the expression of GLP-2R in rat ileum. Data showed that mRNA and protein expressions of GLP-2R were relatively lower in old rats compared to the young counterparts. GLP-2 treatment did not increase the expression of GLP-2R neither in young rats nor in old rats, suggesting that GLP-2R may not entirely depend on the “quantity” to play its expected role.
The protective mechanism of GLP-2 in the intestinal barrier function has not been fully elucidated. According to previous studies, GLP-2R is localized to enteroendocrine cells, subepithelial myofibroblasts and enteric neurons, but is not found in either proliferative crypt cells or enterocytes. These results have led to the conclusion that the growth and functional effects of GLP-2 on the gut are mediated indirectly through other intestinal growth factors (16, 34, 35). As the low GLP-2R levels are difficult to reconcile with the profound effects of GLP-2R activation, it is likely that these factors work in concert to amplify the intestinotrophic actions of GLP-2 (36, 37). Research has shown that GLP-2/GLP-2R signaling pathways include PKA-dependent and PKA-independent cAMP pathways, ERK/PI3-dependent pathway, PI3K-AKT-mTOR pathway, and the PKC-PI3K and Ras/MAPK pathway (38, 39). The exact protective mechanism of GLP-2 in age-related intestinal barrier dysfunction remains to be elucidated.
Aging is a degenerative process which strongly associated with chronic low-grade inflammation. Inflamm-aging involves inflammatory network activation and the release of senescenceassociated factors, including key pro-inflammatory mediators, such as nuclear factor-kappa B (NF-κB) (9, 10). Elevated circulating levels of inflammatory cytokines in the elderly may cause impairment of the intestinal barrier function by chronically altering the structure and localization of TJs. Aging affects properties of the intestinal barrier likely to impact on age-associated disturbances, both locally and systemically (2, 6). GLP-2 enhances barrier formation and attenuates TNF- α-induced changes in a Caco-2 cell model of the intestinal barrier (40). Our study also indicated that GLP-2 not only improves the monolayer permeability of Caco-2 cells, but also reduces the high permeability induced by IFN-γ (data not show). Further evidence is needed to know the effect of GLP-2 in the reduction of intestinal barrier dysfunction caused by chronic inflammation during aging. In addition, the family of proglucagon-derived peptides such as GLP-2 and Glicentin are produced by L-intestinal cells from duodenum to rectum and play some similar roles in intestinal physiology and glucose metabolism (28, 41). Further study to assess its circulating variations in aging animal models offers promising perspectives to investigate its usefulness as non-invasive biomarker in the process of aging and age related disease.
Natural aging animal models are indispensable tools for the investigation of pathogenesis and underlying mechanisms in geriatric patients, which play a key role in the initial development of potential treatment strategies. There are of course few limitations to this current study. Firstly, we did not observe the expression of GLR-2R in different parts of the intestinal tract and inflammatory status in aged rats. Secondly, we did not perform an in-depth analysis of the mechanism of GLP-2 and its associated signaling pathways involved. These problems need to be further investigated and solved in future studies. In addition, a larger sample helps to reduce individual differences.
In conclusion, this study demonstrated that serum GLP-2 level was negatively correlated with small intestinal permeability in 26-month-old rats. GLP-2 treatment improved age-related intestinal barrier dysfunction, reducing intestinal mucosa atrophy, strengthening the TJ structure, increasing the expression of TJ proteins (ZO-1 and occludin), and reducing the small intestinal permeability. GLP-2 may be an effective therapeutic approach for the improvement of intestinal barrier function in elderly patients after clarification of its mechanism of action in appropriate models including native human tissue.
Declaration of interest: The authors declare no competing financial interests. None of the authors affiliated with this manuscript have any commercial or associations that might pose a conflict interest.
Author contribution: Weiying Ren, Jiayu Wu and Li Li participated in performed experiments, experimental design, data assembly, analysis and manuscript writing. Yu Hu participated in experimental design and was responsible for the overall direction of work. Yi Lu and Yikai Shao participated in performed experiments and data assembly. Yiming Qi, Binger Xu, and Yuting He participated in data interpretation and references collection. All authors read and approved the final manuscript.
Acknowledgements: This work was supported by the National Natural Science Foundation of China (grant number 81570795).
Ethical Standards: Ethical approval for research involving animals by animal ethics committee of Zhongshan Hospital, Fudan University.
Conflict of Interest: The authors declare that there is no conflict of interest.
Abbreviations
- GLP-2:
-
glucagon-like peptide-2
- GLP-2R:
-
glucagon-like peptide-2 receptor
- TJ:
-
tight junction
- TEM:
-
transmission electron microscopy
- FITC:
-
fluorescein isothiocyanate
- HE:
-
hematoxylin and eosin
References
Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol 2014; 289: 112–8. https://doi.org/10.1016/j.cellimm.2014.04.001
Nicoletti C. Age-associated changes of the intestinal epithelial barrier: local and systemic implications. Expert Rev Gastroenterol Hepatol 2015; 9: 1467–9. http://dx.doi.org/10.1586/17474124.2015.1092872
Hollander D, Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology 1985; 31:133–7.
Katz D, Hollander D, Said HM, Dadufalza V. Aging-associated increase in intestinal permeability to polyethylene glycol 900. Dig Dis Sci 1987; 32: 285–8.
Tran L, Greenwood-Van Meerveld B. Age-Associated Remodeling of the Intestinal Epithelial Barrier. J Gerontol A Biol Sci Med Sci 2013; 68:1045–56. https://doi.org/10.1093/gerona/glt106
Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat Res 2010; 690: 50–6. https://doi.org/10.1016/j.mrfmmm.2009.07.011
Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care 2003; 9:143–51.
Franceschi C and Campisi J. Chronic inflammation (inflammageing) and its potential contribution to age-associated diseases. J Gerontol A Biol Med Sci 2014; 69 Suppl1: S4–9. https://doi.org/10.1093/gerona/glu057
Gong J, Hu M, Huang Z, Fang K, Wang D, Chen Q, et al. Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front Pharmacol 2017; 8: 42. https://doi.org/10.3389/fphar.2017.00042
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 2015; 21: 10609–20. https://doi.org/10.3748/wjg.v21.i37.10609
Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang SC, et al. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res 2014; 26(2):183–91. https://doi.org/10.1007/s40520-013-0148-0
Drucker DJ, Erlich P, Asa S L, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A 1996; 93(15): 7911–6.
Litvak DA, Hellmich MR, Evers BM, Banker NA, Townsend CM Jr. Glucagon-like peptide 2 is a potent growth factor for small intestine and colon. J Gastrointest Surg 1998; 2(2):146–50.
Chen X, Zhao HX, Fu XS, Li CP, Zhong XL. Glucagonlike peptide 2 protects intestinal barrier in severe acute pancreatitis through regulating intestinal epithelial cell proliferation and apoptosis. Pancreas 2012; 41(7): 1080–5. https://doi. org/10.1097/MPA.0b013e31824966b0
Austin K, Markovic MA, Brubaker PL. Current and potential therapeutic targets of glucagonlike peptide-2. Curr Opin in Pharmacol 2016; 31:13–8. https://doi.org/10.1016/j.coph.2016.08.008
Wu J, Qi KK, Xu ZW. Porcine glucagon-like peptide-2 microspheres ameliorate inflammation in lipopolysaccharide-challenged weaning piglets. J Anim Sci 2016; 94(12):5286–94. https://doi.org/10.2527/jas.2016-1007
Nakame K, Kaji T, Mukai M, Shinyama S, Matsufuji H. The protective and anti-inflammatory effects of glucagon-likepeptide-2 in an experimental rat model of necrotizing enterocolitis. Peptides 2016; 75: 1–7. https://doi.org/10.1016/j.peptides.2015.07.025
Moore SA, Nighot P, Reyes C, Rawat M, McKee J, Lemon D, et al. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg 2016; 51(12):1907–13. http://dx.doi.org/10.1016/j.jpedsurg.2016.09.011
Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil 2016; 28(7):957–65. DOI: 10.1111/nmo.12871
Valensinin L, Ramminger S, Haas V, Postrach E, Werich M, Fischer A, et al. Small intestine permeability in older adults. Physiol Rep 2014; 2(4): e00281. DOI: 10.14814/phy2.281
Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, et al. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci(Lond) 2015; 129(7): 515–27. DOI: 10.1042/CS20150046
Mullin JM, Valenzano MC, Verrecchio JJ, Kothari R. Age-and diet-related increase in transepithelial colon permeability of Fischer 344 rats. Dig Dis Sci 2002; 47(10):2262–70.
Ma TY, Hollander D, Dadufalza V, Krugliak P. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol 1992; 27(3):321–33.
Chen YM, Zhang JS, Duan XL. Changes of microvascular architecture, ultrastructure and permeability of rat jejunal villi at different ages. World J Gastroenterol 2003; 9(4):795–9.
Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 2004; 84(3):282–91.
Meier J, Sturm A. The intestinal epithelial barrier: does it become impaired with age? Dig Dis 2009; 27(3):240–5. https://doi.org/10.1159/000228556
Mabbott, NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS. Aging and the mucosal immune system in the intestine. Biogerontology 2015; 16(2): 133–45. DOI:10.1007/s10522-014-9498-z
Baldassano S, Amato A. GLP-2: What do we know? What are we going to discover? Regul Pept 2014; 194-195:6–10. https://doi.org/10.1016/j.regpep.2014.09.002
Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther 2005; 314(1): 214–20. https://doi.org/10.1124/jpet.105.085373
Zhang W, Zhu W, Zhang J, Li N, Li J. Protective effects of glucagon-like peptide 2 on intestinal ischemia-reperfusion rats. Microsurgery 2008; 28(4):285–90. DOI: 10.1002/micr.20491
Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep 2008; 10(5):443–9.
Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 2000; 119(3): 744–55.
Pedersen J, Pedersen NB, Brix SW, Grunddal KV, Rosenkilde MM, Hartmann B, et al. The glucagon-like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides 2015; 67:20–8. https://doi.org/10.1016/j. peptides.2015.02.007
Rowland KJ, Trivedi S, Lee D, Wan K, Kulkarni RN, Holzenberger M, et al. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulinlike growth factor-1-receptor deletion. Gastroenterology 2011; 141(6): 2166–75. http://dx.doi.org/10.1053/j.gastro.2011.09.014
Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol 2014; 76:561–83. https://doi.org/10.1146/ annurev-physiol-021113-170317
Rowland KJ, Brubaker PL. The «cryptic» mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol 2011; 301(1): G1–8. DOI: 10.1152/ajpgi.00039.2011
Lei Q, Bi J, Wang X, Jiang T, Wu C, Tian F, et al. GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition. Nutrients 2016; 8: 33. DOI: 10.3390/nu8010033
Yu C, Jia G, Deng QH, Zhao H, Chen X, Liu G, et al. The effects of glucagonlike peptide-2 on the tight junction and barrier function in IPEC-J2 cells through phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling pathway. Asian-Australas J Anim Sci 2016; 29(5): 731–8. https://doi. org/10.5713/ajas.15.0415
Qi KK, Sun YQ, Wan J, Deng B, Men XM, Wu J, et al. Effect of porcine glucagonlike peptides-2 on tight junction in GLP-2R + IPEC-J2 cell through the PI 3 k/Akt/mTOR/p70 S6K signalling pathway. J Animl Physiol Anim Nutr (Berl) 2017; doi: 10.1111/jpn.12644.
Moran GW, O’Neill C, McLaughlin JT. GLP-2 enhances barrier formation and attenuates TNFa-induced changes in a Caco-2 cell model of the intestinal barrier. Regul Pept 2012; 178(1-3):95–101. https://doi.org/10.1016/j.regpep.2012.07.002
Raffort J, Lareyre F, Massalou D, Fénichel P, Panaia-Ferrari P, Chinetti G. Insights on glicentin, a promising peptide of the proglucagon family. Biochem Med 2017; 27(2):308–324. https://doi.org/10.11613/BM.2017.034
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, W., Wu, J., Li, L. et al. Glucagon-Like Peptide-2 Improve Intestinal Mucosal Barrier Function in Aged Rats. J Nutr Health Aging 22, 731–738 (2018). https://doi.org/10.1007/s12603-018-1022-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-018-1022-8