Abstract
Background and objectives
Low protein diets supplemented with keto acid (sLPD) are recommended for patients with stage 3–5 chronic kidney disease (CKD). This study assessed whether sLPD is beneficial for patients with steroid-resistant proteinuria during early-stage CKD.
Design, setting, participants, and measurements
A 1-year randomized controlled trial was conducted from 2010 to 2012. In this study, 108 proteinuric patients who were steroid-resistant were assigned to a sLPD group (0.6 g/kg/d with 0.09 g/kg/d keto acids) or a normal protein diet group (NPD, 1.0 g/kg/d). Estimated dietary protein intake, urinary protein excretion, remission rate, renal function, nutritional status, and blood pressure were measured.
Results
Baseline characteristics were comparable between the sLPD group (47 patients) and the NPD group (49 patients). Urinary protein excretion significantly decreased in sLPD compared to NPD in months 6, 9, and 12 (P<0.05). Proteinuria reduction was higher in sLPD than in NPD (P<0.001) at the end of the study. Complete remission and partial remission rates were higher in sLPD than in NPD. Serum albumin and pre-albumin levels were higher in sLPD than in NPD in months 9 and 12 (P<0.05). Serum total cholesterol and triglyceride levels declined more significantly in sLPD than in NPD (P<0.01) at the end of the study. There were no differences in nutritional status, renal function, hemoglobin, or blood pressure between the two groups.
Conclusions
sLPD is both nutritionally safe and beneficial, providing nephroprotective effects for early-stage CKD patients with steroid-resistant proteinuria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteinuria is an independent risk factor for primary glomerular disease (PGD) progressing to end-stage renal disease. In spite of recent immunosuppressive advances in anti-proteinuric therapy, some patients have persistently overt proteinuria. In the past decades, high-protein diet was prescribed to patients with overt proteinuria, intractable edema, or diuretic-resistant oliguria. However, high-protein diets tend to aggravate proteinuria and accelerate the loss in renal function (1). Now normal protein diet (NPD) is conventionally prescribed to patients with overt proteinuria; however, to the best of our knowledge, no studies have evaluated whether NPD is optimal for these patients.
The Kidney Disease Outcomes Quality Initiative (KDOQI) recommends low-protein diet (LPD) for patients with stage 3–5 chronic kidney disease (CKD) (2) because this diet slows the progression of renal failure by limiting the accumulation of nitrogenous wastes, reducing intraglomerular pressure, decreasing glomerular hypertrophy, and exerting nonhemodynamic effects. Ketoacids (KA) are used in LPDs for preserving nutritional status (3). Interestingly, data from animal studies suggest a beneficial effect of LPD and KA on CKD by down-regulating pro-inflammatory factors, (4) reducing oxidative stress, (5) and ameliorating renal tubular epithelial-mesenchymal transition, conditions that are closely associated with the renal damage induced by proteinuria in PGD. Therefore, we hypothesize that KA-supplemented LPD might be beneficial for proteinuric patients with early-stage CKD.
We conducted a single-center, prospective, randomized controlled trial (RCT) to compare the effects between KA-supplemented LPD and NPD on the clinical outcomes of stage 1-2 CKD patients with steroid-resistant proteinuria.
Materials and Methods
Study design
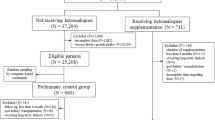
This single-center, prospective RCT was conducted between January 2010 and December 2012 at the Nephrology Department of the First Affiliated Hospital of Dalian Medical University. The study protocol is depicted in Figure 1. Patients with incipient overt proteinuria were diagnosed by renal biopsy with PGD and initiated a one-month standardized steroid screening. Patients who were steroid-resistant were randomly assigned to either a KA-supplemented LPD (sLPD) group with 0.6 g/kg/d diet protein index (DPI) and 0.09 g/kg/d KA (Ketosteril® Fresenius Kabi, Bad Homburg, Germany) or an NPD group with 1.0 g/kg/d DPI. Patients (18–80 yr of age) with an initial estimated GFR (eGFR) ≥60 ml/min/1.73 m2 by MDRD were eligible for the study, whereas patients with urine protein levels <2.0 g/d were excluded. Other exclusion criteria were the presence of secondary glomerular or chronic wasting diseases, acute inflammation, stress, or acute life-threatening diseases; BMI values ≥30 kg/m2 or ≤14 kg/m2; pregnant or nursing patients; refusal to accept renal biopsies; and dubious compliance with sLPD. Withdrawal criteria included the following: LPD or KA intolerance; malnutrition; doubled serum creatinine or eGFR <60 ml/min/1.73 m2; and conditions that affected therapy. Enrolled patients were evaluated in a CKD outpatient clinic four times during one year (at months 3, 6, 9, and 12). Blood pressure, body weight, height, waist circumference, and upper-arm muscular circumference were measured at each follow-up visit. Urinary protein (24 h), serum albumin, creatinine, blood urea nitrogen, total cholesterol, triglyceride, hemoglobin, serum pre-albumin, and urinary urea levels were measured by routine methods.
The study was approved by the Ethical Committee of the First Affiliated Hospital of Dalian Medical University and registered in the Chinese Clinical Trial Registry (ChiCTR number: ChiCTR-TRC-12002820). Written informed consent forms were obtained from each patient prior to randomization.
Dietary compliance
Patients were instructed by dieticians to monitor their protein intakes using food exchange lists. DPI was calculated with the Keto Nutritional Assessment software (version 2.0; Fresenius-Kabi Pharmaceutical Co., Ltd, Beijing, China). The protein equivalent of KA nitrogen was included in the DPI calculations. Total energy intake was expected to be 30–35 kcal/kg/d in both groups. In the sLPD group, caloric balance was achieved by increasing carbohydrate intakes. Compliance to the prescribed protein intake was assessed by 24-h urinary urea nitrogen (UUN) levels according to Mitch-Maroni’s formula: protein intake normalized to weight (g/kg/day) = 6.25 × {[UUN (g/day)] + [0.031 × body weight (kg)]}/body weight (kg). Adherence to LPD was defined as a protein intake reduction of ≥20% (6). Patient compliance was considered optimal if the achieved protein intake was <10% from the recommended protein values.
Medications
Patients in both groups initially received intravenous methylprednisolone pulse therapy (0.5 g, once daily, for three consecutive days), followed by oral prednisone at a dose of 0.8-1.0 mg/kg/d (maximum dose 60 mg/d) for 8 weeks, which was then tapered by 5 mg every two weeks to 20 mg/day, and tapered by 2.5 mg each month to a maintenance dose. Throughout the study, both groups received 3 days of 50 mg leflunomide (Arava®, Suzhou changzheng-xinkai Pharmaceutical, co., LTD) followed by 20 mg/d thereafter. Anti-hypertensive treatment was based on telmisartan (80 mg/d, Micardis®, Boehringer Ingelheim Investment Co., LTD), with additional dihydropyridine calcium channel blockers when necessary. Diuretics were given if the patient had edema. The target blood pressure was ≤130/80 mmHg. Atorvastatin (20 mg/d, Lipitor®, Pfizer Pharmaceutical Co., Ltd) was used for hyperlipidemia. Calcium-supplement agents, proton pumpinhibitive agents, and anti-coagulative agents were used as adjunctive medications with steroids when necessary.
Outcome variables and definitions
The primary outcomes of this study were urinary protein excretion and complete or partial remission rate. Complete remission (CR) was defined by urinary protein levels ≤0.3 g/day; partial remission (PR) was defined by urinary protein levels >0.3 g/day and ≥50% reduction in urinary protein levels; no remission (NR) was defined as <50% reduction in urinary protein levels. The secondary outcomes of this study included serum creatinine, albumin, pre-albumin, cholesterol, and triglycerides levels; eGFR; nutritional status; and adverse effects.
Statistical analyses
Data are expressed as mean ± standard deviation (variables with normal distribution), median and interquartile range (IQR 25–75%; variables with asymmetrical distribution), or percent (binary data). Normality was assessed by the skewness test. To evaluate whether NPD and sLPD groups had similar characteristics at baseline, an independent sample t-test was used for normal quantitative variables, a nonparametric test (Mann-Whitney U-test and Wilcoxon’s rank sum test) was used for non-normal variables, and the Pearson chi-square test was used to compare categorized variables or proportions. Trend differences between the NPD and sLPD groups were assessed by two-way multivariate analysis of variance (MANOVA). One-way analysis of variance (ANOVA) was used to evaluate anthropometric changes during the intervention. Statistical analyses were performed with SPSS 17.0 software (SPSS, Inc, Chicago, IL); P < 0.05 was considered to be statistically significant.
Results
Baseline data
A total of 160 patients were recruited (Figure 2) and 108 patients were enrolled. However, during the study, 12 patients dropped out; therefore, 49 patients in the NPD group and 47 patients in the sLPD group completed the study. The baseline characteristics are summarized in Table 1. There were no significant differences at baseline between the two groups. Medications were similar in the two groups at baseline and follow-ups. Blood pressure was within the predicted target in both groups.
Renal biopsies revealed the presence of membranous nephropathy (48.9% in sLPD and 59.2% in NPD), IgA nephropathy (21.3% in sLPD and 22.4% in NPD), mesangioproliferative glomerulonephritis (17% in sLPD and 12.2% in NPD), and focal segmental glomerulosclerosis (6.4% in sLPD and 4.1% in NPD). The frequency of renal pathological types was similar between the two groups (Table 1).
Dietary intake and compliance
There were no statistical differences in DPI between the two groups at the beginning of the study. During the study, the mean DPI value estimated by 3-day dietary records in NPD and sLPD were 1.10 g/kg/d and 0.75 g/kg/d, respectively, which amounted to 110% and 125%, respectively, of the recommended levels. When estimated by UUN, DPI in NPD and sLPD was 1.06 g/kg/d and 0.73 g/kg/d, respectively, which amounted to 106% and 122%, respectively, of the recommended levels (Table 3).
Urinary protein excretion and outcomes
Figure 3a shows the urinary protein levels during the intervention. Proteinuria had a downward trend after steroid and immunosuppressant therapy; however, the sLPD group had a higher proteinuria reduction rate than NPD, achieving significant reductions in urinary protein levels at months 6, 9, and 12, with a nadir at month 12 (P = 0.027, P = 0.001, and P < 0.001, respectively). MANOVA revealed that sLPD had greater anti-proteinuric effects compared to NPD (P = 0.021). The median urinary protein level in NPD was 3.572 g/d (IQR = 2.566−5.948 g/d) on the first visit and was reduced to 1.263 g/d (IQR = 0.672−1.994 g/d) on the last visit. sLPD was associated with an early and sustained decline in proteinuria from 4.161 g/d (IQR = 2.967−5.238 g/d) on the first visit to 0.363 g/d (IQR = 0.158−0.987 g/d) on the last visit. The reduction rates in proteinuria are shown in Figure 3b. sLPD treatment reduced urinary protein levels by 85.16% ± 13.42% compared to 62.45% ± 23.90% by NPD treatment (P < 0.001). At the end of the study, 22 patients (46.81%) in sLPD and 7 patients (14.29%) in NPD reached CR. PR was achieved by 25 patients (53.19%) in sLPD and 25 patients (51.02%) in NPD. The proportion of patients achieving CR or PR was significantly higher in sLPD than in NPD (Figure 3c). No patient in the sLPD group was recorded as NR; however, 17 patients (34.69%) in NPD remained NR.
(a) Changes in urinary protein levels in NPD and sLPD during the study (NPD•, sLPD□). Proteinuria is expressed as median and IQR. Based on repeated-measures ANOVA, there was a significant time effect for proteinuria compared to month 1 in both the sLPD and NPD groups (P < 0.01). Based on two-way MANOVA, there was a significant group effect for proteinuria between NPD and sLPD in months 6, 9, and 12 (*P < 0.05). (b) The rate of proteinuria reduction in NPD and sLPD at month 12 (NPD•, sLPD▪). Parameters were expressed individually relative to month 1. Median values are depicted by horizontal bars. Proteinuria reduction rates were higher in sLPD than in NPD (85.16% ± 13.42% versus 62.45% ± 23.90%, P < 0.01). (c) Percentage distribution of patients with respect to remission in NPD and sLPD. CR, complete remission; PR, partial remission; NR, no remission.
Blood biochemistry
At the beginning of the study, renal function, assessed by either serum creatinine or eGFR, ranged from normal to mildly impaired (eGFR >60 ml/min/1.73 m2) in both groups. During the intervention, a slight decline in serum creatinine levels and a modest increase in eGFR were observed in sLPD patients compared to NPD patients, who had relatively stable serum creatinine levels and eGFR. However, there were no significant differences between the two groups in serum creatinine levels or eGFR (P > 0.05; Figures 4c and 4d). Hemoglobin levels remained stable in both groups, whereas serum total cholesterol and triglyceride levels decreased (Figures 4e and 4f). The reduction in lipid levels was significantly higher in sLPD patients than in NPD patients (P = 0.003 for total cholesterol; P = 0.001 for triglycerides) by the end of the follow-up period. Systolic and diastolic blood pressure, which did not differ between the two groups at the baseline, remained on target during the entire study.
Nutritional assessment
Table 2 shows the changes in anthropometric parameters during the study. The results revealed that waist circumferences and arm muscle circumferences were within the normal limits in both groups; there were no changes before and after treatment. BMI values remained stable throughout the study in both groups (P > 0.05). No statistical differences were obtained in BMI, waist circumferences, or arm muscle circumferences between the sLPD and NPD groups (P > 0.05).
Plasma albumin, which reflects visceral protein storage, is a common index of nutritional status, whereas plasma prealbumin is a more sensitive indicator of protein malnutrition. Our results revealed that plasma albumin and pre-albumin levels increased in both groups from months 3 to 6. However, plasma albumin and pre-albumin levels were significantly higher in sLPD from months 9 to 12 than in NPD, and the latter had plasma albumin levels of 37–39 g/L (P = 0.032 and P < 0.001, respectively) and plasma pre-albumin levels of 0.37 g/L (both P < 0.001) during this period (Figures 4a and 4b). The increase in plasma albumin and pre-albumin levels was concomitant with a reduction in proteinuria in both groups. MANOVA revealed no differences in serum albumin levels (P = 0.304); however, there were significant differences in prealbumin levels (P = 0.002) between the groups.
Adverse effects
The results showed that none of the patients suffered from malnutrition during the study. No severe adverse effects associated to the medication were observed. No death was registered in any group. The most common complaints included the monotony of the dietary regimen, the need for ‘too many pills’ in addition to the complex polypharmacy, and the economic burden brought by Ketosteril.
Discussion
The KDOQI recommends the use of LPD when eGFR is <60 ml/min/1.73 m2. In patients with early-stage CKD, LPD was not recommended due to lack of evidence on its safety and efficacy (7). Mou S et al studied the effects of LPD with or without KA supplementation on the nutritional status and proteinuria of patients with hepatitis B virus infection and stage 1–2 CKD glomerulonephritis (8). However, one major limitation of the study was that the subjects were not representative of the CKD population. Additionally, the sample size was too small, putting into question the validity of the study.
It is crucial to assess the effects of sLPD on patients with overt proteinuria and hypoproteinemia as a result of the associated uncertain risk of malnutrition and infections, in addition to its uncertain efficacy and practicability in earlystage CKD. In this study, we enrolled stage 1–2 CKD patients with steroid-resistant proteinuria. The patients received either sLPD or NPD for almost 1 year. The results revealed that the early commencement of sLPD is safe and had positive effects on patients with proteinuric CKD. The ability of sLPD to reduce the progression of renal failure without negatively affecting the clinical outcomes or nutritional status of the patients has been reported by other researchers (9). Our study differed in that sLPD was used in early-stage CKD to assess the effects on urinary protein excretion and disease outcomes. Refractory nephrotic syndrome is one of the causative factors for CKD progression. The preservation of renal function largely depends on the degree of proteinuria reduction. We initiated the dietary intervention after the proteinuric patients proved to be resistant to a 4-week steroid therapy to eliminate the effects of glucocorticoids on proteinuria. At follow-up, similar doses of leflunomide, telmisartan, and atorvastatin were given to both groups, and blood pressure was controlled to the same target values to eliminate other possible factors that affect proteinuria levels. Therefore, the beneficial effect of sLPD on proteinuria was independent of these factors. To avoid inadequate amino acid intake and reduce the risk of undernutrition, we administered KA with LPD; KA does not stimulate filtration or change renal hemodynamics (10).
Previous studies had reported difficult compliance to long-term LPD even when intensive counseling was provided. (11) Allowing for a possibly >20% higher of the measured protein intake than the target, a 0.6 g/kg/d DPI was set up in sLPD group. In our study, it was testified that the measured protein intake was roughly 0.75 g/kg/day in sLPD group, which, still within the LPD range, exhibited a substantial reduction compared to the NPD group. Besides, the imprecise compliance to LPD would acceptably compromise to its additive benefit for proteinuria. Future strategies would be employed to improve compliance by (12-14) incorporating compliant patients with favorable psychosocial factors (e.g., satisfaction and comprehension), collaborative efforts of physician and certified dietitian in personalized scheme by training, education, counseling and monitoring, and providing a broader offer of diet options, supplemented with amino and keto acids and substituted with protein-free commercial food.
Proteinuria, which is an indicator of renal damage, (15) was the primary outcome in this study. After 6 months, there was a more significant reduction in proteinuria in the sLPD group than in the NPD group. The statistical significance became more pronounced with time. After 12 months, there was an additional 23% reduction in proteinuria with sLPD compared to NPD, although both groups received steroid and immunosuppressant therapy. Consistent with our results, a previous study involving nephropathic patients who consumed LPD without corticosteroid or cytotoxic agents found a 20% reduction in proteinuria (16). We speculate that the anti-proteinuric effect was likely attributed to LPD and KA and that this effect was independent of steroid and/or immunosuppressant therapy. Similar to the findings obtained in animals, LPD and KA resulted in less proteinuria, which was possibly mediated by lower transforming growth factor-β1, fibronectin, and oxidative damage (5, 8). Therefore, KA supplementation in LPD may have a protective and pharmacological effect.
In this study, we demonstrated that sLPD is safe and adequately maintains the nutritional status of early-stage CKD proteinuric patients for at least one year. Nutritional assessment parameters were either maintained or normalized in both groups; the sLPD group had a more rapid normalization of hypoalbuminemia and hypoprealbuminemia, and higher levels of plasma albumin and pre-albumin during the last 3 months than the NPD group. This result may be attributed to KA supplementation, which corrects negative nitrogen balance and improves protein status by providing essential amino acids (17). The increase in plasma albumin levels was concomitant with the reduction in urinary protein in both groups; however, the significant effects on plasma albumin levels occurred later than the significant change in levels of proteinuria. The increase in plasma albumin levels could be attributed to not only the reduction in urinary protein loss, but also a complex adaptation of protein metabolism associated with postprandial stimulation of protein synthesis and a reduction of whole-body protein catabolism after glucocorticoid therapy. Our results are in accordance with recent small-scale studies using sLPD to treat Chinese patients with hepatitis B virus infection and early-stage chronic glomerulonephritis (8).
Renal function was similar at enrollment and during the study. Even though the sLPD group had lower creatinine levels and higher eGFR values, there were no significant differences by the end of the study, which could be attributed to the short-term treatment period. However, higher GFR values do not always represent a better prognosis of kidney function because glomerular hyperfiltration may occur in the early stages of renal damage. The long-term effects of sLPD should be assessed with longer follow-up periods.
An independent and modifiable risk factor that affects proteinuric CKD is dyslipidemia, (18, 19) which emerges as a result of urinary protein loss. Hyperlipidemia can lead to an over-production of mesangial matrix components and further aggravate glomerulosclerosis. There is evidence that controlling blood lipid levels may slow the progression of renal disease. In this study, sLPD ameliorated the lipid metabolism disorder by significantly lowering serum cholesterol and triglyceride levels. This finding contrasted with another report that stated VLPD+KA increased serum triglycerides levels in patients with advanced renal failure, (12) which was explained as being due to increased carbohydrate intakes. The exact mechanism for the lipid-lowering effects of sLPD in our study are unknown. The effect was probably secondary to proteinuria reduction and better diet control but not to medication because the use of lipid-lowering drugs was similar between the two groups.
This study had some strong points: it demonstrated that sLPD is a feasible, sustainable, and favorable diet. Additionally, there were low drop-out rates. The low drop-out rates emphasized the importance of the ‘patient effect’ in diet choices, thereby arguing against the often-held conception that sLPD is too difficult for patients. The study also demonstrated that sLPD improves short-term prognosis of proteinuric patients and may constitute another efficient therapeutic alternative in the treatment of early-stage CKD.
On the other hand, the study had some limitations. First, we could not differentiate the effects among LPD, KA, and LPD+KA. By assessing the differences between the sLPD and LPD groups we would be able to obtain more information; however, patients on LPD had a risk of protein-energy wasting in the presence of hypoalbuminemia (unpublished data) and low compliance, which required a dietary modification. Secondly, the absence of a run-in phase of more than 3 months before the initiation of dietary treatment does not exclude the possibility of spontaneous improvement in proteinuria in a few patients. However, the sample size was large enough and the possibility was similar in both groups. Third, the achieved protein intake in the sLPD group surpassed the recommendations. Patient compliance is representative of only such clinics that are equipped with specific resources (e.g., dietitians). Fourth, the overlap of nephropathy stages and pathologic types in the subgroup analysis generates the risk of heterogeneity.
In summary, this study showed that LPD supplemented with KA can maintain the nutritional status of proteinuric patients with stage 1–2 CKD and maintain their renal function similarly to a conventional NPD. Moreover, sLPD suppresses proteinuria independent of immunosuppression and RAAS-inhibition and improves serum lipid profiles. Therefore, sLPD might constitute another therapeutic alternative in the treatment of early-stage CKD with severe proteinuria. Further studies, such as larger-scale, multicenter RCTs, with higher statistical power, are needed to confirm the long-term renal benefits associated with sLPD in patients with stage 1–2 CKD.
Disclosures: None.
Acknowledgements: The work was supported by grants from the National Clinical Key Discipline Project (no grant number), and the Ketosteril Research Award by Fresenius Kabi. We thank the physicians, dietitians and nurses of the First Affiliated Hospital of Dalian Medical University for their assistance.
References
Binser-Foucard F, Belot A, Delafosse P, Remontet L, Woronoff A, Bossad N. I. Friedman AN. High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis 2004;44: 950–962
Schena FP. Management of patients with chronic kidney disease. Intern Emerg Med Suppl 2011;1: 77–83
Chang JH, Kim DK, Park JT, Kang EW, Yoo TH, Kim BS, Choi KH, Lee HY, Han DS, Shin SK. Influence of ketoanalogs supplementation on the progression in chronic kidney disease patients who had training on low-protein diet. Nephrology (Carlton) 2009;14: 750–757
Gao X, Huang L, Grosjean F, Esposito V, Wu J, Fu L, Hu H, Tan J, He C, Gray S, Jain MK, Zheng F, Mei C. Low-protein diet supplemented with ketoacids reduces the severity of renal disease in 5/6 nephrectomized rats: a role for KLF15. Kidney Int 2011;79: 987–996
Gao X, Wu J, Dong Z, Hua C, Hu H, Mei C. A low-protein diet supplemented with ketoacids plays a more protective role against oxidative stress of rat kidney tissue with 5/6 nephrectomy than a low-protein diet alone. Br J Nutr 2010;103: 608–616
Paes-Barreto JG, Silva MI, Qureshi AR, Bregman R, Cervante VF, Carrero JJ, Avesani CM. Can renal nutrition education improve adherence to a low-protein diet in patients with stages 3 to 5 chronic kidneydisease? J Ren Nutr 2013;23: 164–171
Di Iorio BR, Bellizzi V, Bellasi A, Torraca S, D’Arrigo G, Tripepi G, Zoccali C. Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol Dial Transplant 2013;28: 632–640
Mou S, Li J, Yu Z, Wang Q, Ni Z. Keto acid-supplemented low-protein diet for treatment of adult patients with hepatitis B virus infection and chronic glomerulonephritis. J Int Med Res 2013;41: 129–137
Brunori G, Viola BF, Parrinello G, De Biase V, Como G, Franco V, Garibotto G, Zubani R, Cancarini GC. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomizedmulticenter controlled study. Am J Kidney Dis 2007;49: 569–580
Mir S, Ozkayin N, Akgun A. The role of keto acids in the supportive treatment of children with chronic renal failure. Pediatr Nephrol 2005;20: 950–955
Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant 2013;28: 2203–2205
Feiten SF, Draibe SA, Watanabe R, Duenhas MR, Baxmann AC, Nerbass FB, Cuppari L. Short-term effects of a very-low-protein diet supplemented with ketoacids in nondialyzed chronic kidney diseasepatients. Eur J Clin Nutr 2005;59: 129–136
Bellizzi V, Bedogni G, Quintaliani G. Compliance with low-protein diet in patients with chronic kidney disease. G Ital Nefrol 2008;25 Suppl 42: S45–49
Piccoli GB, Vigotti FN, Leone F, Capizzi I, Daidola G, Cabiddu G, Avagnina P. Low-protein diets in CKD: how can we achieve them? A narrative, pragmatic review. Clin Kidney J 2015;8: 61–70
Heras M, García-Cosmes P, Fernández-Reyes MJ, Sánchez R. Natural progression of renal function in the elderly: analysis of poor prognosis factors associated with chronic kidney disease. Nefrologia 2013;33: 462–469
Gansevoort RT, de Zeeuw D, de Jong PE. Additive antiproteinuric effect of ACE inhibition and low-protein diet in human renal disease. Nephrol Dial Transplant 1995;10: 497–504
Li H, Long Q, Shao C, Fan H, Yuan L, Huang B, Gu Y, Lin S, Hao C, Chen J. Effect of short-term low-protein diet supplemented with keto acids on hyperphosphatemia in maintenancehemodialysis patients. Blood Purif 2011;31: 33–40
Kent PS. Integrating clinical nutrition practice guidelines in chronic kidney disease. Nutr Clin Pract 2005;20: 213–217
Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int 2001;59: 260–269
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Xie, H., Fang, M. et al. Keto-supplemented low protein diet: A valid therapeutic approach for patients with steroid-resistant proteinuria during early-stage chronic kidney disease. J Nutr Health Aging 20, 420–427 (2016). https://doi.org/10.1007/s12603-015-0612-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-015-0612-y